Breast Cancer and Bone Mineral Density in a U.S. Cohort of Middle-Aged Women: Associations with Phosphate Toxicity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Qualitative Analysis—Grounded Theory Literature Review
“Concept formation in qualitative research is a systematic process whereby the researcher sets definitions for important concepts that emerge during the research. These definitions help to provide the parameters for the qualitative study”.[26]
2.1. Phosphate Toxicity and Tumorigenesis
2.2. Bone Remodeling and Dysregulated Phosphate Metabolism
“Bone remodeling is the process by which bone is renewed to maintain bone strength and mineral homeostasis. Remodeling involves continuous removal of discrete packets of old bone, replacement of these packets with newly synthesized proteinaceous matrix, and subsequent mineralization of the matrix to form new bone. The remodeling process resorbs old bone and forms new bone to prevent accumulation of bone microdamage”.[43]
“PTH can produce catabolic or anabolic effect(s) on bone metabolism depending on the level of the hormone, periodicity, and duration of exposure”.[48]
2.3. Metastatic Breast Cancer
“Metastases leading to overall bone loss are classified as osteolytic. Those leading to excess bone deposition are considered osteoblastic. However, both bone degradation and deposition likely occur early in the metastatic process. The majority of breast cancer metastases ultimately cause bone loss”.[51]
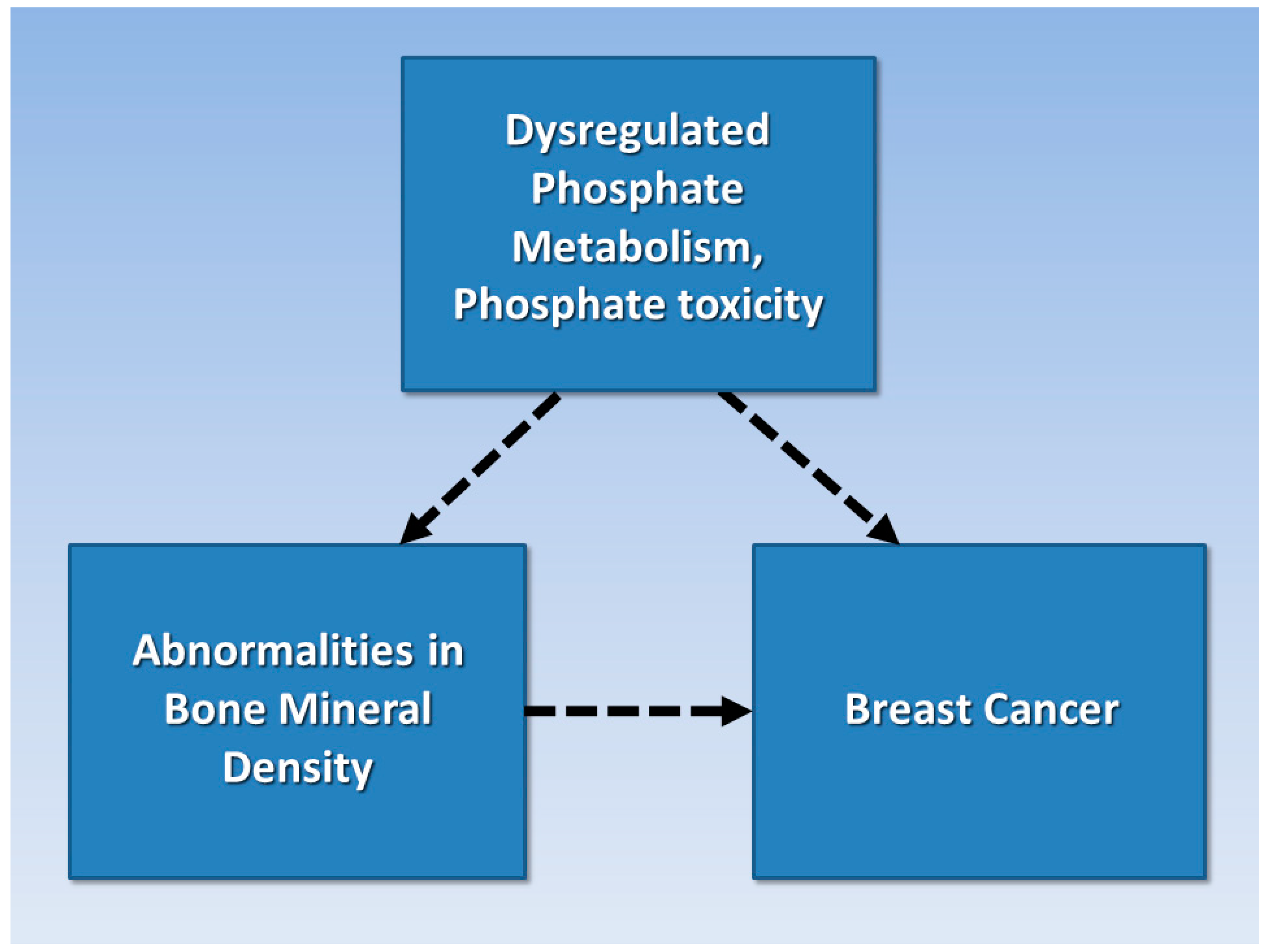
2.4. Hypothesis
3. Quantitative Analysis—Mixed-Effects Model
Quantitative Model Selection
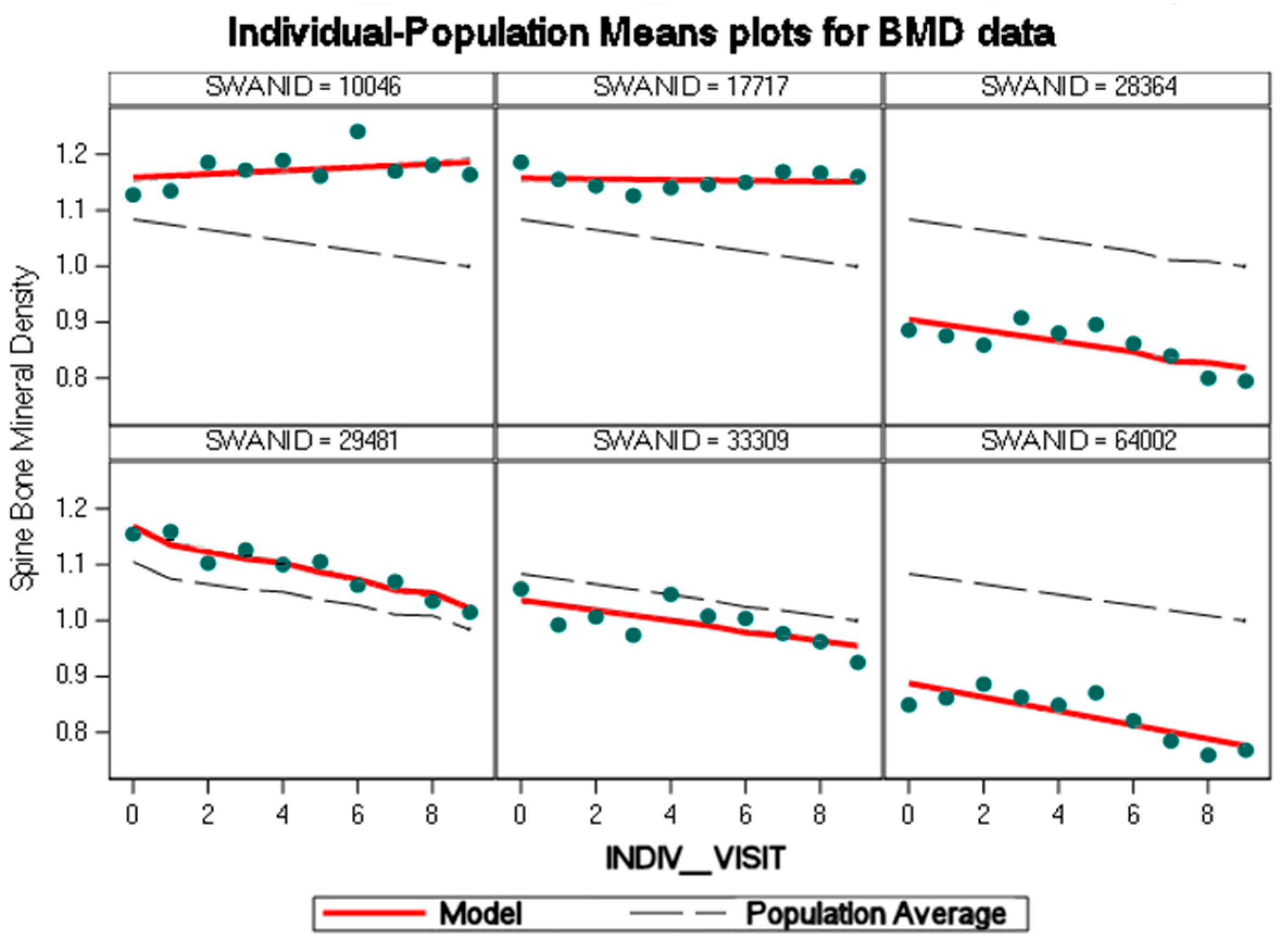
4. Results
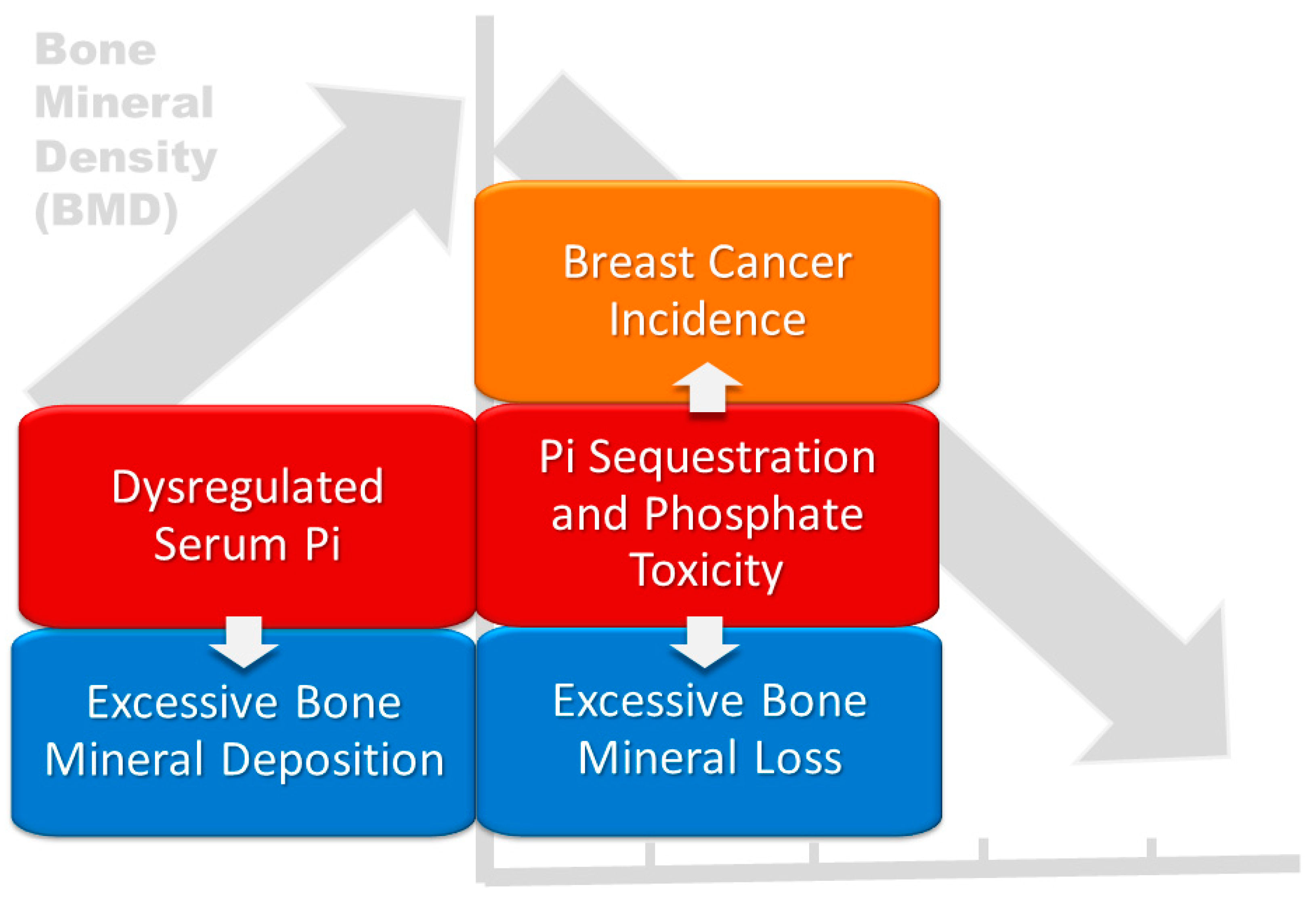
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraenkel, M.; Novack, V.; Mizrakli, Y.; Koretz, M.; Siris, E.; Norton, L.; Shafat, T.; Geffen, D.B. Bone mineral density in women newly diagnosed with breast cancer: A prospective cohort study. NPJ Breast Cancer 2022, 8, 21. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, X.; Qin, A.; Liu, G.; Zhai, Z.; Hao, Y.; Li, H.; Zhu, Z.; Dai, K. Bone mineral density and risk of breast cancer in postmenopausal women. Breast Cancer Res. Treat. 2013, 138, 261–271. [Google Scholar] [CrossRef]
- Fraenkel, M.; Novack, V.; Liel, Y.; Koretz, M.; Siris, E.; Norton, L.; Shafat, T.; Shany, S.; Geffen, D.B. Association between bone mineral density and incidence of breast cancer. PLoS ONE 2013, 8, e70980. [Google Scholar] [CrossRef]
- Gonçalves, L.V.; Martins, K.A.; Godinho-Mota, J.C.M.; Schincaglia, R.M.; Sousa, A.L.L.; Freitas-Junior, R. High Bone Mineral Density of the Lumbar Spine Is Positively Associated with Breast Cancer. Biomed Res. Int. 2019, 2019, 8010356. [Google Scholar] [CrossRef]
- Muhammad, A.; Mada, S.B.; Malami, I.; Forcados, G.E.; Erukainure, O.L.; Sani, H.; Abubakar, I.B. Postmenopausal osteoporosis and breast cancer: The biochemical links and beneficial effects of functional foods. Biomed. Pharmacother. 2018, 107, 571–582. [Google Scholar] [CrossRef]
- Taxel, P.; Faircloth, E.; Idrees, S.; Van Poznak, C. Cancer Treatment–Induced Bone Loss in Women with Breast Cancer and Men with Prostate Cancer. J. Endocr. Soc. 2018, 2, 574–588. [Google Scholar] [CrossRef]
- Canadian Cancer Society. All about Hormone Replacement Therapy (HRT). Available online: https://cancer.ca/en/cancer-information/reduce-your-risk/understand-hormones/all-about-hormone-replacement-therapy-hrt (accessed on 22 March 2022).
- Trémollieres, F.; Pouillès, J.-M.; Laparra, J.; Ribot, C. Bone mineral density at menopause does not predict breast cancer incidence. Osteoporos. Int. 2008, 19, 1497–1504. [Google Scholar] [CrossRef]
- Nagel, G.; Peter, R.S.; Klotz, E.; Brozek, W.; Concin, H. Bone mineral density and breast cancer risk: Results from the Vorarlberg Health Monitoring & Prevention Program and meta-analysis. Bone Rep. 2017, 7, 83–89. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Shepherd, J.; Creasman, J.; Tice, J.A.; Ziv, E.; Cummings, S.R. Are Breast Density and Bone Mineral Density Independent Risk Factors for Breast Cancer? JNCI J. Natl. Cancer Inst. 2005, 97, 368–374. [Google Scholar] [CrossRef]
- Stewart, A.; Kumar, V.; Torgerson, D.J.; Fraser, W.D.; Gilbert, F.J.; Reid, D.M. Axial BMD, change in BMD and bone turnover do not predict breast cancer incidence in early postmenopausal women. Osteoporos. Int. 2005, 16, 1627–1632. [Google Scholar] [CrossRef]
- Hardcastle, S.A.; Gregson, C.L.; Deere, K.C.; Davey Smith, G.; Dieppe, P.; Tobias, J.H. High bone mass is associated with an increased prevalence of joint replacement: A case-control study. Rheumatology 2013, 52, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Paccou, J.; Javier, R.M.; Henry-Desailly, I.; Ternynck, C.; Nottez, A.; Legroux-Gérot, I.; Robin, F.; Fardellone, P.; Lespessailles, E.; Roux, C.; et al. The French multicentre elevated bone mass study: Prevalence and causes. Osteoporos. Int. 2021, 32, 1763–1775. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B.; Razzaque, M.S. Phosphate toxicity and tumorigenesis. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2018, 1869, 303–309. [Google Scholar]
- Mahd, A.A.; Brown, R.B.; Razzaque, M.S. Osteoporosis in Populations with High Calcium Intake: Does Phosphate Toxicity Explain the Paradox? Indian J. Clin. Biochem. 2015, 30, 365–367. [Google Scholar]
- Brown, R.B.; Razzaque, M.S. Dysregulation of phosphate metabolism and conditions associated with phosphate toxicity. BoneKEy Rep. 2015, 4, 705. [Google Scholar]
- Hartman, M.-L.; Groppo, F.; Ohnishi, M.; Goodson, J.M.; Hasturk, H.; Tavares, M.; Yaskell, T.; Floros, C.; Behbehani, K.; Razzaque, M.S. Can salivary phosphate levels be an early biomarker to monitor the evolvement of obesity. In Phosphate and Vitamin D in Chronic Kidney Disease; Karger Publishers: Basel, Switzerland, 2013; Volume 180, pp. 138–148. [Google Scholar]
- Movilli, E.; Feliciani, A.; Camerini, C.; Brunori, G.; Zubani, R.; Scolari, F.; Parrinello, G.; Cancarini, G.C. A high calcium-phosphate product is associated with high C-reactive protein concentrations in hemodialysis patients. Nephron Clin. Pract. 2005, 101, c161–c167. [Google Scholar] [CrossRef]
- Greendale, G.A.; Jackson, N.J.; Han, W.; Huang, M.; Cauley, J.A.; Karvonen-Gutierrez, C.; Karlamangla, A.S. Increase in C-Reactive Protein Predicts Increase in Rate of Bone Mineral Density Loss: The Study of Women’s Health Across the Nation. JBMR Plus 2021, 5, e10480. [Google Scholar] [CrossRef]
- Shorten, A.; Smith, J. Mixed methods research: Expanding the evidence base. Evid. Based Nurs. 2017, 20, 74–75. [Google Scholar] [CrossRef]
- Sowers, M.F.R.; Crawford, S.L.; Sternfeld, B.; Morganstein, D.; Gold, E.B.; Greendale, G.A.; Evans, D.A.; Neer, R.; Matthews, K.A.; Sherman, S. Chapter 11—SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In Menopause; Academic Press: San Diego, CA, USA, 2000; pp. 175–188. [Google Scholar]
- Brown, R.B.; Bigelow, P.; Dubin, J.A.; Mielke, J.G. High Dietary Phosphorus Is Associated with Increased Breast Cancer Risk in a U.S. Cohort of Middle-Aged Women. Nutrients 2023, 15, 3735. [Google Scholar] [CrossRef]
- Howell Smith, M.C.; Babchuk, W.A.; Stevens, J.; Garrett, A.L.; Wang, S.C.; Guetterman, T.C. Modeling the Use of Mixed Methods–Grounded Theory: Developing Scales for a New Measurement Model. J. Mix. Methods Res. 2020, 14, 184–206. [Google Scholar] [CrossRef]
- Shim, M.; Johnson, B.; Bradt, J.; Gasson, S. A Mixed Methods–Grounded Theory Design for Producing More Refined Theoretical Models. J. Mix. Methods Res. 2021, 15, 61–86. [Google Scholar] [CrossRef]
- Wolfswinkel, J.F.; Furtmueller, E.; Wilderom, C.P.M. Using grounded theory as a method for rigorously reviewing literature. Eur. J. Inf. Syst. 2013, 22, 45–55. [Google Scholar] [CrossRef]
- Peters, B. Qualitative Methods in Monitroing and Evaluation: Concept Formation and Operationalization. Available online: https://programs.online.american.edu/msme/masters-in-measurement-and-evaluation/resources/concept-formation-and-operationalization (accessed on 25 March 2022).
- Bowen, G.A. Grounded Theory and Sensitizing Concepts. Int. J. Qual. Methods 2006, 5, 12–23. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Lin, Y.; McKinnon, K.E.; Ha, S.W.; Beck, G.R., Jr. Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression. Mol. Carcinog. 2015, 54, 926–934. [Google Scholar] [CrossRef]
- Ward, D.N.; Griffin, A.C. Phosphorus incorporation into nucleic acids and proteins of liver nuclei of normal and azo dye-fed rats. Cancer Res. 1955, 15, 456–461. [Google Scholar] [PubMed]
- Levan, K.; Mehryar, M.; Mateoiu, C.; Albertsson, P.; Bäck, T.; Sundfeldt, K. Immunohistochemical evaluation of epithelial ovarian carcinomas identifies three different expression patterns of the MX35 antigen, NaPi2b. BMC Cancer 2017, 17, 303. [Google Scholar]
- D’Arcangelo, M.; Brustugun, O.; Xiao, Y.; Choi, Y.; Behrens, C.; Solis, L.; Wang, Y.; Firestein, R.; Boyle, T.; Lund-Iversen, M. 194P prevalence and prognostic significance of sodium-dependent phosphate transporter 2B (NAPI2B) protein expression in non-small cell lung cancer (NSCLC). Ann. Oncol. 2014, 25, iv66–iv67. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Cosentino-Gomes, D.; Nascimento, M.T.C.; Carvalho-Kelly, L.F.; Gomes, T.; Rodrigues, M.F.; König, S.; Rumjanek, F.D.; Monteiro, R.Q.; et al. H(+)-dependent inorganic phosphate transporter in breast cancer cells: Possible functions in the tumor microenvironment. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2180–2188. [Google Scholar] [CrossRef]
- Low-Beer, B.V. Surface Measurements of Radioactive Phosphorus in Breast Tumors as a Possible Diagnostic Method. Science 1946, 104, 399. [Google Scholar] [CrossRef]
- Brown, R.B. Cancer Cachexia and Dysregulated Phosphate Metabolism: Insights from Mutant p53 and Mutant Klotho Mouse Models. Metabolites 2022, 12, 1284. [Google Scholar] [CrossRef]
- Chudek, J.; Nagy, A.; Kokot, F.; Podwinski, A.; Wiecek, A.; Ritz, E.; Kovacs, G. Phosphatemia is related to chromosomal aberrations of parathyroid glands in patients with hyperparathyroidism. J. Nephrol. 2007, 20, 164–172. [Google Scholar]
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Dhimitruka, I.; Evans, J.; Mohammad, R.; Tchekneva, E.E.; Dikov, M.M.; Khramtsov, V.V. Interstitial inorganic phosphate as a tumor microenvironment marker for tumor progression. Sci. Rep. 2017, 7, 41233. [Google Scholar]
- Wilson, K.M.; Shui, I.M.; Mucci, L.A.; Giovannucci, E. Calcium and phosphorus intake and prostate cancer risk: A 24-y follow-up study. Am. J. Clin. Nutr. 2015, 101, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Papaloucas, C.; Papaloucas, M.; Kouloulias, V.; Neanidis, K.; Pistevou-Gompaki, K.; Kouvaris, J.; Zygogianni, A.; Mystakidou, K.; Papaloucas, A. Measurement of blood phosphorus: A quick and inexpensive method for detection of the existence of cancer in the body. Too good to be true, or forgotten knowledge of the past? Med. Hypotheses 2014, 82, 24–25. [Google Scholar] [PubMed]
- Jin, H.; Xu, C.-X.; Lim, H.-T.; Park, S.-J.; Shin, J.-Y.; Chung, Y.-S.; Park, S.-C.; Chang, S.-H.; Youn, H.-J.; Lee, K.-H. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68. [Google Scholar] [PubMed]
- Camalier, C.E.; Young, M.R.; Bobe, G.; Perella, C.M.; Colburn, N.H.; Beck, G.R. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev. Res. 2010, 3, 359–370. [Google Scholar]
- Elser, J.J.; Kyle, M.M.; Smith, M.S.; Nagy, J.D. Biological stoichiometry in human cancer. PLoS ONE 2007, 2, e1028. [Google Scholar]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef]
- Rowe, P.; Koller, A.; Sharma, S. Physiology, Bone Remodeling. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tasnim, N.; Dutta, P.; Nayeem, J.; Masud, P.; Ferdousi, A.; Ghosh, A.S.; Hossain, M.; Rajia, S.; Kubra, K.T.; Sakibuzzaman, M.; et al. Osteoporosis, an Inevitable Circumstance of Chronic Kidney Disease: A Systematic Review. Cureus 2021, 13, e18488. [Google Scholar] [CrossRef]
- NIDDK. Mineral & Bone Disorder in Chronic Kidney Disease. Available online: https://www.niddk.nih.gov/health-information/kidney-disease/mineral-bone-disorder (accessed on 29 July 2023).
- Cai, J.H.; Zheng, J.H.; Lin, X.Q.; Lin, W.X.; Zou, J.; Chen, Y.K.; Li, Z.Y.; Chen, Y.X. Individualized treatment of breast cancer with chronic renal failure: A case report and review of literature. World J. Clin. Cases 2021, 9, 10345–10354. [Google Scholar] [CrossRef]
- Khedr, A. Skeletal Manifestations of Hyperparathyroidism. In Anatomy, Posture, Prevalence, Pain, Treatment and Interventions of Musculoskeletal Disorders; IntechOpen: London, UK, 2018. [Google Scholar]
- Fang, J.; Xu, Q. Differences of osteoblastic bone metastases and osteolytic bone metastases in clinical features and molecular characteristics. Clin. Transl. Oncol. 2015, 17, 173–179. [Google Scholar] [CrossRef] [PubMed]
- David Roodman, G.; Silbermann, R. Mechanisms of osteolytic and osteoblastic skeletal lesions. BoneKEy Rep. 2015, 4, 753. [Google Scholar] [CrossRef]
- Chen, Y.C.; Sosnoski, D.M.; Mastro, A.M. Breast cancer metastasis to the bone: Mechanisms of bone loss. Breast Cancer Res. 2010, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Gregson, C.L.; Hardcastle, S.A.; Cooper, C.; Tobias, J.H. Friend or foe: High bone mineral density on routine bone density scanning, a review of causes and management. Rheumatology 2013, 52, 968–985. [Google Scholar] [CrossRef]
- Bartl, R.; Frisch, B. Osteosclerosis. In Biopsy of Bone in Internal Medicine: An Atlas and Sourcebook; Bartl, R., Frisch, B., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 121–129. [Google Scholar] [CrossRef]
- Friedman, E.A. Consequences and management of hyperphosphatemia in patients with renal insufficiency. Kidney Int. Suppl. 2005, 67, S1–S7. [Google Scholar] [CrossRef]
- Raikou, V.D. Serum phosphate and chronic kidney and cardiovascular disease: Phosphorus potential implications in general population. World J. Nephrol. 2021, 10, 76–87. [Google Scholar] [CrossRef]
- Ali, M.A.; Czene, K.; Hall, P.; Humphreys, K. Association of Microcalcification Clusters with Short-term Invasive Breast Cancer Risk and Breast Cancer Risk Factors. Sci. Rep. 2019, 9, 14604. [Google Scholar] [CrossRef]
- Franca Gois, P.H.; Wolley, M.; Ranganathan, D.; Seguro, A.C. Vitamin D Deficiency in Chronic Kidney Disease: Recent Evidence and Controversies. Int. J. Environ. Res. Public Health 2018, 15, 1773. [Google Scholar] [CrossRef]
- Atoum, M.; Alzoughool, F. Vitamin D and breast cancer: Latest evidence and future steps. Breast Cancer Basic Clin. Res. 2017, 11, 1178223417749816. [Google Scholar] [CrossRef]
- Williams, J.D.; Aggarwal, A.; Swami, S.; Krishnan, A.V.; Ji, L.; Albertelli, M.A.; Feldman, B.J. Tumor Autonomous Effects of Vitamin D Deficiency Promote Breast Cancer Metastasis. Endocrinology 2016, 157, 1341–1347. [Google Scholar] [CrossRef]
- Ewendt, F.; Feger, M.; Föller, M. Role of Fibroblast Growth Factor 23 (FGF23) and αKlotho in Cancer. Front. Cell Dev. Biol. 2020, 8, 601006. [Google Scholar] [CrossRef]
- Palmieri, S.; Roggero, L.; Cairoli, E.; Morelli, V.; Scillitani, A.; Chiodini, I.; Eller-Vainicher, C. Occurrence of malignant neoplasia in patients with primary hyperparathyroidism. Eur. J. Intern. Med. 2017, 43, 77–82. [Google Scholar]
- Guité-Verret, A.; Vachon, M. The incurable metastatic breast cancer experience through metaphors: The fight and the unveiling. Int. J. Qual. Stud. Health Well-Being 2021, 16, 1971597. [Google Scholar] [CrossRef]
- Pulido, C.; Vendrell, I.; Ferreira, A.R.; Casimiro, S.; Mansinho, A.; Alho, I.; Costa, L. Bone metastasis risk factors in breast cancer. Ecancermedicalscience 2017, 11, 715. [Google Scholar] [CrossRef]
- Venetis, K.; Piciotti, R.; Sajjadi, E.; Invernizzi, M.; Morganti, S.; Criscitiello, C.; Fusco, N. Breast Cancer with Bone Metastasis: Molecular Insights and Clinical Management. Cells 2021, 10, 1377. [Google Scholar] [CrossRef]
- Ramirez, C.P.; Fiedler, D. Investigating the role of inorganic phosphate in tumor metabolism and metastasis. Cancer Metab. 2014, 2 (Suppl. S1), P55. [Google Scholar] [CrossRef]
- Gazes, R.P.; Chee, N.W.; Hampton, R.R. Cognitive mechanisms for transitive inference performance in rhesus monkeys: Measuring the influence of associative strength and inferred order. J. Exp. Psychol. Anim. Behav. Process. 2012, 38, 331–345. [Google Scholar] [CrossRef]
- Swanstudy.org. SWAN: Study of Women’s Health Across the Nation. Available online: https://www.swanstudy.org/ (accessed on 2 July 2023).
- Santoro, N.; Sutton-Tyrrell, K. The SWAN song: Study of Women’s Health Across the Nation’s recurring themes. Obstet. Gynecol. Clin. North Am. 2011, 38, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Sowers, M.; Han, W.; Huang, M.-H.; Finkelstein, J.S.; Crandall, C.J.; Lee, J.S.; Karlamangla, A.S. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: Results from the Study of Women’s Health Across the Nation (SWAN). J. Bone Miner. Res. 2012, 27, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Salkind, N.J. Fixed-Effects Models. Available online: https://methods.sagepub.com/reference/encyc-of-research-design/n155.xml (accessed on 14 January 2022).
- Salkind, N.J. Mixed- and Random-Effects Models. Available online: https://methods.sagepub.com/reference/encyc-of-research-design/n244.xml (accessed on 14 January 2022).
- Hedeker, D.; Gibbons, R.D. Longitudinal Data Analysis; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Portet, S. A primer on model selection using the Akaike Information Criterion. Infect. Dis. Model. 2020, 5, 111–128. [Google Scholar] [CrossRef]
- Bevans, R. Akaike Information Criterion|When & How to Use It. Available online: https://www.scribbr.com/statistics/akaike-information-criterion/ (accessed on 17 March 2022).
- Glen, S. Maximum Likelihood and Maximum Likelihood Estimation. Available online: https://www.statisticshowto.com/maximum-likelihood-estimation/ (accessed on 21 March 2022).
- Methods.sagepub.com. Interaction Effect. Available online: https://methods.sagepub.com/reference/encyclopedia-of-survey-research-methods (accessed on 30 March 2022).
- Allison, P.D. Missing Data; SAGE Publications: Thousand Oaks, CA, USA, 2002. [Google Scholar] [CrossRef]
- Allison, P.D. Handling Missing Data by Maximum Likelihood. In Proceedings of the SAS Global Forum 2012, Orlando, FL, USA, 22–25 April 2012. [Google Scholar]
- Glassock, R.J.; Winearls, C. Ageing and the glomerular filtration rate: Truths and consequences. Trans. Am. Clin. Climatol. Assoc. 2009, 120, 419–428. [Google Scholar] [PubMed]
- Ahmed, S.B.; Culleton, B.F.; Tonelli, M.; Klarenbach, S.W.; MacRae, J.M.; Zhang, J.; Hemmelgarn, B.R. Oral estrogen therapy in postmenopausal women is associated with loss of kidney function. Kidney Int. 2008, 74, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Au, K.; Francis, R.S.; Mudge, D.W.; Johnson, D.W.; Pillans, P.I. Phosphate binders in patients with chronic kidney disease. Aust. Prescr. 2017, 40, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Hjemås, B.J.; Bøvre, K.; Mathiesen, L.; Lindstrøm, J.C.; Bjerknes, K. Interventional study to improve adherence to phosphate binder treatment in dialysis patients. BMC Nephrol. 2019, 20, 178. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Wolf, M. Dietary phosphorus restriction in advanced chronic kidney disease: Merits, challenges, and emerging strategies. Semin. Dialysis 2010, 23, 401–406. [Google Scholar] [CrossRef]


| −2 Log Likelihood | −60,154.1 |
| AIC (Smaller is Better) | −60,138.1 |
| AICC (Smaller is Better) | −60,138.1 |
| BIC (Smaller is Better) | −60,089.8 |
| Effect | Breast Cancer | Estimate | Std Error | DF | t Value | Pr > |t| |
|---|---|---|---|---|---|---|
| Intercept | 1.0837 | 0.003106 | 2212 | 348.88 | <0.0001 | |
| INDIV_VISIT | −0.00937 | 0.000201 | 2121 | −46.58 | <0.0001 | |
| BRSTCAN | Yes | 0.02130 | 0.007439 | 13E3 | 2.86 | 0.0042 |
| BRSTCAN | No [Ref] | 0 | ||||
| INDIV_VISIT × BRSTCAN | Yes | −0.00411 | 0.001302 | 13E3 | −3.15 | 0.0016 |
| INDIV_VISIT × BRSTCAN | No [Ref] | 0 |
| Visit | Breast Cancer Yes | Breast Cancer No | Difference |
|---|---|---|---|
| 1 | 1.105 | 1.0837 | 0.0213 |
| 2 | 1.09152 | 1.07433 | 0.01719 |
| 3 | 1.07804 | 1.06496 | 0.01308 |
| 4 | 1.06456 | 1.05559 | 0.00897 |
| 5 | 1.05108 | 1.04622 | 0.00486 |
| 6 | 1.0376 | 1.03685 | 0.00075 |
| 7 | 1.02412 | 1.02748 | −0.00336 |
| 8 | 1.01064 | 1.01811 | −0.007046 |
| 9 | 0.99716 | 1.00874 | −0.01158 |
| 10 | 0.98368 | 0.99937 | −0.01569 |
| Mean | 1.04434 | 1.04154 | 0.0028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.B.; Bigelow, P.; Dubin, J.A. Breast Cancer and Bone Mineral Density in a U.S. Cohort of Middle-Aged Women: Associations with Phosphate Toxicity. Cancers 2023, 15, 5093. https://doi.org/10.3390/cancers15205093
Brown RB, Bigelow P, Dubin JA. Breast Cancer and Bone Mineral Density in a U.S. Cohort of Middle-Aged Women: Associations with Phosphate Toxicity. Cancers. 2023; 15(20):5093. https://doi.org/10.3390/cancers15205093
Chicago/Turabian StyleBrown, Ronald B., Philip Bigelow, and Joel A. Dubin. 2023. "Breast Cancer and Bone Mineral Density in a U.S. Cohort of Middle-Aged Women: Associations with Phosphate Toxicity" Cancers 15, no. 20: 5093. https://doi.org/10.3390/cancers15205093







