Don’t Judge a Book by Its Cover: The Role of Statins in Liver Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
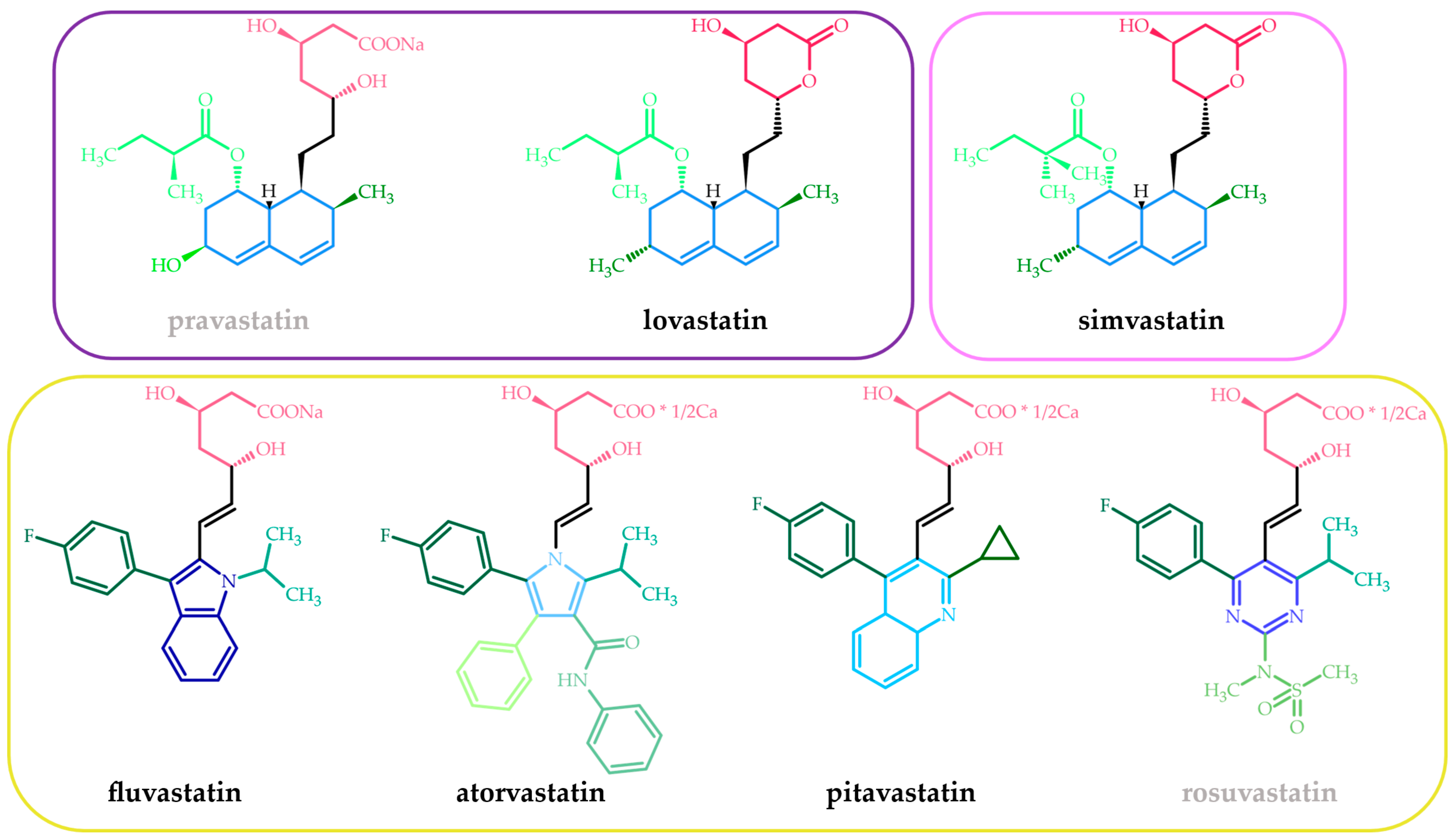
2. Statins—Chemical, Pharmacokinetic, and Pharmacodynamic Features—A Short Presentation
3. Statins in Primary and Secondary Prevention of Cardiovascular Diseases
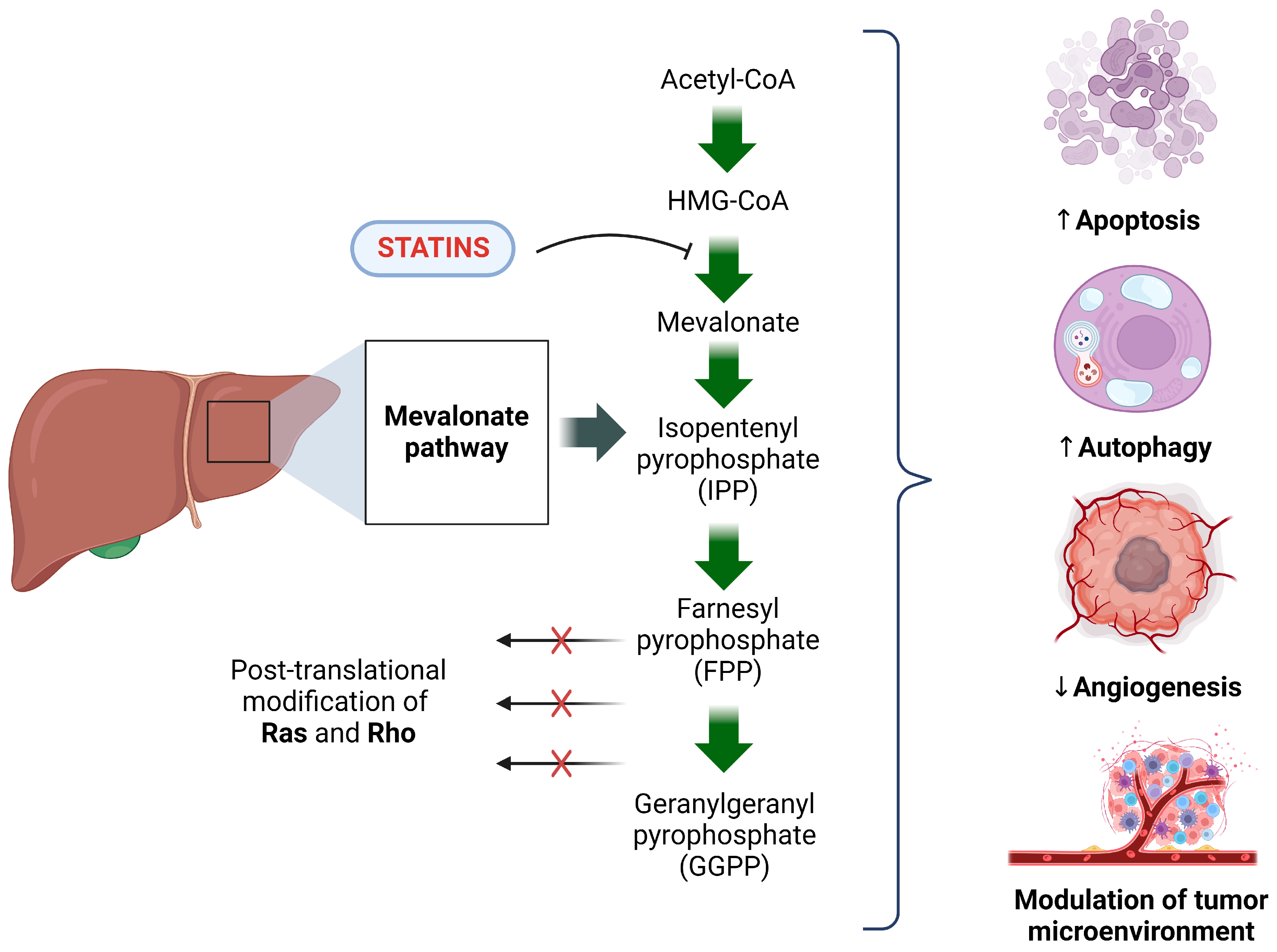
4. Basic Understanding of the Molecular Mechanism of the Anti-Cancer Properties of Statins
5. Statins in HCC Prevention
| Study | Design | Population | Follow-Up (Years) | Findings |
|---|---|---|---|---|
| Friis et al. [94] | Retrospective cohort study 1998–2002 | n = 348,262 | 3.3 | No beneficial effect for statin use (HR 1.16; 95% CI: 0.46–2.90) |
| McGlynn et al. [73] | Case control study 1988–2011 | n = 5835 | - | Significant HCC risk reduction for patients with liver disease and diabetes (aOR, 0.55; 95% CI: 0.45–0.69) |
| Tran et al. [96] | Case-control study 2000–2011 | n = 9852 | 2 | Significant HCC risk reduction (aOR, 0.44 (95% CI: 0.33–0.58) |
| Kim et al. [103] | Case-control study 2002–2013 | n = 1374 | 12 | Significant HCC risk reduction in patients with liver disease and incident TD2M (aOR = 0.27, 95% CI: 0.14–0.50) |
| Goh et al. [97] | Retrospective cohort study 2008–2012 | n = 7713 HBV-infected patients | 7.8 | Statin use is associated with lower risk of HCC (aHR 0.36, 95% CI: 0.19–0.68) |
| Hsiang et al. [98] | Retrospective cohort study | n = 53,513 HBV-infected patients | 4.6 | Statin users had a 32% risk reduction for HCC (wSHR 0.68; 95% CI: 0.48–0.97) |
| Tsan et al. [86] | Retrospective cohort study 1999–2010 | n = 260,864 HCV-infected patients | 10.7 | Reduction in HCC risk in a dose-dependent manner: aHRs, 0.66, 0.47 and 0.33 for statin use of 28 to 89, 90 to 180, and >180 cDDDs per year |
| Pinyopornpanish et al. [99] | Retrospective cohort study 2002–2016 | n = 1072 NASH-related liver fibrosis (F3 or cirrhosis) | 4.6 | Statin use associated with lower risk of HCC (HR, 0.40; 95% CI: 0.24–0.67) |
| Khazaaleh S et al. [100] | Meta-analysis | n = 2,668,497 | - | Significant HCC reduction in statin users vs. non-users (OR 0.573; 95% CI: 0.491–0.668, I2 = 86.57%) |
| Wang et al. [101] | Meta-analysis | n = 4,963,518 | - | Significant HCC reduction in statin users vs. non-users (aOR, 0.58; 95% CI: 0.51–0.67) |
| Singh S et al. [87] | Meta-analysis | n = 1,459,417 | - | Statin users were less likely to develop HCC than statin non-users (aOR, 0.63; 95% CI: 0.52–0.76) |
| Zeng R et al. [102] | Meta-analysis | n = 1,774,476 | - | Statin use was associated with HCC risk reduction in all subgroup analysis (HR: 0.52; 95% CI: 0.37–0.72) |
| NCT02968810, United States [105] | Randomized prospective clinical trial | Patients with liver cirrhosis | - | Simvastatin for preventing liver cancer development in liver cirrhosis patients |
| NCT03024684, Taiwan [104] | Randomized prospective clinical trial | Patients with HCC BCLC 0, A | - | Atorvastatin for prevention of HCC recurrence after curative treatment |
6. Statins in HCC Treatment
7. Statins in CCA Prevention
8. Statins in CCA Treatment
9. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Marin, J.J.G.; Reviejo, M.; Soto, M.; Lozano, E.; Asensio, M.; Ortiz-Rivero, S.; Berasain, C.; Avila, M.A.; Herraez, E. Impact of Alternative Splicing Variants on Liver Cancer Biology. Cancers 2022, 14, 18. [Google Scholar] [CrossRef]
- Mocan, L.P.; Rusu, I.; Melincovici, C.S.; Boșca, B.A.; Mocan, T.; Crăciun, R.; Spârchez, Z.; Iacobescu, M.; Mihu, C.M. The Role of Immunohistochemistry in the Differential Diagnosis between Intrahepatic Cholangiocarcinoma, Hepatocellular Carcinoma and Liver Metastasis, as Well as Its Prognostic Value. Diagnostics 2023, 13, 1542. [Google Scholar] [CrossRef]
- Castelli, G.; Pelosi, E.; Testa, U. Liver Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Cancers 2017, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.A.; Kodali, S.; Abdelrahim, M.; Javle, M.M.; Brombosz, E.W.; Ghobrial, R.M. Intrahepatic cholangiocarcinoma: The role of liver transplantation, adjunctive treatments, and prognostic biomarkers. Front. Oncol. 2022, 12, 996710. [Google Scholar] [CrossRef]
- Chamseddine, S.; LaPelusa, M.; Kaseb, A.O. Systemic Neoadjuvant and Adjuvant Therapies in the Management of Hepatocellular Carcinoma—A Narrative Review. Cancers 2023, 15, 3508. [Google Scholar] [CrossRef] [PubMed]
- Bupathi, M.; Ahn, D.H.; Bekaii-Saab, T. Therapeutic options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Elvevi, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristoferi, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann. Hepatol. 2022, 27, 100737. [Google Scholar] [CrossRef]
- Feng, M.; Pan, Y.; Kong, R.; Shu, S. Therapy of Primary Liver Cancer. Innovation 2020, 1, 100032. [Google Scholar] [CrossRef]
- Gairing, S.; Thol, F.; Müller, L.; Hahn, F.; Thomaidis, T.; Czauderna, C.; Bartsch, F.; Pitton, M.; Marquardt, J.; Wörns, M.-A.; et al. The addition of transarterial chemoembolization to palliative chemotherapy extends survival in intrahepatic cholangiocarcinoma. J. Clin. Med. 2021, 10, 2732. [Google Scholar] [CrossRef]
- Miyayama, S. Treatment Strategy of Transarterial Chemoembolization for Hepatocellular Carcinoma. Appl. Sci. 2020, 10, 7337. [Google Scholar] [CrossRef]
- Ramjeesingh, R.; Chaudhury, P.; Tam, V.C.; Roberge, D.; Lim, H.J.; Knox, J.J.; Asselah, J.; Doucette, S.; Chhiber, N.; Goodwin, R. A Practical Guide for the Systemic Treatment of Biliary Tract Cancer in Canada. Curr. Oncol. 2023, 30, 7132–7150. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Gupta, A.; Kurzrock, R.; Adashek, J.J. Evolution of the Targeted Therapy Landscape for Cholangiocarcinoma: Is Cholangiocarcinoma the ‘NSCLC’ of GI Oncology? Cancers 2023, 15, 1578. [Google Scholar] [CrossRef]
- Abdelmalak, J.; Tan, N.; Con, D.; Eslick, G.; Majeed, A.; Kemp, W.; Roberts, S.K. The Effect of Aspirin Use on Incident Hepatocellular Carcinoma—An Updated Systematic Review and Meta-Analysis. Cancers 2023, 15, 3518. [Google Scholar] [CrossRef] [PubMed]
- Athuluri-Divakar, S.K.; Hoshida, Y. Generic chemoprevention of hepatocellular carcinoma. Ann. N. Y. Acad. Sci. 2019, 1440, 23. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.V.; Coluzzi, F.; Colucci, R.D.; Olsson, H.; LeQuang, J.A.; Al-Saadi, J.; Magnusson, P. Statins and muscle pain. Expert Rev. Clin. Pharmacol. 2020, 13, 299–310. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Gharbi, S.; Rajani, H.F.; Farzaneh, A.; Mahjoob, G.; Hoseinsalari, A.; Korsching, E. Statins: Complex outcomes but increasingly helpful treatment options for patients. Eur. J. Pharmacol. 2019, 863, 172704. [Google Scholar] [CrossRef]
- Food and Drug Administration. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations; US Food and Drug Administration (FDA): Silver Spring, MD, USA, 1985. [Google Scholar]
- Istvan, E. Statin inhibition of HMG-CoA reductase: A 3-dimensional view. Atheroscler. Suppl. 2003, 4, 3–8. [Google Scholar] [CrossRef]
- Ahmadi, M.; Amiri, S.; Pecic, S.; Machaj, F.; Rosik, J.; Łos, M.J.; Alizadeh, J.; Mahdian, R.; da Silva Rosa, S.C.; Schaafsma, D.; et al. Pleiotropic effects of statins: A focus on cancer. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165968. [Google Scholar] [CrossRef]
- Evans, M.D.; McDowell, S.A. Pleiotropic Effects of Statins: New Therapeutic Approaches to Chronic, Recurrent Infection by Staphylococcus aureus. Pharmaceutics 2021, 13, 2047. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Sehgal, V.S.; Kashfi, K. Molecular targets of statins and their potential side effects: Not all the glitter is gold. Eur. J. Pharmacol. 2022, 922, 174906. [Google Scholar] [CrossRef]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Istvan, E.S. Structural mechanism for statin inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Am. Heart J. 2002, 144, S27–S32. [Google Scholar] [CrossRef]
- Sirtori, C.R. The pharmacology of statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity: Mechanistic Insights and Clinical Implications. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.W. Statins in therapy: Understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. Eur. J. Med. Chem. 2014, 85, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Kellick, K.A.; Bottorff, M.; Toth, P.P. A clinician’s guide to statin drug-drug interactions. J. Clin. Lipidol. 2014, 8, S30–S46. [Google Scholar] [CrossRef]
- Barbalata, C.I.; Tefas, L.R.; Achim, M.; Tomuta, I.; Porfire, A.S. Statins in risk-reduction and treatment of cancer. World J. Clin. Oncol. 2020, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Gales, L.; Forsea, L.; Mitrea, D.; Stefanica, I.; Stanculescu, I.; Mitrica, R.; Georgescu, M.; Trifanescu, O.; Anghel, R.; Serbanescu, L. Antidiabetics, Anthelmintics, Statins, and Beta-Blockers as Co-Adjuvant Drugs in Cancer Therapy. Medicina 2022, 58, 1239. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.W.; He, X.R.; Jin, W.L.; He, X.Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef]
- Pun, N.T.; Jeong, C.H. Statin as a Potential Chemotherapeutic Agent: Current Updates as a Monotherapy, Combination Therapy, and Treatment for Anti-Cancer Drug Resistance. Pharmaceuticals 2021, 14, 470. [Google Scholar] [CrossRef]
- Rushworth, L.K.; Loveridge, C.; Salji, M.; MacLeod, M.; Mui, E.; Sumpton, D.; Neilson, M.; Hedley, A.; Alexander, L.; McCartney, E.; et al. Phase II proof-of-concept study of atorvastatin in castration-resistant prostate cancer. BJU Int. 2023, 131, 236–243. [Google Scholar] [CrossRef]
- Di Bello, E.; Zwergel, C.; Mai, A.; Valente, S. The Innovative Potential of Statins in Cancer: New Targets for New Therapies. Front. Chem. 2020, 8, 516. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, F.; Lombardi, R.; Milone, M.R.; Pucci, B.; De Rienzo, S.; Budillon, A.; Bruzzese, F. Targeting Mevalonate Pathway in Cancer Treatment: Repurposing of Statins. Recent Pat. Anti-Cancer Drug Discov. 2018, 13, 184–200. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur. Heart J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef]
- Byrne, P.; Cullinan, J.; Smith, A.; Smith, S.M. Statins for the primary prevention of cardiovascular disease: An overview of systematic reviews. BMJ Open 2019, 9, e023085. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Morin, L.; Jarbøl, D.E.; Andersen, J.H.; Ernst, M.T.; Nielsen, J.B.; Haastrup, P.; Schmidt, M.; Pottegård, A. Statin Discontinuation and Cardiovascular Events among Older People in Denmark. JAMA Netw. Open 2021, 4, e2136802. [Google Scholar] [CrossRef]
- Major, R.W.; Cheung, C.K.; Gray, L.J.; Brunskill, N.J. Statins and cardiovascular primary prevention in CKD: A meta-analysis. Clin. J. Am. Soc. Nephrol. 2015, 10, 732–739. [Google Scholar] [CrossRef]
- Hodkinson, A.; Tsimpida, D.; Kontopantelis, E.; Rutter, M.K.; Mamas, M.A.; Panagioti, M. Comparative effectiveness of statins on non-high density lipoprotein cholesterol in people with diabetes and at risk of cardiovascular disease: Systematic review and network meta-analysis. BMJ 2022, 376, e067731. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Abel, L.; Langford, O.; Monaghan, G.; Aronson, J.K.; Stevens, R.J.; Lay-Flurrie, S.; Koshiaris, C.; McManus, R.J.; Hobbs, F.D.R.; et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: Systematic review with pairwise, network, and dose-response meta-analyses. BMJ 2021, 374, n1537. [Google Scholar] [CrossRef]
- Strandberg, T.E. Role of Statin Therapy in Primary Prevention of Cardiovascular Disease in Elderly Patients. Curr. Atheroscler. Rep. 2019, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Force, U.P.S.T.; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Jaén, C.R.; et al. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 328, 746–753. [Google Scholar] [CrossRef]
- Yourman, L.C.; Cenzer, I.S.; Boscardin, W.J.; Nguyen, B.T.; Smith, A.K.; Schonberg, M.A.; Schoenborn, N.L.; Widera, E.W.; Orkaby, A.; Rodriguez, A.; et al. Evaluation of Time to Benefit of Statins for the Primary Prevention of Cardiovascular Events in Adults Aged 50 to 75 Years: A Meta-analysis. JAMA Intern. Med. 2021, 181, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-Y.; Lin, C.-H.; Wang, K.-Y.; Huang, J.-L.; Hsieh, Y.-C.; Loh, E.-W.; Lan, T.-H.; Chou, P.; Ting, C.-T.; Wu, T.-J. Dosage of statin, cardiovascular comorbidities, and risk of atrial fibrillation: A nationwide population-based cohort study. Int. J. Cardiol. 2013, 168, 1131–1136. [Google Scholar] [CrossRef]
- Oraii, A.; Vasheghani-Farahani, A.; Oraii, S.; Roayaei, P.; Balali, P.; Masoudkabir, F. Update on the efficacy of statins in primary and secondary prevention of atrial fibrillation. Rev. Port. Cardiol. 2021, 40, 509–518. [Google Scholar] [CrossRef]
- Laleman, N.; Henrard, S.; Akker, M.v.D.; Goderis, G.; Buntinx, F.; Van Pottelbergh, G.; Vaes, B. Time trends in statin use and incidence of recurrent cardiovascular events in secondary prevention between 1999 and 2013: A registry-based study. BMC Cardiovasc. Disord. 2018, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- Tecson, K.M.; Kluger, A.Y.; Cassidy-Bushrow, A.E.; Liu, B.; Coleman, C.M.; Jones, L.K.; Jefferson, C.R.; VanWormer, J.J.; McCullough, P.A. Usefulness of Statins as Secondary Prevention Against Recurrent and Terminal Major Adverse Cardiovascular Events. Am. J. Cardiol. 2022, 176, 37–42. [Google Scholar] [CrossRef]
- Thalmann, I.; Preiss, D.; Schlackow, I.; Gray, A.; Mihaylova, B. Population-wide cohort study of statin use for the secondary cardiovascular disease prevention in Scotland in 2009–2017. Heart 2023, 109, 388–395. [Google Scholar] [CrossRef]
- Tramacere, I.; Boncoraglio, G.B.; Banzi, R.; Del Giovane, C.; Kwag, K.H.; Squizzato, A.; Moja, L. Comparison of statins for secondary prevention in patients with ischemic stroke or transient ischemic attack: A systematic review and network meta-analysis. BMC Med. 2019, 17, 67. [Google Scholar] [CrossRef]
- Sigglekow, F.; Horsburgh, S.; Parkin, L. Statin adherence is lower in primary than secondary prevention: A national follow-up study of new users. PLoS ONE 2020, 15, e0242424. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, H.; Xu, T.; Liu, X.; Liu, X.; Sun, L.; Pan, X.-F.; Mai, W.; Cai, X.; Huang, Y. Effects of statins in primary and secondary prevention for venous thromboembolism events: A meta analysis. Vascul. Pharmacol. 2022, 142, 106931. [Google Scholar] [CrossRef]
- Guerra, B.; Recio, C.; Aranda-Tavío, H.; Guerra-Rodríguez, M.; García-Castellano, J.M.; Fernández-Pérez, L. The Mevalonate Pathway, a Metabolic Target in Cancer Therapy. Front. Oncol. 2021, 11, 626971. [Google Scholar] [CrossRef] [PubMed]
- Juarez, D.; Fruman, D.A. Targeting the Mevalonate Pathway in Cancer. Trends Cancer 2021, 7, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Altwairgi, A.K. Statins are potential anticancerous agents (review). Oncol. Rep. 2015, 33, 1019–1039. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Rauner, M.; Hofbauer, L.C.; Rachner, T.D. Cholesterol and beyond—The role of the mevalonate pathway in cancer biology. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2020, 1873, 188351. [Google Scholar] [CrossRef]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Waller, D.D.; Park, J.; Tsantrizos, Y.S. Inhibition of farnesyl pyrophosphate (FPP) and/or geranylgeranyl pyrophosphate (GGPP) biosynthesis and its implication in the treatment of cancers. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 41–60. [Google Scholar] [CrossRef]
- Liu, C.; Chen, H.; Hu, B.; Shi, J.; Chen, Y.; Huang, K. New insights into the therapeutic potentials of statins in cancer. Front. Pharmacol. 2023, 14, 1188926. [Google Scholar] [CrossRef]
- Bokhari, S.M.Z.; Hamar, P. Vascular Endothelial Growth Factor-D (VEGF-D): An Angiogenesis Bypass in Malignant Tumors. Int. J. Mol. Sci. 2023, 24, 13317. [Google Scholar] [CrossRef]
- Dulak, J.; Jozkowicz, A. Anti-angiogenic and anti-inflammatory effects of statins: Relevance to anti-cancer therapy. Curr. Cancer Drug Targets 2005, 5, 579–594. [Google Scholar] [CrossRef]
- Muehlebach, M.E.; Holstein, S.A. Geranylgeranyl diphosphate synthase: Role in human health, disease and potential therapeutic target. Clin. Transl. Med. 2023, 13, e1167. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Cai, H.; Long, Y.; Sirka, O.K.; Padmanaban, V.; Ewald, A.J.; Devreotes, P.N. Statin-induced GGPP depletion blocks macropinocytosis and starves cells with oncogenic defects. Proc. Natl. Acad. Sci. USA 2020, 117, 4158–4168. [Google Scholar] [CrossRef] [PubMed]
- Mansourian, P.G.; Yoneda, M.; Rao, M.K.; Martinez, F.J.; Thomas, E.; Schiff, E.R. Effects of Statins on the Risk of Hepatocellular Carcinoma. Gastroenterol. Hepatol. (N. Y.) 2014, 10, 417. [Google Scholar] [PubMed]
- Dongoran, R.A.; Wang, K.H.; Lin, T.J.; Yuan, T.C.; Liu, C.H. Anti-Proliferative Effect of Statins Is Mediated by DNMT1 Inhibition and p21 Expression in OSCC Cells. Cancers 2020, 12, 2084. [Google Scholar] [CrossRef]
- Kodach, L.L.; Jacobs, R.J.; Voorneveld, P.W.; Wildenberg, M.E.; Verspaget, H.W.; van Wezel, T.; Morreau, H.; Hommes, D.W.; Peppelenbosch, M.P.; Van den Brink, G.R.; et al. Statins augment the chemosensitivity of colorectal cancer cells inducing epigenetic reprogramming and reducing colorectal cancer cell “stemness” via the bone morphogenetic protein pathway. Gut 2011, 60, 1544–1553. [Google Scholar] [CrossRef]
- Miller, T.; Yang, F.; Wise, C.E.; Meng, F.; Priester, S.; Munshi, K.; Guerrier, M.; Dostal, D.E.; Glaser, S.S. Simvastatin stimulates apoptosis in cholangiocarcinoma by inhibition of Rac1 activity. Dig. Liver Dis. 2011, 43, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Seeree, P.; Janvilisri, T.; Kangsamaksin, T.; Tohtong, R.; Kumkate, S. Downregulation of ABCA1 and ABCG1 transporters by simvastatin in cholangiocarcinoma cells. Oncol. Lett. 2019, 18, 5173. [Google Scholar] [CrossRef] [PubMed]
- Collisson, E.A.; Kleer, C.; Wu, M.; De, A.; Gambhir, S.S.; Merajver, S.D.; Kolodney, M.S. Atorvastatin prevents RhoC isoprenylation, invasion, and metastasis in human melanoma cells. Mol. Cancer Ther. 2003, 2, 941. [Google Scholar]
- Fujiwara, D.; Tsubaki, M.; Takeda, T.; Tomonari, Y.; Koumoto, Y.-I.; Sakaguchi, K.; Nishida, S. Statins induce apoptosis through inhibition of Ras signaling pathways and enhancement of Bim and p27 expression in human hematopoietic tumor cells. Tumour Biol. 2017, 39, 1–12. [Google Scholar] [CrossRef]
- Soriano, O.; Alcón-Pérez, M.; Vicente-Manzanares, M.; Castellano, E. The Crossroads between RAS and RHO Signaling Pathways in Cellular Transformation, Motility and Contraction. Genes 2021, 12, 819. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Colio, L.M.; Villa, A.; Ortego, M.; Hernández-Presa, M.A.; Pascual, A.; Plaza, J.J.; Egido, J. 3-Hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors, atorvastatin and simvastatin, induce apoptosis of vascular smooth muscle cells by downregulation of Bcl-2 expression and Rho A prenylation. Atherosclerosis 2002, 161, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Hong, E.M.; Kim, M.; Kim, J.H.; Jang, J.; Park, S.W.; Byun, H.W.; Koh, D.H.; Choi, M.H.; Kae, S.H.; et al. Simvastatin induces heme oxygenase-1 via NF-E2-related factor 2 (Nrf2) activation through ERK and PI3K/Akt pathway in colon cancer. Oncotarget 2016, 7, 46219–46229. [Google Scholar] [CrossRef] [PubMed]
- Cerezo-Guisado, M.I.; García-Román, N.; García-Marín, L.J.; Álvarez-Barrientos, A.; Bragado, M.J.; Lorenzo, M.J. Lovastatin inhibits the extracellular-signal-regulated kinase pathway in immortalized rat brain neuroblasts. Biochem. J. 2007, 401, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Ro, S.W. MAPK/ERK Signaling Pathway in Hepatocellular Carcinoma. Cancers 2021, 13, 3026. [Google Scholar] [CrossRef]
- Dehnavi, S.; Kiani, A.; Sadeghi, M.; Biregani, A.F.; Banach, M.; Atkin, S.L.; Jamialahmadi, T.; Sahebkar, A. Targeting AMPK by Statins: A Potential Therapeutic Approach. Drugs 2021, 81, 923–933. [Google Scholar] [CrossRef]
- Mengual, D.; Medrano, L.E.; Villamizar-Villamizar, W.; Osorio-Llanes, E.; Mendoza-Torres, E.; Bolívar, S. Novel Effects of Statins on Cancer via Autophagy. Pharmaceuticals 2022, 15, 648. [Google Scholar] [CrossRef]
- Cui, J.; Shen, H.M.; Lim, L.H.K. The Role of Autophagy in Liver Cancer: Crosstalk in Signaling Pathways and Potential Therapeutic Targets. Pharmaceuticals 2020, 13, 432. [Google Scholar] [CrossRef]
- Che, L.; Chi, W.; Qiao, Y.; Zhang, J.; Song, X.; Liu, Y.; Li, L.; Jia, J.; Pilo, M.G.; Wang, J.; et al. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut 2020, 69, 177–186. [Google Scholar] [CrossRef]
- Merlo, L.M.F.; Pepper, J.W.; Reid, B.J.; Maley, C.C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 2006, 6, 924–935. [Google Scholar] [CrossRef]
- Cai, D.; Wang, J.; Gao, B.; Li, J.; Wu, F.; Zou, J.X.; Xu, J.; Jiang, Y.; Zou, H.; Huang, Z.; et al. RORγ is a targetable master regulator of cholesterol biosynthesis in a cancer subtype. Nat. Commun. 2019, 10, 4621. [Google Scholar] [CrossRef]
- Lewis, C.A.; Brault, C.; Peck, B.; Bensaad, K.; Griffiths, B.; Mitter, R.; Chakravarty, P.; East, P.; Dankworth, B.; Alibhai, D.; et al. SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene 2015, 34, 5128–5140. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Tsan, Y.-T.; Lee, C.-H.; Wang, J.-D.; Chen, P.-C. Statins and the Risk of Hepatocellular Carcinoma in Patients with Hepatitis B Virus Infection. J. Clin. Oncol. 2012, 30, 623–630. [Google Scholar] [CrossRef]
- Tsan, Y.-T.; Lee, C.-H.; Ho, W.-C.; Lin, M.-H.; Wang, J.-D.; Chen, P.-C. Statins and the Risk of Hepatocellular Carcinoma in Patients with Hepatitis C Virus Infection. J. Clin. Oncol. 2013, 31, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Statins Are Associated with a Reduced Risk of Hepatocellular Cancer: A Systematic Review and Meta-analysis. Gastroenterology 2013, 144, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.D.; Achacoso, N.; Fireman, B.; Habel, L.A. Statins and Reduced Risk of Liver Cancer: Evidence for Confounding: Table 1. J. Natl. Cancer Inst. 2016, 108, djw109. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Kanwal, F. Obesity and hepatocellular carcinoma: Hype and reality. Hepatology 2014, 60, 779–781. [Google Scholar] [CrossRef]
- Averbukh, L.D.; Turshudzhyan, A.; Wu, D.C.; Wu, G.Y. Statin-induced Liver Injury Patterns: A Clinical Review. J. Clin. Transl. Hepatol. 2022, 10, 543–552. [Google Scholar] [CrossRef]
- Mohammadzadeh, N.; Montecucco, F.; Carbone, F.; Xu, S.; Al-Rasadi, K.; Sahebkar, A. Statins: Epidrugs with effects on endothelial health? Eur. J. Clin. Investig. 2020, 50, e13388. [Google Scholar] [CrossRef]
- Wu, J.; Wong, W.W.-L.; Khosravi, F.; Minden, M.D.; Penn, L.Z. Blocking the Raf/MEK/ERK Pathway Sensitizes Acute Myelogenous Leukemia Cells to Lovastatin-Induced Apoptosis. Cancer Res. 2004, 64, 6461–6468. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Fan-Minogue, H.; Bellovin, D.I.; Yevtodiyenko, A.; Arzeno, J.; Yang, Q.; Gambhir, S.S.; Felsher, D.W. MYC Phosphorylation, Activation, and Tumorigenic Potential in Hepatocellular Carcinoma Are Regulated by HMG-CoA Reductase. Cancer Res. 2011, 71, 2286–2297. [Google Scholar] [CrossRef]
- Friis, S.; Poulsen, A.H.; Johnsen, S.P.; McLaughlin, J.K.; Fryzek, J.P.; Dalton, S.O.; Sørensen, H.T.; Olsen, J.H. Cancer risk among statin users: A population-based cohort study. Int. J. Cancer 2005, 114, 643–647. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Hagberg, K.; Chen, J.; Graubard, B.I.; London, W.T.; Jick, S.; Sahasrabuddhe, V.V. Statin use and risk of primary liver cancer in the Clinical Practice Research Datalink. J. Natl. Cancer Inst. 2015, 107, djv009. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.; McMenamin, Ú.C.; Coleman, H.G.; Cardwell, C.R.; Murchie, P.; Iversen, L.; Lee, A.J.; Thrift, A.P. Statin use and risk of liver cancer: Evidence from two population-based studies. Int. J. Cancer 2020, 146, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.J.; Sinn, D.H.; Kim, S.; Woo, S.Y.; Cho, H.; Kang, W.; Gwak, G.; Paik, Y.; Choi, M.S.; Lee, J.H.; et al. Statin Use and the Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. Hepatology 2020, 71, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, J.C.; Wong, G.L.H.; Tse, Y.K.; Wong, V.W.S.; Yip, T.C.F.; Chan, H.L.Y. Statin and the risk of hepatocellular carcinoma and death in a hospital-based hepatitis B-infected population: A propensity score landmark analysis. J. Hepatol. 2015, 63, 1190–1197. [Google Scholar] [CrossRef]
- Pinyopornpanish, K.; Al-Yaman, W.; Butler, R.S.; Carey, W.; McCullough, A.; Romero-Marrero, C. Chemopreventive Effect of Statin on Hepatocellular Carcinoma in Patients with Nonalcoholic Steatohepatitis Cirrhosis. Am. J. Gastroenterol. 2021, 116, 2258–2269. [Google Scholar] [CrossRef]
- Khazaaleh, S.; Sarmini, M.T.; Alomari, M.; Al Momani, L.; El Kurdi, B.; Asfari, M.; Almomani, Z.; Romero-Marrero, C. Statin Use Reduces the Risk of Hepatocellular Carcinoma: An Updated Meta-Analysis and Systematic Review. Cureus 2022, 14, e27032. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, M.; Shi, J.; Jia, X.; Dang, S. A Meta-Analysis of Statin Use and Risk of Hepatocellular Carcinoma. Can. J. Gastroenterol. Hepatol. 2022, 2022, 5389044. [Google Scholar] [CrossRef]
- Zeng, R.W.; Yong, J.N.; Tan, D.J.H.; Fu, C.E.; Lim, W.H.; Xiao, J.; Chan, K.E.; Tan, C.; Goh, X.L.; Chee, D.; et al. Meta-analysis: Chemoprevention of hepatocellular carcinoma with statins, aspirin and metformin. Aliment. Pharmacol. Ther. 2023, 57, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Jang, S.; Han, E.; Lee, Y.; Park, S.; Nam, C.M.; Kang, E.S. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: A nationwide nested case-control study. Int. J. Cancer 2017, 140, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y. Statin for Preventing Hepatocellular Carcinoma Recurrence after Curative Treatment: A Double-Blind Randomized Placebo-controlled Trial; ClinicalTrialsGov Identifier: NCT03024684; Chiayi Christian Hospital: Chiayi, Taiwan, 2022. [Google Scholar]
- Goodman, M.T. Simvastatin in Preventing Liver Cancer in Patients with Liver Cirrhosis; ClinicalTrialsGov Identifier: NCT02968810; National Cancer Institute (NCI): Bethesda, MD, USA, 2023. [Google Scholar]
- Chen, C.-I.; Kuan, C.-F.; Fang, Y.-A.; Liu, S.-H.; Liu, J.-C.; Wu, L.-L.; Chang, C.-J.; Yang, H.-C.; Hwang, J.; Miser, J.S.; et al. Cancer Risk in HBV Patients with Statin and Metformin Use. Medicine 2015, 94, e462. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Jang, S.-Y.; Nam, C.M.; Kang, E.S. Statin use and the risk of hepatocellular carcinoma in patients at high risk: A nationwide nested case-control study. J. Hepatol. 2018, 68, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Duberg, A.-S.; Aleman, S.; Hagstrom, H.; Nguyen, L.H.; Khalili, H.; Chung, R.T.; Ludvigsson, J.F. Lipophilic Statins and Risk for Hepatocellular Carcinoma and Death in Patients with Chronic Viral Hepatitis: Results from a Nationwide Swedish Population. Ann. Intern. Med. 2019, 171, 318. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Sugihara, M.; Kaburagi, J.; Ozawa, M.; Iwashita, M.; Yoshida, S.; Saito, H.; Hattori, Y. Pravastatin use and cancer risk: A meta-analysis of individual patient data from long-term prospective controlled trials in Japan. Pharmacoepidemiol. Drug Saf. 2010, 19, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Ajiki, W.; Kobayashi, T.; Awata, N. Pravastatin use and the five-year incidence of cancer in coronary heart disease patients: From the prevention of coronary sclerosis study. J. Epidemiol. 2006, 16, 201–206. [Google Scholar] [CrossRef]
- Yi, S.-W.; Kim, S.H.; Han, K.J.; Yi, J.-J.; Ohrr, H. Higher cholesterol levels, not statin use, are associated with a lower risk of hepatocellular carcinoma. Br. J. Cancer 2020, 122, 630–633. [Google Scholar] [CrossRef]
- Bosch, J.; Gracia-Sancho, J.; Abraldes, J.G. Cirrhosis as new indication for statins. Gut 2020, 69, 953–962. [Google Scholar] [CrossRef]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R.; et al. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef]
- Sutter, A.P.; Maaser, K.; Höpfner, M.; Huether, A.; Schuppan, D.; Scherübl, H. Cell cycle arrest and apoptosis induction in hepatocellular carcinoma cells by HMG-CoA reductase inhibitors. Synergistic antiproliferative action with ligands of the peripheral benzodiazepine receptor. J. Hepatol. 2005, 43, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Zhang, P.; Hu, J.; Hou, H.; Xiong, M.; Xiong, J.; Yan, N. Synergistic effect of receptor-interacting protein 140 and simvastatin on the inhibition of proliferation and survival of hepatocellular carcinoma cells. Oncol. Lett. 2018, 15, 4344–4350. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Hwang, J.W.; Yim, H.; Yim, H.J.; Woo, S.U.; Suh, S.J.; Hyun, J.J.; Jung, S.W.; Koo, J.S.; Kim, J.H.; et al. Synergistic effect of simvastatin plus NS398 on inhibition of proliferation and survival in hepatocellular carcinoma cell line. J. Gastroenterol. Hepatol. 2014, 29, 1299–1307. [Google Scholar] [CrossRef]
- Elleithi, Y.A.; El-Gayar, A.M.; Amin, M.N. Simvastatin Induces Apoptosis and Suppresses Hepatocellular Carcinoma Induced in Rats. Appl. Biochem. Biotechnol. 2023, 195, 1656–1674. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Blanco, C.; Fondevila, F.; García-Palomo, A.; González-Gallego, J.; Mauriz, J.L. Sorafenib resistance in hepatocarcinoma: Role of hypoxia-inducible factors. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Zhou, T.-Y.; Zhuang, L.-H.; Hu, Y.; Zhou, Y.-L.; Lin, W.-K.; Wang, D.-D.; Wan, Z.-Q.; Chang, L.-L.; Chen, Y.; Ying, M.-D.; et al. Inactivation of hypoxia-induced YAP by statins overcomes hypoxic resistance tosorafenib in hepatocellular carcinoma cells. Sci. Rep. 2016, 6, 30483. [Google Scholar] [CrossRef]
- Feng, J.; Dai, W.; Mao, Y.; Wu, L.; Li, J.; Chen, K.; Yu, Q.; Kong, R.; Li, S.; Zhang, J.; et al. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J. Exp. Clin. Cancer Res. 2020, 39, 24. [Google Scholar] [CrossRef]
- Zhou, T.-Y.; Zhou, Y.-L.; Qian, M.-J.; Fang, Y.-Z.; Ye, S.; Xin, W.-X.; Yang, X.-C.; Wu, H.-H. Interleukin-6 induced by YAP in hepatocellular carcinoma cells recruits tumor-associated macrophages. J. Pharmacol. Sci. 2018, 138, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Guo, J.; Liu, Y.; Wang, M.; Liu, Z.; Gao, Y.; Huang, L. Nano delivery of simvastatin targets liver sinusoidal endothelial cells to remodel tumor microenvironment for hepatocellular carcinoma. J. Nanobiotechnol. 2022, 20, 9. [Google Scholar] [CrossRef]
- Shwe, T.H.; Pothacharoen, P.; Phitak, T.; Wudtiwai, B.; Kongtawelert, P. Atorvastatin Attenuates Programmed Death Ligand-1 (PD-L1) Induction in Human Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 8755. [Google Scholar] [CrossRef]
- Kawata, S.; Yamasaki, E.; Nagase, T.; Inui, Y.; Ito, N.; Matsuda, Y.; Inada, M.; Tamura, S.; Noda, S.; Imai, Y.; et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br. J. Cancer 2001, 84, 886–891. [Google Scholar] [CrossRef]
- Graf, H.; Jüngst, C.; Straub, G.; Dogan, S.; Hoffmann, R.-T.; Jakobs, T.; Reiser, M.; Waggershauser, T.; Helmberger, T.; Walter, A.; et al. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion 2008, 78, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Jouve, J.-L.; Lecomte, T.; Bouché, O.; Barbier, E.; Akouz, F.K.; Riachi, G.; Khac, E.N.; Ollivier-Hourmand, I.; Debette-Gratien, M.; Faroux, R.; et al. Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J. Hepatol. 2019, 71, 516–522. [Google Scholar] [CrossRef]
- Blanc, J.F.; Khemissa, F.; Bronowicki, J.P.; Monterymard, C.; Perarnau, J.M.; Bourgeois, V.; Obled, S.; Abdelghani, M.B.; Mabile-Archambeaud, I.; Faroux, R.; et al. Phase 2 trial comparing sorafenib, pravastatin, their combination or supportive care in HCC with Child-Pugh B cirrhosis. Hepatol. Int. 2021, 15, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Björkhem-Bergman, L. Is There a Role for Statins in Palliative Care for Patients Suffering from Hepatocellular Carcinoma? J. Palliat. Care 2015, 31, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.Y.H.; Lee, F.P.; Chang, C.L.; Wu, S.Y. Statin-Based Palliative Therapy for Hepatocellular Carcinoma. Medicine 2015, 94, e1801. [Google Scholar] [CrossRef]
- Lund, J.L.; Montomoli, J. Common flaws in pharmacoepidemiologic study design and analysis. J. Clin. Oncol. 2013, 31, 4161–4162. [Google Scholar] [CrossRef]
- Nishio, T.; Taura, K.; Nakamura, N.; Seo, S.; Yasuchika, K.; Kaido, T.; Okajima, H.; Hatano, E.; Uemoto, S. Impact of statin use on the prognosis of patients with hepatocellular carcinoma undergoing liver resection: A subgroup analysis of patients without chronic hepatitis viral infection. Surgery 2018, 163, 264–269. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Wang, C.-C.; Chen, K.-D.; Liu, Y.-W.; Lin, C.-C.; Chuang, C.-H.; Tsai, Y.-C.; Yao, C.-C.; Yen, Y.-H.; Hsiao, C.-C.; et al. Statin use is associated with a lower risk of recurrence after curative resection in BCLC stage 0-A hepatocellular carcinoma. BMC Cancer 2021, 21, 70. [Google Scholar] [CrossRef]
- Khajeh, E.; Moghadam, A.D.; Eslami, P.; Ali-Hasan-Al-Saegh, S.; Ramouz, A.; Shafiei, S.; Ghamarnejad, O.; Dezfouli, S.A.; Rupp, C.; Springfeld, C.; et al. Statin use is associated with the reduction in hepatocellular carcinoma recurrence after liver surgery. BMC Cancer 2022, 22, 91. [Google Scholar] [CrossRef]
- Wu, L.L.; Hsieh, M.C.; Chow, J.M.; Liu, S.H.; Chang, C.L.; Wu, S.Y. Statins improve outcomes of nonsurgical curative treatments in hepatocellular carcinoma patients. Medicine 2016, 95, e4639. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, M.S.; Nam, C.M.; Kang, E.S. Statin Use is Associated with Decreased Hepatocellular Carcinoma Recurrence in Liver Transplant Patients. Sci. Rep. 2019, 9, 1467. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Lee, S.W.; Jang, J.W.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; Choi, H.J.; Na, G.H.; You, Y.K.; Park, I.Y.; et al. Anticancer Effect of Statins in Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma. Liver Transpl. 2022, 28, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Turati, F.; La Vecchia, C. Prevention of Biliary Cancer with Statins: Still a Long Way to Go. Gastroenterology 2019, 157, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lin, C.; Hsu, W.; Chang, C.; Yeh, H.; Tung, C.; Wu, Y.; Sung, F.; Kao, C. Statins are associated with a reduced risk of cholangiocarcinoma: A population-based case-control study. Br. J. Clin. Pharmacol. 2015, 80, 755–761. [Google Scholar] [CrossRef]
- Wijarnpreecha, K.; Aby, E.S.; Ghoz, H.; Cheungpasitporn, W.; Lukens, F.J.; Harnois, D.M.; Ungprasert, P. Statins and Risk of Cholangiocarcinoma: A Systematic Review and Meta-analysis. J. Gastrointestin. Liver Dis. 2020, 29, 629–635. [Google Scholar] [CrossRef]
- Cheung, K.S.; Yeung, Y.W.M.; Wong, W.S.; Li, B.; Seto, W.K.; Leung, W.K. Statins associate with lower risk of biliary tract cancers: A systematic review and meta-analysis. Cancer Med. 2023, 12, 557–568. [Google Scholar] [CrossRef]
- Friedman, G.D.; Flick, E.D.; Udaltsova, N.; Chan, J.; Quesenberry, C.P.; Habel, L.A. Screening statins for possible carcinogenic risk: Up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol. Drug Saf. 2008, 17, 27–36. [Google Scholar] [CrossRef]
- Burr, N.E.; Talboys, R.J.; Savva, S.; Clark, A.; Phillips, M.; Metcalfe, M.; Dennison, A.; Robinson, R.; Lewis, M.P.; Rhodes, M.; et al. Aspirin may prevent cholangiocarcinoma: A case-control study from the United kingdom. Dig. Dis. Sci. 2014, 59, 1567–1572. [Google Scholar] [CrossRef]
- Marcano-Bonilla, L.; Schleck, C.D.; Harmsen, W.S.; Sadr-Azodi, O.; Borad, M.J.; Patel, T.; Petersen, G.M.; Therneau, T.M.; Roberts, L.R.; Brusselaers, N. Aspirin, Statins, Non-aspirin NSAIDs, Metformin, and the Risk of Biliary Cancer: A Swedish Population-Based Cohort Study. Cancer Epidemiol. Biomark. Prev. 2022, 31, 804–810. [Google Scholar] [CrossRef]
- Liu, Z.; Alsaggaf, R.; McGlynn, K.A.; Anderson, L.A.; Tsai, H.-T.; Zhu, B.; Zhu, Y.; Mbulaiteye, S.M.; Gadalla, S.M.; Koshiol, J. Statin use and reduced risk of biliary tract cancers in the UK Clinical Practice Research Datalink. Gut 2019, 68, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Prasai, K.; Tella, S.H.; Yadav, S.; Kommalapati, A.; Mara, K.; Mady, M.; Hassan, M.A.; Wongjarupong, N.; Rodriguez-Payan, N.; Borad, M.; et al. Aspirin and Statin Use and the Risk of Gallbladder Cancer. Cancers 2021, 13, 1186. [Google Scholar] [CrossRef] [PubMed]
- Lavu, S.; Therneau, T.M.; Harmsen, W.S.; Mara, K.C.; Wongjarupong, N.; Hassan, M.; Ali, H.A.; Antwi, S.; Giama, N.H.; Miyabe, K.; et al. Effect of Statins on the Risk of Extrahepatic Cholangiocarcinoma. Hepatology 2020, 72, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Chaiteerakij, R.; Yang, J.D.; Harmsen, W.S.; Slettedahl, S.W.; Mettler, T.A.; Fredericksen, Z.S.; Kim, W.R.; Gores, G.J.; Roberts, R.O.; Olson, J.E.; et al. Risk factors for intrahepatic cholangiocarcinoma: Association between metformin use and reduced cancer risk. Hepatology 2013, 57, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Petrick, J.L.; Yang, B.; Altekruse, S.F.; Van Dyke, A.L.; Koshiol, J.; Graubard, B.I.; McGlynn, K.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS ONE 2017, 12, e0186643. [Google Scholar] [CrossRef]
- Schottenfeld, D.; Beebe-Dimmer, J. Chronic inflammation: A common and important factor in the pathogenesis of neoplasia. CA Cancer J. Clin. 2006, 56, 69–83. [Google Scholar] [CrossRef]
- Chang, J.S.; Tsai, C.R.; Chen, L.T. Medical risk factors associated with cholangiocarcinoma in Taiwan: A population-based case-control study. PLoS ONE 2013, 8, e69981. [Google Scholar] [CrossRef]
- Sripa, B.; Deenonpoe, R.; Brindley, P.J. Co-infections with liver fluke and Helicobacter species: A paradigm change in pathogenesis of opisthorchiasis and cholangiocarcinoma? Parasitol. Int. 2017, 66, 383–389. [Google Scholar] [CrossRef]
- Kumagai, S.; Sobue, T.; Makiuchi, T.; Kubo, S.; Uehara, S.; Hayashi, T.; Sato, K.K.; Endo, G. Relationship between cumulative exposure to 1,2-dichloropropane and incidence risk of cholangiocarcinoma among offset printing workers. Occup. Environ. Med. 2016, 73, 545–552. [Google Scholar] [CrossRef]
- Yang, S.-H.; Lin, H.-Y.; Changou, C.A.; Chen, C.-H.; Liu, Y.-R.; Wang, J.; Jiang, X.; Luh, F.; Yen, Y. Integrin β3 and LKB1 are independently involved in the inhibition of proliferation by lovastatin in human intrahepatic cholangiocarcinoma. Oncotarget 2016, 7, 362–373. [Google Scholar] [CrossRef]
- Yang, S.-H.; Lin, H.-Y.; Chang, V.H.; Chen, C.-C.; Liu, Y.-R.; Wang, J.; Zhang, K.; Jiang, X.; Yen, Y. Lovastatin overcomes gefitinib resistance through TNF-α signaling in human cholangiocarcinomas with different LKB1 statuses in vitro and in vivo. Oncotarget 2015, 6, 23857–23873. [Google Scholar] [CrossRef] [PubMed]
- Kamigaki, M.; Sasaki, T.; Serikawa, M.; Inoue, M.; Kobayashi, K.; Itsuki, H.; Minami, T.; Yukutake, M.; Okazaki, A.; Ishigaki, T.; et al. Statins induce apoptosis and inhibit proliferation in cholangiocarcinoma cells. Int. J. Oncol. 2011, 39, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Moriya, K.; Kaji, K.; Saikawa, S.; Sato, S.; Nishimura, N.; Namisaki, T.; Akahane, T.; Mitoro, A.; Yoshiji, H. Atorvastatin Augments Gemcitabine-Mediated Anti-Cancer Effects by Inhibiting Yes-Associated Protein in Human Cholangiocarcinoma Cells. Int. J. Mol. Sci. 2020, 21, 7588. [Google Scholar] [CrossRef]
- Buranrat, B.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, A.V. Effects of Simvastatin in Combination with Anticancer Drugs on Proliferation and Migration in Cholangiocarcinoma Cells. Indian J. Pharm. Sci. 2022, 84, 72–79. [Google Scholar] [CrossRef]
- Buranrat, B.; Senggunprai, L.; Prawan, A.; Kukongviriyapan, V. Simvastatin and atorvastatin as inhibitors of proliferation and inducers of apoptosis in human cholangiocarcinoma cells. Life Sci. 2016, 153, 41–49. [Google Scholar] [CrossRef]
- Gunchick, V.; Mcdevitt, R.L.; Choi, E.; Winslow, K.; Zalupski, M.M.; Sahai, V. Survival Analysis of 1140 Patients with Biliary Cancer and Benefit from Concurrent Renin-Angiotensin Antagonists, Statins, or Aspirin with Systemic Therapy. Oncologist 2023, 28, 531–541. [Google Scholar] [CrossRef]
- Rossi, A.; Filetti, M.; Salimbeni, B.T.; Piras, M.; Rizzo, F.; Giusti, R.; Marchetti, P. Statins and immunotherapy: Togetherness makes strength the potential effect of statins on immunotherapy for NSCLC. Cancer Rep. 2021, 4, e1368. [Google Scholar] [CrossRef]
| Author and Year | Country/ Region | Study Design | Sample Size | Main Findings | |
|---|---|---|---|---|---|
| Statin Users | Statin Non-Users | ||||
| Friedman 2008 [141] | USA/ West | Cohort territory-wide healthcare delivery program database | 361,859 | 3,860,801 | There is no strong evidence of either the causation or prevention of cancer by statins. |
| Burr 2014 [142] | UK/ West | Case control 2 territory hospitals | 81 | 275 | There were no significant associations between the development of cholangiocarcinoma and statins (OR 0.58; 95% CI: 0.28–1.19) |
| Peng 2015 [138] | Taiwan/ East | Case control nationwide NIHRD | 1560 | 4788 | The overall adjusted OR of statin use-associated CCA was 0.80 (95% CI: 0.71–0.90) and lowered for those with longer medications. A stronger dose-response association was seen when using lovastatin. |
| Marcano-Bonilla 2022 [143] | Sweden/ West | Population-based cohort nationwide drug registry database | 950,635 | 4,809,847 | Statins were associated with a lower risk of BTC (HR, 0.66; 95% CI: 0.56–0.78), iCCA (HR, 0.69; 95% CI: 0.50–0.95), eCCA (HR 0.54; 95% CI: 0.38–0.76), and gallbladder cancer (HR, 0.72; 95% CI: 0.57–0.91) |
| Liu, Alsaggaf 2019 [144] | UK/ West | Case control nationwide CPRD GOLD database | 5544 | 13,093 | Compared with the nonuse of statins, current statin use is associated with a 12% lower risk of BTCs. |
| Prasai 2019 [145] | USA/ West | Case Control | 633 | 1266 | In multivariate analysis, statin use was not associated with a reduced risk of gallbladder carcinoma. |
| Lavu 2020 [146] | USA/ West | Case control at the Mayo Clinic in Rochester | 482 | 716 | Statin use was significantly associated with a 4-fold reduction in the risk of eCCA. The risk reduction was observed among the two subtypes of ECC to varying degrees: 3-fold in pCCA and 16-fold in dCCA. |
| Tran 2020 [96] | UK/ West | Prospective cohort PCCIU of Scotland, UK biobank of England, Scotland, Wales | 395,301 | 76,550 | Statin use was associated with a 39% lower risk of liver cancer in the PCCIU |
| Chaiteerakij 2013 [147] | USA/ West | Case control Mayo clinic of Rochester | 237 | 969 | There is no association between statin use and iCCA risk among patients with hyperlipidemia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekuś-Słomka, N.; Mocan, L.P.; Shkreli, R.; Grapă, C.; Denkiewicz, K.; Wesolowska, O.; Kornek, M.; Spârchez, Z.; Słomka, A.; Crăciun, R.; et al. Don’t Judge a Book by Its Cover: The Role of Statins in Liver Cancer. Cancers 2023, 15, 5100. https://doi.org/10.3390/cancers15205100
Piekuś-Słomka N, Mocan LP, Shkreli R, Grapă C, Denkiewicz K, Wesolowska O, Kornek M, Spârchez Z, Słomka A, Crăciun R, et al. Don’t Judge a Book by Its Cover: The Role of Statins in Liver Cancer. Cancers. 2023; 15(20):5100. https://doi.org/10.3390/cancers15205100
Chicago/Turabian StylePiekuś-Słomka, Natalia, Lavinia Patricia Mocan, Rezarta Shkreli, Cristiana Grapă, Kinga Denkiewicz, Oliwia Wesolowska, Miroslaw Kornek, Zeno Spârchez, Artur Słomka, Rareș Crăciun, and et al. 2023. "Don’t Judge a Book by Its Cover: The Role of Statins in Liver Cancer" Cancers 15, no. 20: 5100. https://doi.org/10.3390/cancers15205100






