Rectum and Bladder Toxicity in Postoperative Prostate Bed Irradiation: Dose–Volume Parameters Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
Statistical Analysis
3. Results
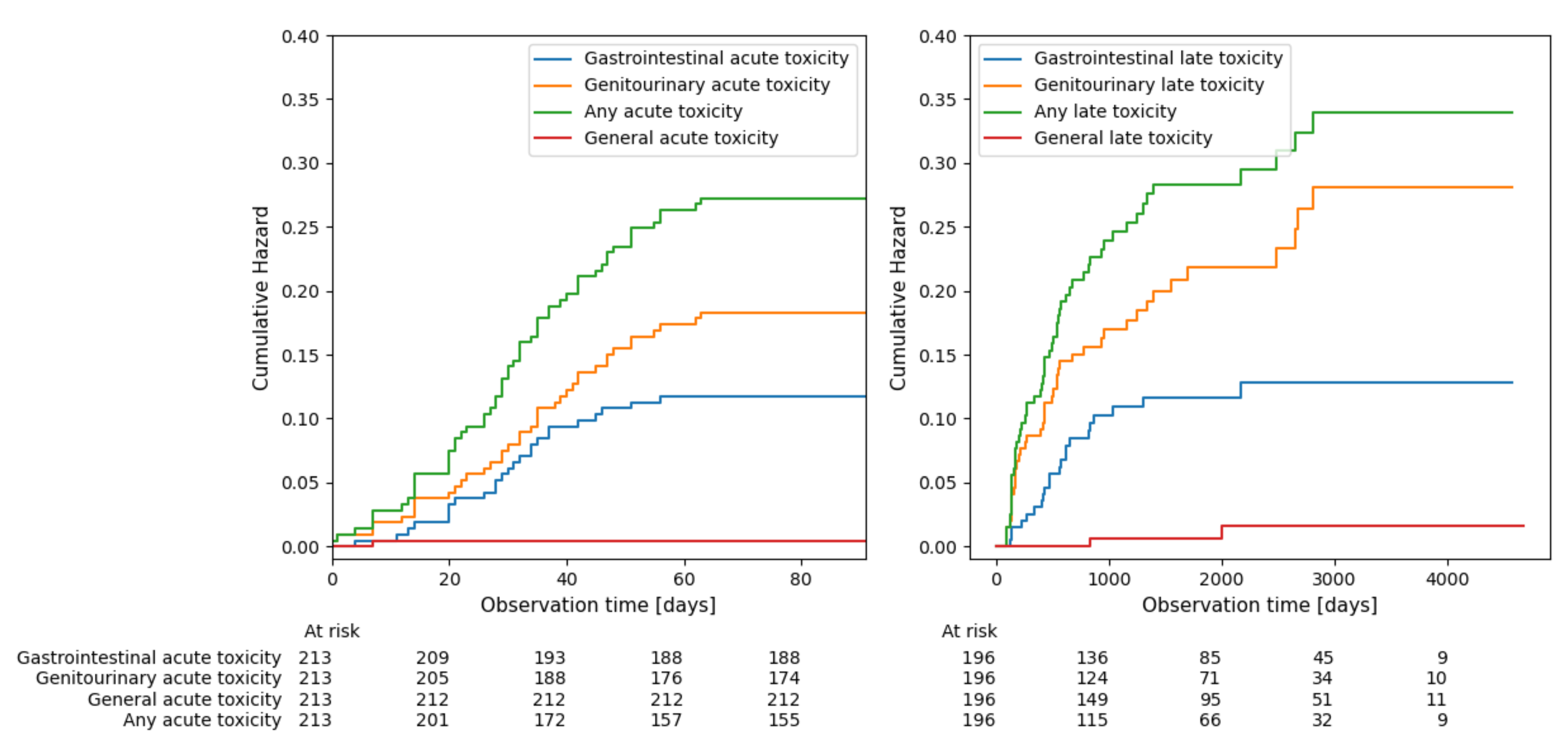
3.1. Overall Treatment Toxicity
3.2. Association between ≥G2 Toxicity Occurrence and Respective DVPs
3.3. Association between QUANTEC DVCs and Organ Toxicity
3.4. Association between ≥G2 Toxicity Occurrence and Clinical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kneebone, A.; Fraser-Browne, C.; Duchesne, G.M.; Fisher, R.; Frydenberg, M.; Herschtal, A.; Williams, S.G.; Brown, C.; Delprado, W.; Haworth, A.; et al. Adjuvant Radiotherapy versus Early Salvage Radiotherapy Following Radical Prostatectomy (TROG 08.03/ANZUP RAVES): A Randomised, Controlled, Phase 3, Non-Inferiority Trial. Lancet Oncol. 2020, 21, 1331–1340. [Google Scholar] [CrossRef]
- Parker, C.C.; Clarke, N.W.; Cook, A.D.; Kynaston, H.G.; Petersen, P.M.; Catton, C.; Cross, W.; Logue, J.; Parulekar, W.; Payne, H.; et al. Timing of Radiotherapy after Radical Prostatectomy (RADICALS-RT): A Randomised, Controlled Phase 3 Trial. Lancet 2020, 396, 1413–1421. [Google Scholar] [CrossRef]
- Sargos, P.; Chabaud, S.; Latorzeff, I.; Magné, N.; Benyoucef, A.; Supiot, S.; Pasquier, D.; Abdiche, M.S.; Gilliot, O.; Graff-Cailleaud, P.; et al. Adjuvant Radiotherapy versus Early Salvage Radiotherapy plus Short-Term Androgen Deprivation Therapy in Men with Localised Prostate Cancer after Radical Prostatectomy (GETUG-AFU 17): A Randomised, Phase 3 Trial. Lancet Oncol. 2020, 21, 1341–1352. [Google Scholar] [CrossRef]
- Prostate Cancer—INTRODUCTION—Uroweb. Available online: https://uroweb.org/guidelines/prostate-cancer/chapter/introduction (accessed on 7 November 2023).
- Gay, H.A.; Barthold, H.J.; O’Meara, E.; Bosch, W.R.; El Naqa, I.; Al-Lozi, R.; Rosenthal, S.A.; Lawton, C.; Lee, W.R.; Sandler, H.; et al. Pelvic Normal Tissue Contouring Guidelines for Radiation Therapy: A Radiation Therapy Oncology Group Consensus Panel Atlas. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e353–e362. [Google Scholar] [CrossRef]
- Szymanski, K.M.; Wei, J.T.; Dunn, R.L.; Sanda, M.G. Development and Validation of an Abbreviated Version of the Expanded Prostate Cancer Index Composite Instrument for Measuring Health-Related Quality of Life among Prostate Cancer Survivors. Urology 2010, 76, 1245–1250. [Google Scholar] [CrossRef]
- Litwin, M.S.; Hays, R.D.; Fink, A.; Ganz, P.A.; Leake, B.; Brook, R.H. The UCLA Prostate Cancer Index: Development, Reliability, and Validity of a Health-Related Quality of Life Measure. Med. Care 1998, 36, 1002–1012. [Google Scholar] [CrossRef]
- Schick, U.; Latorzeff, I.; Sargos, P. Postoperative Radiotherapy in Prostate Cancer: Dose and Volumes. Cancer/Radiothérapie 2021, 25, 674–678. [Google Scholar] [CrossRef]
- Miszczyk, L.; Namysł-Kaletka, A.; Napieralska, A.; Kraszkiewicz, M.; Miszczyk, M.; Woźniak, G.; Stąpór-Fudzińska, M.; Głowacki, G.; Tukiendorf, A. Stereotactic Ablative Radiotherapy for Prostate Cancer—The Treatment Results of 500 Patients and Analysis of Failures. Technol. Cancer Res. Treat. 2019, 18, 1533033819870815. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE). 2017; p. 155. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 7 November 2023).
- Viswanathan, A.N.; Yorke, E.D.; Marks, L.B.; Eifel, P.J.; Shipley, W.U. Radiation Dose–Volume Effects of the Urinary Bladder. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S116–S122. [Google Scholar] [CrossRef]
- Michalski, J.M.; Gay, H.; Jackson, A.; Tucker, S.L.; Deasy, J.O. Radiation Dose–Volume Effects in Radiation-Induced Rectal Injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S123–S129. [Google Scholar] [CrossRef]
- What Is a Serious Adverse Event? | FDA. Available online: https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event (accessed on 7 November 2023).
- Vale, C.L.; Fisher, D.; Kneebone, A.; Parker, C.; Pearse, M.; Richaud, P.; Sargos, P.; Sydes, M.R.; Brawley, C.; Brihoum, M.; et al. Adjuvant or Early Salvage Radiotherapy for the Treatment of Localised and Locally Advanced Prostate Cancer: A Prospectively Planned Systematic Review and Meta-Analysis of Aggregate Data. Lancet 2020, 396, 1422–1431. [Google Scholar] [CrossRef]
- Cozzarini, C.; Fiorino, C.; Da Pozzo, L.F.; Alongi, F.; Berardi, G.; Bolognesi, A.; Briganti, A.; Broggi, S.; Deli, A.; Guazzoni, G.; et al. Clinical Factors Predicting Late Severe Urinary Toxicity after Postoperative Radiotherapy for Prostate Carcinoma: A Single-Institute Analysis of 742 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 191–199. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Levin, E.J.; Hunt, M.; Yamada, Y.; Shippy, A.M.; Jackson, A.; Amols, H.I. Incidence of Late Rectal and Urinary Toxicities After Three-Dimensional Conformal Radiotherapy and Intensity-Modulated Radiotherapy for Localized Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1124–1129. [Google Scholar] [CrossRef]
- Alicikus, Z.A.; Yamada, Y.; Zhang, Z.; Pei, X.; Hunt, M.; Kollmeier, M.; Cox, B.; Zelefsky, M.J. Ten-Year Outcomes of High-Dose, Intensity-Modulated Radiotherapy for Localized Prostate Cancer. Cancer 2011, 117, 1429–1437. [Google Scholar] [CrossRef]
- Hamstra, D.A.; Stenmark, M.H.; Ritter, T.; Litzenberg, D.; Jackson, W.; Johnson, S.; Albrecht-Unger, L.; Donaghy, A.; Phelps, L.; Blas, K.; et al. Age and Comorbid Illness Are Associated With Late Rectal Toxicity Following Dose-Escalated Radiation Therapy for Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1246–1253. [Google Scholar] [CrossRef]
- Tucker, S.L.; Dong, L.; Bosch, W.R.; Michalski, J.; Winter, K.; Mohan, R.; Purdy, J.A.; Kuban, D.; Lee, A.K.; Cheung, M.R.; et al. Late Rectal Toxicity on RTOG 94-06: Analysis Using a Mixture Lyman Model. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1253–1260. [Google Scholar] [CrossRef]
- Cozzarini, C.; Rancati, T.; Carillo, V.; Civardi, F.; Garibaldi, E.; Franco, P.; Avuzzi, B.; Esposti, C.D.; Girelli, G.; Iotti, C.; et al. Multi-Variable Models Predicting Specific Patient-Reported Acute Urinary Symptoms after Radiotherapy for Prostate Cancer: Results of a Cohort Study. Radiother. Oncol. 2015, 116, 185–191. [Google Scholar] [CrossRef]
- Yahya, N.; Ebert, M.A.; Bulsara, M.; Haworth, A.; Kennedy, A.; Joseph, D.J.; Denham, J.W. Dosimetry, Clinical Factors and Medication Intake Influencing Urinary Symptoms after Prostate Radiotherapy: An Analysis of Data from the RADAR Prostate Radiotherapy Trial. Radiother. Oncol. 2015, 116, 112–118. [Google Scholar] [CrossRef]
- Jereczek-Fossa, B.A.; Zerini, D.; Fodor, C.; Santoro, L.; Serafini, F.; Cambria, R.; Vavassori, A.; Cattani, F.; Garibaldi, C.; Gherardi, F.; et al. Correlation Between Acute and Late Toxicity in 973 Prostate Cancer Patients Treated With Three-Dimensional Conformal External Beam Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 26–34. [Google Scholar] [CrossRef]
- Heemsbergen, W.D.; Peeters, S.T.H.; Koper, P.C.M.; Hoogeman, M.S.; Lebesque, J.V. Acute and Late Gastrointestinal Toxicity after Radiotherapy in Prostate Cancer Patients: Consequential Late Damage. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 3–10. [Google Scholar] [CrossRef]
- Peach, M.S.; Showalter, T.N.; Ohri, N. Systematic Review of the Relationship between Acute and Late Gastrointestinal Toxicity after Radiotherapy for Prostate Cancer. Prostate Cancer 2015, 2015, e624736. [Google Scholar] [CrossRef]
- Borghetti, P.; Spiazzi, L.; Cozzaglio, C.; Pedretti, S.; Caraffini, B.; Triggiani, L.; Greco, D.; Bardoscia, L.; Barbera, F.; Buglione, M.; et al. Postoperative Radiotherapy for Prostate Cancer: The Sooner the Better and Potential to Reduce Toxicity Even Further. Radiol. Med. 2018, 123, 63–70. [Google Scholar] [CrossRef]
- Pederson, A.W.; Fricano, J.; Correa, D.; Pelizzari, C.A.; Liauw, S.L. Late Toxicity after Intensity-Modulated Radiation Therapy for Localized Prostate Cancer: An Exploration of Dose-Volume Histogram Parameters to Limit Genitourinary and Gastrointestinal Toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 235–241. [Google Scholar] [CrossRef]
- Landoni, V.; Fiorino, C.; Cozzarini, C.; Sanguineti, G.; Valdagni, R.; Rancati, T. Predicting Toxicity in Radiotherapy for Prostate Cancer. Phys. Med. 2016, 32, 521–532. [Google Scholar] [CrossRef]
- Catucci, F.; Alitto, A.R.; Masciocchi, C.; Dinapoli, N.; Gatta, R.; Martino, A.; Mazzarella, C.; Fionda, B.; Frascino, V.; Piras, A.; et al. Predicting Radiotherapy Impact on Late Bladder Toxicity in Prostate Cancer Patients: An Observational Study. Cancers 2021, 13, 175. [Google Scholar] [CrossRef]
- Schaake, W.; van der Schaaf, A.; van Dijk, L.V.; van den Bergh, A.C.M.; Langendijk, J.A. Development of a Prediction Model for Late Urinary Incontinence, Hematuria, Pain and Voiding Frequency among Irradiated Prostate Cancer Patients. PLoS ONE 2018, 13, e0197757. [Google Scholar] [CrossRef]
- Shirai, K.; Suzuki, M.; Akahane, K.; Takahashi, Y.; Kawahara, M.; Yamada, E.; Wakatsuki, M.; Ogawa, K.; Takahashi, S.; Minato, K.; et al. Dose-Volume Histogram-Based Predictors for Hematuria and Rectal Hemorrhage in Patients Receiving Radiotherapy After Radical Prostatectomy. In Vivo 2020, 34, 1289–1295. [Google Scholar] [CrossRef]
- Sanfratello, A.; Cusumano, D.; Piras, A.; Boldrini, L.; D’Aviero, A.; Fricano, P.; Messina, M.; Vaglica, M.; Galanti, D.; Spada, M.; et al. New Dosimetric Parameters to Predict Ano-Rectal Toxicity during Radiotherapy Treatment. Phys. Med. 2022, 99, 55–60. [Google Scholar] [CrossRef]
- Tucker, S.L.; Dong, L.; Cheung, R.; Johnson, J.; Mohan, R.; Huang, E.H.; Liu, H.H.; Thames, H.D.; Kuban, D. Comparison of Rectal Dose–Wall Histogram versus Dose–Volume Histogram for Modeling the Incidence of Late Rectal Bleeding after Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 1589–1601. [Google Scholar] [CrossRef]
- Bakkal, B.H.; Elmas, O. Dosimetric Comparison of Organs at Risk in 5 Different Radiotherapy Plans in Patients with Preoperatively Irradiated Rectal Cancer. Medicine 2021, 100, e24266. [Google Scholar] [CrossRef]
- Buettner, F.; Gulliford, S.L.; Webb, S.; Sydes, M.R.; Dearnaley, D.P.; Partridge, M. Assessing Correlations between the Spatial Distribution of the Dose to the Rectal Wall and Late Rectal Toxicity after Prostate Radiotherapy: An Analysis of Data from the MRC RT01 Trial (ISRCTN 47772397). Phys. Med. Biol. 2009, 54, 6535. [Google Scholar] [CrossRef]
- Fokdal, L.; Honoré, H.; Høyer, M.; von der Maase, H. Dose–Volume Histograms Associated to Long-Term Colorectal Functions in Patients Receiving Pelvic Radiotherapy. Radiother. Oncol. 2005, 74, 203–210. [Google Scholar] [CrossRef]
- Cozzarini, C.; Fiorino, C.; Ceresoli, G.L.; Cattaneo, G.M.; Bolognesi, A.; Calandrino, R.; Villa, E. Significant Correlation between Rectal DVH and Late Bleeding in Patients Treated after Radical Prostatectomy with Conformal or Conventional Radiotherapy (66.6–70.2 Gy). Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 688–694. [Google Scholar] [CrossRef]
- Fenwick, J.D.; Khoo, V.S.; Nahum, A.E.; Sanchez-Nieto, B.; Dearnaley, D.P. Correlations between Dose-Surface Histograms and the Incidence of Long-Term Rectal Bleeding Following Conformal or Conventional Radiotherapy Treatment of Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 473–480. [Google Scholar] [CrossRef]
- Gulliford, S.L.; Foo, K.; Morgan, R.C.; Aird, E.G.; Bidmead, A.M.; Critchley, H.; Evans, P.M.; Gianolini, S.; Mayles, W.P.; Moore, A.R.; et al. Dose-Volume Constraints to Reduce Rectal Side Effects from Prostate Radiotherapy: Evidence from MRC RT01 Trial ISRCTN 47772397. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 747–754. [Google Scholar] [CrossRef]
- Chennupati, S.K.; Pelizzari, C.A.; Kunnavakkam, R.; Liauw, S.L. Late Toxicity and Quality of Life after Definitive Treatment of Prostate Cancer: Redefining Optimal Rectal Sparing Constraints for Intensity-Modulated Radiation Therapy. Cancer Med. 2014, 3, 954–961. [Google Scholar] [CrossRef]
- Peterson, J.L.; Buskirk, S.J.; Heckman, M.G.; Diehl, N.N.; Bernard, J.R.; Tzou, K.S.; Casale, H.E.; Bellefontaine, L.P.; Serago, C.; Kim, S.; et al. Image-Guided Intensity-Modulated Radiotherapy for Prostate Cancer: Dose Constraints for the Anterior Rectal Wall to Minimize Rectal Toxicity. Med. Dosim 2014, 39, 12–17. [Google Scholar] [CrossRef]
- Chua, B.; Min, M.; Wood, M.; Edwards, S.; Hoffmann, M.; Greenham, S.; Kovendy, A.; McKay, M.J.; Shakespeare, T.P. Implementation of an Image Guided Intensity-Modulated Protocol for Post-Prostatectomy Radiotherapy: Planning Data and Acute Toxicity Outcomes. J. Med. Imaging Radiat. Oncol. 2013, 57, 482–489. [Google Scholar] [CrossRef]
- NCCN Guidelines® Insights—Prostate Cancer, Version 1.2023 | NCCN Continuing Education. Available online: https://education.nccn.org/node/91128 (accessed on 21 May 2023).
- Fonteyne, V.; Ost, P.; Vanpachtenbeke, F.; Colman, R.; Sadeghi, S.; Villeirs, G.; Decaestecker, K.; De Meerleer, G. Rectal Toxicity after Intensity Modulated Radiotherapy for Prostate Cancer: Which Rectal Dose Volume Constraints Should We Use? Radiother. Oncol. 2014, 113, 398–403. [Google Scholar] [CrossRef]
- Gómez, L.; Andrés, C.; Ruiz, A. Dosimetric Impact in the Dose-Volume Histograms of Rectal and Vesical Wall Contouring in Prostate Cancer IMRT Treatments. Rep. Pract. Oncol. Radiother. 2017, 22, 223–230. [Google Scholar] [CrossRef][Green Version]
| Number of Cases (All/Adjuvant/Salvage) | N = 213 | N = 101 (47.4%) | N = 112 (52.6%) |
| * Age [years] | 63.6 (59.8–68.4) | 62.3 (58.8–66.3) | 65.2 (60.5–70.2) |
| * Follow-up [months] | 61.4 (38.1–78.4) | 64.9 (38.1–86.6) | 57.6 (38.5–74.4) |
| * Time from RP 1 to RT 2 [months] | 6.2 (3.4–25.8) | 3.7 (3.0–4.8) | 22.6 (7.0–46.2) |
| Total dose (prostate bed) [Gy] | 70. (70–74); Min 62, Max 76 | 70 (70–72); Min 62, Max 76 | 74 (70–76); Min 66, Max 76 |
| * max PSA pre-RP [ng/mL] | 9.24 (6.75–14.61) | 8.87 (6.70–14.39) | 10.33 (7.00–14.69) |
| * max PSA post-RP [ng/mL] | 0.27 (0.04–1.14) | 0.03 (0.01–0.10) | 1.00 (0.40–2.38) |
| * pT stage pT2a pT2b pT2c pT3a pT3b | 32 (15%) 12 (5.6%) 79 (37.1%) 39 (18.3%) 44 (20.7%) | 6 (5.9%) 4 (4.0%) 39 (38.6%) 27 (26.7%) 24 (23.8%) | 26 (23.2%) 8 (7.1%) 40 (35.7%) 12 (10.7%) 20 (17.9%) |
| ADT 3 (receiving) | 83 (39.0%) | 35 (34.7%) | 48 (42.9%) |
| ISUP 4 grade group | |||
| 1 | 83 (39.0%) | 33 (32.7%) | 50 (44.6%) |
| 2 | 72 (33.8%) | 41 (40.6%) | 31 (27.7%) |
| 3 | 31 (14.6%) | 14 (13.9%) | 17 (15.2%) |
| 4 | 12 (5.6%) | 5 (5.0%) | 7 (6.3%) |
| 5 | 11 (5.2%) | 7 (6.9%) | 4 (3.6%) |
| Radiotherapy modality ^ | |||
| IMRT 5 | 117 (54.9%) | 58 (57.4%) | 59 (52.7%) |
| 3DCRT 6 | 38 (17.8%) | 16 (15.8%) | 22 (19.6%) |
| VMAT 7 | 35 (16.4%) | 16 (15.8%) | 19 (17.0%) |
| Tomotherapy | 8 (3.8%) | 3 (3.0%) | 5 (4.5%) |
| IMRT + VMAT | 6 (2.8%) | 3 (3.0%) | 3 (2.7%) |
| 3DCRT + IMRT | 5 (2.3%) | 3 (3.0%) | 2 (1.8%) |
| 3DCRT + VMAT | 4 (1.9%) | 2 (2.0%) | 2 (1.8%) |
| Pelvic lymph node irradiation | 58 (27.2%) | 33 (32.7%) | 25 (22.3%) |
| N (%) | |
|---|---|
| grade ≥ 2 GI acute | 25 (11.7%) |
| grade ≥ 2 GU acute | 40 (18.7%) |
| grade ≥ 2 other acute | 1 (0.5%) |
| grade ≥ 2 GI late | 22 (11.2%) |
| grade ≥ 2 GU late | 42 (21.3%) |
| grade ≥ 2 other late | 2 (1.0%) |
| DVCs | ||||||
|---|---|---|---|---|---|---|
| Rectum | V50 < 50% | V50 > 50% | V70 < 35% | V70 > 35% | V75 < 25% | V75 > 25% |
| ≥G2 acute toxicity rate | 10.3% | 26.3% | 11.3% | 15.8% | 11.1% | 16.7% |
| ≥G2 late toxicity rate | 9.3% | 21.1% | 9.8% | 15.8% | 8.5% | 25.0% |
| DVCs | ||
|---|---|---|
| Rectum wall | V50 < 50% | V50 > 50% |
| ≥G2 late toxicity rate | 9.1% | 28.6% |
| Bladder wall | Dmin < 3% | Dmin > 3% |
| ≥G2 late toxicity rate | 14.7% | 28.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasterok, M.; Szołtysik, M.; Nowicka, Z.; Goc, B.; Gräupner, D.; Majewski, W.; Rasławski, K.; Rajwa, P.; Jabłońska, I.; Magrowski, Ł.; et al. Rectum and Bladder Toxicity in Postoperative Prostate Bed Irradiation: Dose–Volume Parameters Analysis. Cancers 2023, 15, 5334. https://doi.org/10.3390/cancers15225334
Hasterok M, Szołtysik M, Nowicka Z, Goc B, Gräupner D, Majewski W, Rasławski K, Rajwa P, Jabłońska I, Magrowski Ł, et al. Rectum and Bladder Toxicity in Postoperative Prostate Bed Irradiation: Dose–Volume Parameters Analysis. Cancers. 2023; 15(22):5334. https://doi.org/10.3390/cancers15225334
Chicago/Turabian StyleHasterok, Maja, Monika Szołtysik, Zuzanna Nowicka, Bartłomiej Goc, Donata Gräupner, Wojciech Majewski, Konrad Rasławski, Paweł Rajwa, Iwona Jabłońska, Łukasz Magrowski, and et al. 2023. "Rectum and Bladder Toxicity in Postoperative Prostate Bed Irradiation: Dose–Volume Parameters Analysis" Cancers 15, no. 22: 5334. https://doi.org/10.3390/cancers15225334
APA StyleHasterok, M., Szołtysik, M., Nowicka, Z., Goc, B., Gräupner, D., Majewski, W., Rasławski, K., Rajwa, P., Jabłońska, I., Magrowski, Ł., Przydacz, M., Krajewski, W., Masri, O., & Miszczyk, M. (2023). Rectum and Bladder Toxicity in Postoperative Prostate Bed Irradiation: Dose–Volume Parameters Analysis. Cancers, 15(22), 5334. https://doi.org/10.3390/cancers15225334







