Fertility and Pregnancy Outcomes after Fertility-Sparing Surgery for Early-Stage Borderline Ovarian Tumors and Epithelial Ovarian Cancer: A Single-Center Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Results
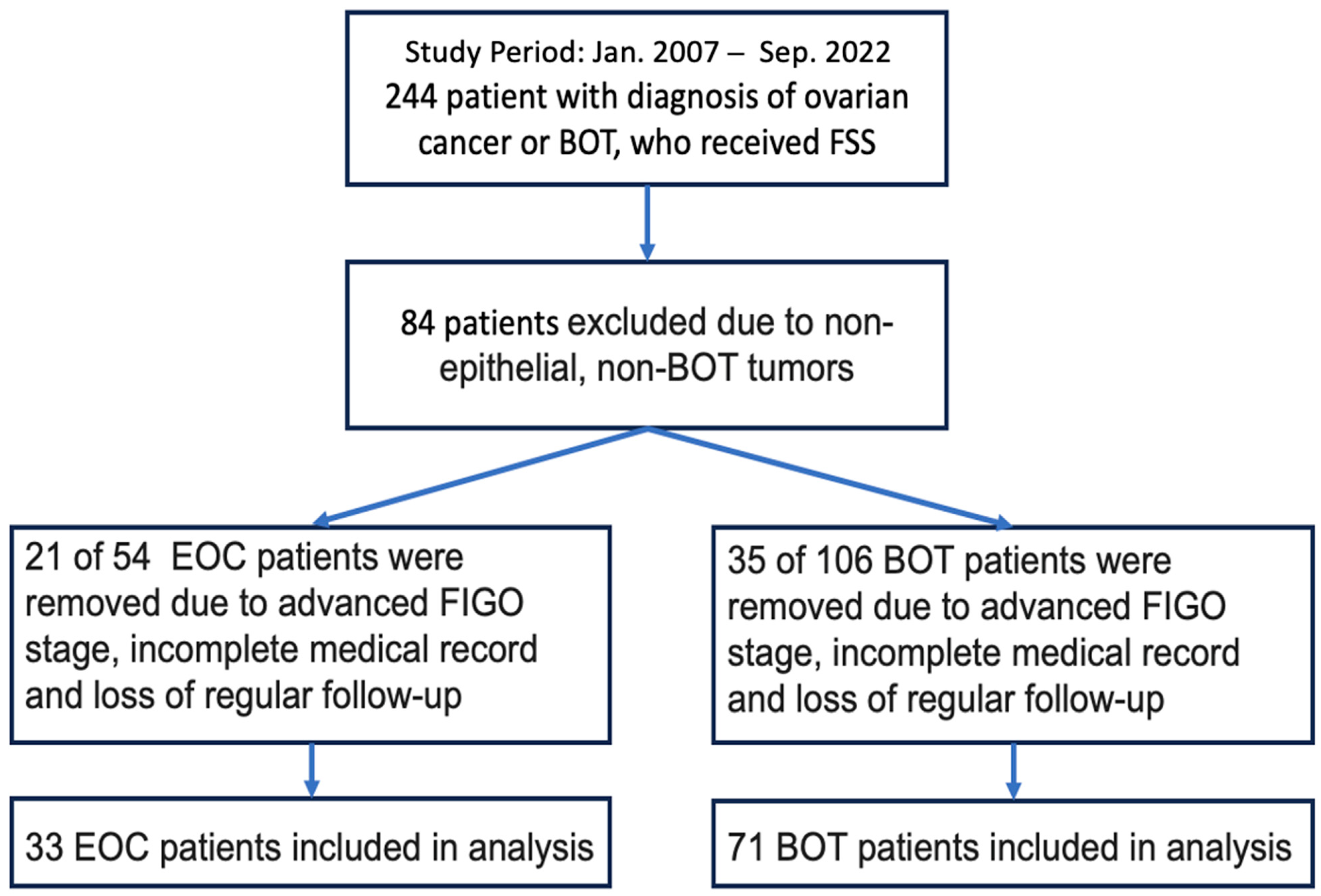
3.1. Study Cohort
3.2. Clinicopathological Characteristics of Included Patients
3.3. Tumor Recurrence
3.4. Menstrual Regularity
3.5. Pregnancy Outcomes
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Duska, L.R.; Chang, Y.C.; Flynn, C.E.; Chen, A.H.; Goodman, A.; Fuller, A.F.; Nikrui, N. Epithelial ovarian carcinoma in the reproductive age group. Cancer 1999, 85, 2623–2629. [Google Scholar] [CrossRef]
- Heintz, A.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Benedet, J.L.; Creasman, W.T.; Ngan, H.; Pecorelli, S.; Beller, U. Carcinoma of the Ovary. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), S161–S192. [Google Scholar] [CrossRef]
- Fischerova, D.; Zikan, M.; Dundr, P.; Cibula, D. Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist 2012, 17, 1515–1533. [Google Scholar] [CrossRef]
- May, J.; Skorupskaite, K.; Congiu, M.; Ghaoui, N.; Walker, G.A.; Fegan, S.; Martin, C.W.; O’Donnell, R.L. Borderline Ovarian Tumors: Fifteen Years’ Experience at a Scottish Tertiary Cancer Center. Int. J. Gynecol. Cancer 2018, 28, 1683–1691. [Google Scholar] [CrossRef]
- Harter, P.; Gershenson, D.; Lhomme, C.; Lecuru, F.; Ledermann, J.; Provencher, D.M.; Mezzanzanica, D.; Quinn, M.; Maenpaa, J.; Kim, J.W.; et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian tumors of low malignant potential (borderline ovarian tumors). Int. J. Gynecol. Cancer 2014, 24 (Suppl. 3), S5–S8. [Google Scholar] [CrossRef]
- Huchon, C.; Bourdel, N.; Wahab, C.A.; Azaïs, H.; Bendifallah, S.; Bolze, P.A.; Brun, J.L.; Canlorbe, G.; Chauvet, P.; Chereau, E.; et al. Borderline ovarian tumors: French guidelines from the CNGOF. Part 1. Epidemiology, biopathology, imaging and biomarkers. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101965. [Google Scholar] [CrossRef]
- Van de Kaa, D.J. Europe’s second demographic transition. Popul Bull. 1987, 42, 1–59. [Google Scholar]
- Tough, S.C.; Newburn-Cook, C.; Johnston, D.W.; Svenson, L.W.; Rose, S.; Belik, J. Delayed childbearing and its impact on population rate changes in lower birth weight, multiple birth, and preterm delivery. Pediatrics 2002, 109, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C.; ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. 6), vi24–vi32, Erratum in: Ann. Oncol. 2018, 29 (Suppl 4), iv259. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K.; Drake, P. Births: Final Data for 2016. Natl. Vital. Stat. Rep. 2018, 67, 1–55. [Google Scholar] [PubMed]
- Kajiyama, H.; Suzuki, S.; Yoshikawa, N.; Kawai, M.; Mizuno, K.; Yamamuro, O.; Nagasaka, T.; Shibata, K.; Kikkawa, F. Fertility-sparing surgery and oncologic outcome among patients with early-stage ovarian cancer ~propensity score- matched analysis. BMC Cancer 2019, 19, 1235. [Google Scholar] [CrossRef]
- Watanabe, T.; Soeda, S.; Nishiyama, H.; Kiko, Y.; Tokunaga, H.; Shigeta, S.; Yaegashi, N.; Yamada, H.; Ohta, T.; Nagase, S.; et al. Clinical and reproductive outcomes of fertility-sparing surgery in stage I epithelial ovarian cancer. Mol. Clin. Oncol. 2020, 12, 44–50. [Google Scholar] [CrossRef]
- Liu, D.; Cai, J.; Gao, A.; Wang, Z.; Cai, L. Fertility sparing surgery vs radical surgery for epithelial ovarian cancer: A meta-analysis of overall survival and disease-free survival. BMC Cancer 2020, 20, 320. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, F.; Cromi, A.; Fanfani, F.; Malzoni, M.; Ditto, A.; De Iaco, P.; Uccella, S.; Gallotta, V.; Raspagliesi, F.; Scambia, G. Laparoscopic fertility-sparing surgery for early ovarian epithelial cancer: A multi-institutional experience. Gynecol. Oncol. 2016, 141, 461–465. [Google Scholar] [CrossRef]
- Nasioudis, D.; Heyward, Q.D.; Ko, E.M.; Haggerty, A.F.; Cory, L.; Giuntoli Ii, R.L.; Kim, S.H.; Latif, N.A. Fertility-sparing surgery for patients with stage IC2 or IC3 epithelial ovarian carcinoma: Any evidence of safety? Int. J. Gynecol. Cancer 2022, 32, 165–171. [Google Scholar] [CrossRef]
- Karlsen, N.M.S.; Karlsen, M.A.; Høgdall, E.; Nedergaard, L.; Christensen, I.J.; Høgdall, C. Relapse and disease specific survival in 1143 Danish women diagnosed with borderline ovarian tumours (BOT). Gynecol. Oncol. 2016, 142, 50–53. [Google Scholar] [CrossRef]
- Helpman, L.; Beiner, M.E.; Aviel-Ronen, S.; Perri, T.; Hogen, L.; Jakobson-Setton, A.; Ben-Baruch, G.; Korach, J. Safety of ovarian conservation and fertility preservation in advanced borderline ovarian tumors. Fertil. Steril. 2015, 104, 138–144. [Google Scholar] [CrossRef]
- Lu, Z.; Li, B.; Gu, C. Outcomes of fertility-sparing surgery for stage II and III serous borderline ovarian tumors. J. Int. Med. Res. 2019, 47, 4895–4903. [Google Scholar] [CrossRef] [PubMed]
- Fauvet, R.; Boccara, J.; Dufournet, C.; Poncelet, C.; Daraï, E. Laparoscopic management of borderline ovarian tumors: Results of a French multicenter study. Ann. Oncol. 2005, 16, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kasaven, L.S.; Chawla, M.; Jones, B.P.; Al-Memar, M.; Galazis, N.; Ahmed-Salim, Y.; El-Bahrawy, M.; Lavery, S.; Saso, S.; Yazbek, J. Fertility Sparing Surgery and Borderline Ovarian Tumours. Cancers 2022, 14, 1485. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.Z.; Xiang, Y.; Yang, J.J.; Shi, J.H.; Jia, C.W.; Leng, J.H. Oncofertility outcomes after fertility-sparing treatment of bilateral serous borderline ovarian tumors: Results of a large retrospective study. Hum. Reprod. 2020, 35, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhong, Q.; Tang, Q.; Wang, H. Second fertility-sparing surgery and fertility-outcomes in patients with recurrent borderline ovarian tumors. Arch. Gynecol. Obstet. 2022, 306, 1177–1183. [Google Scholar] [CrossRef]
- Satoh, T.; Hatae, M.; Watanabe, Y.; Yaegashi, N.; Ishiko, O.; Kodama, S.; Yamaguchi, S.; Ochiai, K.; Takano, M.; Yokota, H.; et al. Outcomes of fertility-sparing surgery for stage I epithelial ovarian cancer: A proposal for patient selection. J. Clin. Oncol. 2010, 28, 1727–1732, Erratum in: J. Clin. Oncol. 2011, 29, 4725. [Google Scholar] [CrossRef]
- Zapardiel, I.; Diestro, M.D.; Aletti, G. Conservative treatment of early stage ovarian cancer: Oncological and fertility outcomes. Eur. J. Surg. Oncol. 2014, 40, 387–393. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, J.; Yu, M.; Xie, W.; Cao, D.; Wu, M.; Pan, L.; Huang, H.; You, Y.; Shen, K. Oncofertility in patients with stage I epithelial ovarian cancer: Fertility-sparing surgery in young women of reproductive age. World J. Surg. Oncol. 2017, 15, 154. [Google Scholar] [CrossRef]
- Fruscio, R.; Corso, S.; Ceppi, L.; Garavaglia, D.; Garbi, A.; Floriani, I.; Franchi, D.; Cantù, M.G.; Bonazzi, C.M.; Milani, R.; et al. Conservative management of early-stage epithelial ovarian cancer: Results of a large retrospective series. Ann. Oncol. 2013, 24, 138–144. [Google Scholar] [CrossRef]
- Hayford, S.R.; Guzzo, K.B. Racial and ethnic variation in unmarried young adults’ motivation to avoid pregnancy. Perspect. Sex Reprod. Health 2013, 45, 41–51. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.F.; Zhang, Y.; Yang, B.; Ai, J.H.; Wang, X.Y.; Cheng, X.D.; Li, K.Z. Oncological and Reproductive Outcomes of Fertility-sparing Surgery in Women with Early-stage Epithelial Ovarian Carcinoma: A Multicenter Retrospective Study. Curr. Med. Sci. 2020, 40, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Bercow, A.; Nitecki, R.; Brady, P.C.; Rauh-Hain, J.A. Outcomes after Fertility-sparing Surgery for Women with Ovarian Cancer: A Systematic Review of the Literature. J. Minim. Invasive Gynecol. 2021, 28, 527–536.e1. [Google Scholar] [CrossRef] [PubMed]
- Delle Marchette, M.; Ceppi, L.; Andreano, A.; Bonazzi, C.M.; Buda, A.; Grassi, T.; Giuliani, D.; Sina, F.; Lamanna, M.; Bianchi, T.; et al. Oncologic and fertility impact of surgical approach for borderline ovarian tumours treated with fertility sparing surgery. Eur. J. Cancer 2019, 111, 61–68. [Google Scholar] [CrossRef]
- Canlorbe, G.; Chabbert-Buffet, N.; Uzan, C. Fertility-Sparing Surgery for Ovarian Cancer. J. Clin. Med. 2021, 10, 4235. [Google Scholar] [CrossRef] [PubMed]
- Zapardiel, I.; Cruz, M.; Diestro, M.D.; Requena, A.; Garcia-Velasco, J.A. Assisted reproductive techniques after fertility-sparing treatments in gynaecological cancers. Hum. Reprod. Update 2016, 22, 281–305. [Google Scholar] [CrossRef] [PubMed]
| Epithelial Ovarian Cancer | Borderline Ovarian Tumors | |
|---|---|---|
| Total | 33 | 71 |
| Median age (years) | 34 (22–42) | 30 (19–44) |
| Median follow-up interval (month) | 97 (3–180) | 71 (6–152) |
| Follow-up interval ≥ 24 months (%) | 87.9 (29/33) | 87.3 (62/71) |
| FIGO stage | ||
| IA (%) | 45.5 (15/33) | 55.0 (39/71) |
| IB (%) | - | 2.8 (2/71) |
| IC (%) | 54.5 (18/33) | 42.2 (30/71) |
| Histology | ||
| Mucinous (%) | 45.5 (15/33) | 73.2 (52/71) |
| Serous (%) | 3.0 (1/33) | 21.1(15/71) |
| Clear Cell (%) | 30.3 (10/33) | - |
| Endometrioid (%) | 21.2 (7/33) | - |
| Seromucinous (%) | - | 5.6 (4/71) |
| Lesion site | ||
| Right (%) | 18.2 (6/33) | 19.7 (14/71) |
| Left (%) | 81.8 (27/33) | 73.2 (52/71) |
| Bilateral (%) | - | 7.1(5/71) |
| Diameter | ||
| Median diameter (cm) | 16 (3–40) | 15 (5–50) |
| <10 cm (%) | 33.3 (11/33) | 21.1 (15/71) |
| ≥10 cm (%) | 66.7 (22/33) | 78.9 (56/71) |
| Preoperative CA-125 > 35 IU/mL (%) | 51.5 (17/33) | 54.9 (39/71) |
| Surgical approach | ||
| Laparoscopy (%) | 15.2 (5/33) | 33.8 (24/71) |
| Laparotomy (%) | 84.8 (28/33) | 66.2 (47/71) |
| Comprehensiveness of surgery | ||
| Comprehensive FSS (%) | 57.6 (19/33) | 38.0 (27/71) |
| Simple adnexectomy/cystectomy (%) | 42.4 (14/33) | 62.0 (44/71) |
| Intraoperative rupture (%) | 36.4 (12/33) | 30.1 (22/71) |
| Lymphadenectomy (%) | 54.5 (18/33) | 28.2 (20/71) |
| Omentectomy (%) | 63.6 (21/33) | 35.2 (25/71) |
| Adjuvant chemotherapy (%) | 60.6 (20/33) | 4.2 (3/71) |
| Recurrence (%) | 3.0 (1/33) | 11.3 (8/71) |
| Death (%) | - | 1.4 (1/71) |
| Recurrence (%) | p Value | ||||
|---|---|---|---|---|---|
| FIGO stage | Ia | Ib | Ic | 0.0354 | |
| 2.63(1/38) | 0 (0/2) | 22.58 (7/31) | |||
| Histology | Mucinous | Serous | Seromucinous | 0.0179 | |
| 5.77 (3/52) | 33.33 (5/15) | 0 (0/4) | |||
| Preoperative CA-125 | <35 U/mL | 35–100 U/mL | >100 U/mL | 0.0238 | |
| 3.13 (1/32) | 15.79 (3/19) | 20 (4/20) | |||
| Comprehensiveness of FSS | sFSS | cFSS | 0.7014 | ||
| 13.64 (6/44) | 7.41 (2/27) | ||||
| Extent of ovarian surgery in FSS | Salpingo-oophorectomy only | Inclusion of cystectomy | 0.189 | ||
| 7.55 (4/53) | 22.22 (4/18) | ||||
| Lymphadenectomy | Yes | No | 0.4267 | ||
| 5 (1/20) | 13.73 (7/51) | ||||
| Omentectomy | Yes | No | 0.8033 | ||
| 12 (3/25) | 10.87 (5/46) | ||||
| Adjuvant chemotherapy | Yes | No | - | ||
| 0 (0/3) | 11.76 (8/68) | ||||
| Remaining Married | Getting Married | Divorced | Single | |
|---|---|---|---|---|
| Epithelial Ovarian Cancer (n = 33) | 7 | 7 | 1 | 18 |
| Borderline Ovarian Tumors (n = 71) | 23 | 12 | 2 | 34 |
| Borderline Ovarian Tumor | |||||||
|---|---|---|---|---|---|---|---|
| Number of Patients | Number of Patients Achieving Pregnancy | Number of Pregnancies | Live Birth | Abortion | Preterm | ART * | |
| Married with prior childbirth | 18 | 6 | 7 | 6 | 1 | 0 | 0 |
| Married without prior childbirth | 5 | 3 | 6 | 4 | 2 | 0 | 1 |
| Married after treatment | 12 | 11 | 13 | 13 | 0 | 1 ** | 0 |
| Age | FIGO Stage | FSS Procedure | Histology Type | Recurrence (Month) | Treatment | Prior Live Birth | Achieved Pregnancy | Achieved Live Birth | |
|---|---|---|---|---|---|---|---|---|---|
| EOC1 | 35 | IC1 | LSO + BPLNS + omentectomy | Clear cell | 21 | Loss of follow-up | 0 | 0 | 0 |
| BOT1 | 33 | IC2 | RSO | Mucinous | 23 * | Debulking surgery and C/T | 1 | 1 | 1 |
| BOT2 | 35 | IC1 | LSO + appendectomy + omental biopsy + LPLNS | Mucinous | 37 M | Complete staging | 0 | 0 | 0 |
| BOT3 | 25 | IA | LSC RSO | Serous | 70 M | Left cystectomy | 0 | 1 | 1 |
| BOT4 | 22 | IC1 | LSO | Serous | 71 M | LSC RPO | 0 | 1 | 1 |
| BOT5 | 23 | IC1 | LSO | Serous | 66 M | RSO | 0 | 1 | 1 |
| BOT6 | 35 | IC1 | LSC left cystectomy | Mucinous | 17 M | LSO | 0 | 3 | 1 |
| BOT7 | 28 | IC2 | RSO + omental biopsy | Serous | 36 M | LSO | 0 | 0 | 0 |
| BOT8 | 29 | IC3 | LSO + Right cystectomy + omentectomy | Serous | 69 M | RPO | 0 | 0 | 0 |
| Epithelial Ovarian Cancer | |||||||
|---|---|---|---|---|---|---|---|
| Number of Patients | Number of Patients Achieving Pregnancy | Number of Pregnancies | Live Birth | Abortion | Preterm | ART * | |
| Married with prior childbirth | 4 | 4 | 4 | 4 | 0 | 0 | 0 |
| Married without prior childbirth | 3 | 2 | 2 | 2 | 0 | 0 | 1 |
| Married after treatment | 7 | 6 | 9 | 10 | 1 | 2 ** | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, M.-E.; Lin, Y.-H.; Huang, K.-J.; Chang, W.-C.; Sheu, B.-C. Fertility and Pregnancy Outcomes after Fertility-Sparing Surgery for Early-Stage Borderline Ovarian Tumors and Epithelial Ovarian Cancer: A Single-Center Study. Cancers 2023, 15, 5327. https://doi.org/10.3390/cancers15225327
Ko M-E, Lin Y-H, Huang K-J, Chang W-C, Sheu B-C. Fertility and Pregnancy Outcomes after Fertility-Sparing Surgery for Early-Stage Borderline Ovarian Tumors and Epithelial Ovarian Cancer: A Single-Center Study. Cancers. 2023; 15(22):5327. https://doi.org/10.3390/cancers15225327
Chicago/Turabian StyleKo, Mu-En, Yi-Heng Lin, Kuan-Ju Huang, Wen-Chun Chang, and Bor-Ching Sheu. 2023. "Fertility and Pregnancy Outcomes after Fertility-Sparing Surgery for Early-Stage Borderline Ovarian Tumors and Epithelial Ovarian Cancer: A Single-Center Study" Cancers 15, no. 22: 5327. https://doi.org/10.3390/cancers15225327
APA StyleKo, M.-E., Lin, Y.-H., Huang, K.-J., Chang, W.-C., & Sheu, B.-C. (2023). Fertility and Pregnancy Outcomes after Fertility-Sparing Surgery for Early-Stage Borderline Ovarian Tumors and Epithelial Ovarian Cancer: A Single-Center Study. Cancers, 15(22), 5327. https://doi.org/10.3390/cancers15225327





