Prognostic Significance of C-Reactive Protein in Lenvatinib-Treated Unresectable Hepatocellular Carcinoma: A Multi-Institutional Study
Abstract
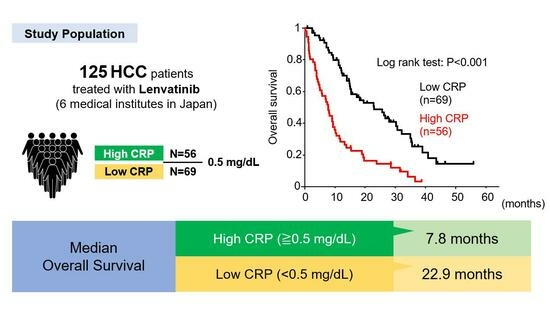
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Diagnosis and Treatment of HCC
2.3. Lenvatinib Treatment
2.4. Adverse Events
2.5. Ethics
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Factors Associated with OS in All Patients
3.3. Factors Associated with OS in Patients with ALBI Grade 1 and 2a
3.4. Factors Associated with OS in Patients with ALBI Grades of 2b and 3
3.5. Factors Associated with PFS in All Patients
3.6. Factors Associated with TTF in All Patients
3.7. Association between Reasons of Treatment Failure and CRP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef]
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J.; et al. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology 2021, 73 (Suppl. 1), 158–191. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Szkandera, J.; Stotz, M.; Absenger, G.; Stojakovic, T.; Samonigg, H.; Kornprat, P.; Schaberl-Moser, R.; Alzoughbi, W.; Lackner, C.; Ress, A.L.; et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br. J. Cancer 2014, 110, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Tateishi, K.; Sonehara, K.; Hirota, S.; Komatsu, M.; Yamamoto, M.; Kanda, S.; Kuraishi, H.; Hanaoka, M.; Koizumi, T. Clinical utility of the C-reactive protein:albumin ratio in non-small cell lung cancer patients treated with nivolumab. Thorac. Cancer 2021, 12, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Shibata, M.; Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. C-reactive protein can predict dose intensity, time to treatment failure and overall survival in HCC treated with lenvatinib. PLoS ONE 2020, 15, e0244370. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr. 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Ikeda, K.; Kudo, M.; Kawazoe, S.; Osaki, Y.; Ikeda, M.; Okusaka, T.; Tamai, T.; Suzuki, T.; Hisai, T.; Hayato, S.; et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Shirai, Y.; Toiyama, Y.; Saigusa, S.; Hishida, A.; Yokoe, T.; Tanaka, K.; Tanaka, M.; Yasuda, H.; Fujikawa, H.; et al. Clinical Burden of Modified Glasgow Prognostic Scale in Colorectal Cancer. Anticancer. Res. 2018, 38, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Toiyama, Y.; Miki, C.; Inoue, Y.; Tanaka, K.; Mohri, Y.; Kusunoki, M. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp. Ther. Med. 2011, 2, 95–101. [Google Scholar] [CrossRef]
- Scheiner, B.; Pomej, K.; Kirstein, M.M.; Hucke, F.; Finkelmeier, F.; Waidmann, O.; Himmelsbach, V.; Schulze, K.; von Felden, J.; Fründt, T.W.; et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy—Development and validation of the CRAFITY score. J. Hepatol. 2022, 76, 353–363. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tsuji, K.; Takaguchi, K.; Itobayashi, E.; Kariyama, K.; Ochi, H.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Validation of Modified ALBI Grade for More Detailed Assessment of Hepatic Function in Hepatocellular Carcinoma Patients: A Multicenter Analysis. Liver Cancer 2019, 8, 121–129. [Google Scholar] [CrossRef]
- Warschkow, R.; Ukegjini, K.; Tarantino, I.; Steffen, T.; Müller, S.A.; Schmied, B.M.; Marti, L. Diagnostic study and meta-analysis of C-reactive protein as a predictor of postoperative inflammatory complications after pancreatic surgery. J. Hepatobiliary Pancreat. Sci. 2012, 19, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, T.; Saeki, H.; Sugimachi, K. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am. J. Surg. 2001, 182, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Hiraoka, A.; Hirooka, M.; Kariyama, K.; Tani, J.; Atsukawa, M.; Takaguchi, K.; Itobayashi, E.; Fukunishi, S.; et al. C-reactive protein to albumin ratio predicts survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Sci. Rep. 2022, 12, 8421. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, L.; He, Z.; Xu, Z.; Xiang, Z.; Nie, R.C.; Lin, W.; Chen, W.; Zhou, J.; Yin, Y.; et al. C-Reactive Protein Levels Predict Responses to PD-1 Inhibitors in Hepatocellular Carcinoma Patients. Front. Immunol. 2022, 13, 808101. [Google Scholar] [CrossRef]
- Du, J.; Hu, W.; Yang, C.; Wang, Y.; Wang, X.; Yang, P. C-reactive protein is associated with the development of tongue squamous cell carcinoma. Acta Biochim. Biophys. Sin. 2018, 50, 238–245. [Google Scholar] [CrossRef]
- Hidayat, F.; Labeda, I.; Sampetoding, S.; Pattelongi, I.J.; Lusikooy, R.E.; Warsinggih; Dani, M.I.; Mappincara; Kusuma, M.I.; Uwuratuw, J.A.; et al. Correlation of interleukin-6 and C-reactive protein levels in plasma with the stage and differentiation of colorectal cancer: A cross-sectional study in East Indonesia. Ann. Med. Surg. 2021, 62, 334–340. [Google Scholar] [CrossRef]
- Masjedi, A.; Hashemi, V.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Azizi, G.; Yousefi, M.; Jadidi-Niaragh, F. The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed. Pharmacother. 2018, 108, 1415–1424. [Google Scholar] [CrossRef]
- Song, W.; Mazzieri, R.; Yang, T.; Gobe, G.C. Translational Significance for Tumor Metastasis of Tumor-Associated Macrophages and Epithelial-Mesenchymal Transition. Front. Immunol. 2017, 8, 1106. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, L.; Li, D.; Andl, T.; Zhang, Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front. Cell Dev. Biol. 2019, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kang, B.; Ha, Y.; Lee, S.H.; Kim, I.; Kim, H.; Lee, W.S.; Kim, G.; Jung, S.; Rha, S.Y.; et al. High serum IL-6 correlates with reduced clinical benefit of atezolizumab and bevacizumab in unresectable hepatocellular carcinoma. JHEP Rep. 2023, 5, 100672. [Google Scholar] [CrossRef] [PubMed]




| All Patients (n = 125) | High-CRP CRP ≥ 0.5 mg/dL (n = 56) | Low-CRP CRP < 0.5 mg/dL (n = 69) | p-Value * | |
|---|---|---|---|---|
| Median (IQR) | ||||
| Age (years) | 74 (69–78) | 75 (70–77) | 73 (69–80) | 0.907 |
| Male, n (%) | 94 (75.2) | 47 (83.9) | 47 (68.1) | 0.191 |
| ECOG-PS, 0/1/2, n | 99/18/8 | 37/12/7 | 62/6/1 | 0.151 |
| Etiology, HCV/HBV/other, n | 41/13/71 | 11/4/41 | 30/9/30 | 0.108 |
| Total bilirubin (mg/dL) | 0.78 (0.60–1.07) | 0.80 (0.62–1.19) | 0.70 (0.59–0.99) | 0.078 |
| Albumin (g/dL) | 3.6 (3.2–4.0) | 3.3 (3.0–3.7) | 3.8 (3.5–4.1) | <0.001 |
| AST, U/L | 44 (30–63) | 54 (39–78) | 35 (25–46) | <0.001 |
| ALT, U/L | 29 (18–48) | 32 (22–56) | 26 (17–36) | 0.039 |
| Platelets, ×104/μL | 14.5 (10.4–21.1) | 17.4 (11.5–25.6) | 12.5 (10.1–17.4) | 0.001 |
| Prothrombin, (%) | 88 (78–97) | 85 (77–91) | 92 (81–101) | 0.006 |
| CRP (mg/dL) | 0.29 (0.10–1.11) | 1.45 (0.81–3.42) | 0.12 (0.06–0.23) | <0.001 |
| AFP (ng/mL) | 57.8 (5.4–868.5) | 177.5 (6.3–1981.5) | 12.0 (4.7–234.1) | 0.019 |
| DCP (mAU/mL) | 182.0 (36.5–2559.8) | 589.7 (110.2–15462.7) | 85.0 (23.0–323.0) | <0.001 |
| Child–Pugh score, 5/6/≥7, n | 72/41/12 | 19/25/12 | 53/16/0 | 0.007 |
| ALBI grade | −2.35 (−2.63 to −1.97) | −2.05 (−2.45 to −1.74) | −2.47 (−2.78 to −2.30) | <0.001 |
| mALBI grade, 1/2a/2b/3, n | 37/36/45/7 | 9/10/30/7 | 28/26/15/0 | <0.001 |
| BCLC stage, A/B/C/D, n | 3/65/56/1 | 1/23/31/1 | 2/42/25/0 | 0.101 |
| Initial dose, 4 mg/8 mg/12 mg, n | 17/65/43 | 9/26/21 | 8/39/22 | 0.053 |
| Treatment line, 1st/2nd/3rd/4th/5th | 93/22/6/3/1 | 39/12/2/2/1 | 54/10/4/1/0 | 0.128 |
| Follow-up duration (months) | 11.6 (5.5–25.8) | 7.8 (3.5–13.5) | 15.4 (9.5–28.7) | <0.001 |
| Treatment response, CR/PR/SD/PD/NE, n | 2/35/33/35/20 | 0/16/14/13/13 | 2/19/19/22/7 | 0.136 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Treatment line | 1.39 (1.06–1.81) | 0.016 | ||
| Performance status | 1.33 (1.08–1.63) | 0.006 | 1.35 (1.07–1.71) | 0.011 |
| BCLC stage | 1.65 (1.14–2.39) | 0.008 | ||
| Total bilirubin, mg/dL | 1.52 (1.26–1.84) | <0.001 | 1.55 (1.25–1.91) | <0.001 |
| Albumin, g/dL | 0.41 (0.28–0.61) | <0.001 | 0.60 (0.39–0.92) | 0.018 |
| Prothrombin, % | 0.98 (0.97–0.99) | 0.003 | ||
| CRP, mg/dL | 1.74 (1.39–2.19) | <0.001 | 1.63 (1.25–2.13) | <0.001 |
| Multivariable | ||
|---|---|---|
| HR (95% CI) | p-Value | |
| Male | 1.17 (0.69–1.96) | 0.561 |
| AFP, ng/mL | 1.00 (0.99–1.01) | 0.257 |
| Treatment line | 1.29 (0.99–1.70) | 0.062 |
| mALBI grade | 1.53 (1.17–1.99) | 0.002 |
| Age | 1.05 (1.02–1.08) | <0.001 |
| CRP, mg/dL | 2.21 (1.38–3.55) | <0.001 |
| High-CRP (n = 56) CRP ≥ 0.5 mg/dL | High-CRP-1st (n = 22) 0.5≤ CRP < 1.0 mg/dL | High-CRP-2nd (n = 34) CRP ≥ 1.0 mg/dL | * p-Value | |
|---|---|---|---|---|
| Median (IQR) | ||||
| Age (years) | 75 (70–77) | 72 (64–77) | 76 (70–78) | 0.121 |
| Male, n (%) | 47 (83.9) | 19 (86.4) | 28 (82.4) | 0.689 |
| ECOG-PS, 0/1/2, n | 37/12/7 | 16/2/4 | 21/10/3 | 0.153 |
| Etiology, HCV/HBV/other, n (%) | 11/4/41 | 4/1/17 | 7/3/24 | 0.793 |
| Total bilirubin (mg/dL) | 0.80 (0.62–1.19) | 0.9 (0.8–1.2) | 0.7 (0.6–1.1) | 0.067 |
| Albumin (g/dL) | 3.3 (3.0–3.7) | 3.4 (3.1–3.8) | 3.3 (2.9–3.6) | 0.274 |
| AST, U/L | 54 (39–78) | 51 (30–71) | 56 (40–103) | 0.182 |
| ALT, U/L | 32 (22–56) | 28 (21–42) | 38 (23–64) | 0.161 |
| Platelets, ×104/μL | 17.4 (11.5–25.6) | 13.0 (10.2–16.8) | 21.8 (14.5–30.5) | 0.003 |
| Prothrombin, (%) | 85 (77–91) | 87 (83–95) | 83 (76–87) | 0.122 |
| CRP (mg/dL) | 1.45 (0.81–3.42) | 0.71 (0.64–0.82) | 2.81 (1.55–4.76) | <0.001 |
| AFP (ng/mL) | 177.5 (6.3–1981.5) | 165.6 (6.0–1878.0) | 177.5 (6.5–5466.8) | 0.973 |
| DCP (mAU/mL) | 589.7 (110.2–15462.7) | 161.0 (58.9–2559.8) | 5374.0 (306.0–36246.0) | 0.008 |
| Child–Pugh score, 5/6/≥7, n | 19/25/12 | 19/2/1 | 0/23/11 | <0.001 |
| ALBI grade | −2.05 (−2.45 to −1.74) | −2.08 (−2.48 to −1.88) | −2.05 (−2.43 to −1.45) | 0.491 |
| mALBI grade, 1/2a/2b/3, n | 9/10/30/7 | 4/4/13/1 | 5/6/17/6 | 0.543 |
| BCLC stage, A/B/C/D, n | 1/23/31/1 | 1/11/9/1 | 0/12/22/0 | 0.161 |
| Initial dose, 4 mg/8 mg/12 mg, n | 9/26/21 | 2/10/10 | 7/16/11 | 0.424 |
| Treatment line, 1st/2nd/3rd/4th/5th | 39/12/2/2/1 | 17/3/0/2/0 | 22/9/2/0/1 | 0.173 |
| Treatment response, CR/PR/SD/PD/NE, n | 0/16/14/13/13 | 0/6/5/7/4 | 0/10/9/6/9 | 0.801 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.04 (1.01–1.08) | 0.007 | 1.05 (1.01–1.08) | 0.006 |
| AST, U/mL | 1.004 (1.000–1.008) | 0.044 | ||
| CRP, mg/dL | 1.49 (1.06–2.11) | 0.022 | 1.53 (1.08–2.16) | 0.016 |
| DCP, mAU/mL | 1.001 (1.000–1.001) | 0.010 | ||
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Performance status | 1.49 (1.10–2.03) | 0.011 | 1.44 (1.02–2.03) | 0.036 |
| Total bilirubin, mg/dL | 1.33 (1.07–1.64) | 0.008 | 1.38 (1.09–1.75) | 0.006 |
| CRP, mg/dL | 1.58 (1.11–2.26) | 0.011 | 1.78 (1.22–2.61) | 0.003 |
| AST, U/mL | 1.005 (1.001–1.009) | 0.017 | ||
| BCLC stage | 1.63 (1.03–2.57) | 0.036 | ||
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.030 (1.006–1.053) | 0.012 | 1.03 (1.01–1.06) | 0.008 |
| Hemoglobin, g/dL | 0.89 (0.81–0.98) | 0.017 | ||
| Albumin, g/dL | 0.51 (0.36–0.74) | <0.001 | 0.55 (0.39–0.79) | 0.001 |
| Total bilirubin, mg/dL | 1.32 (1.11–1.58) | 0.002 | 1.28 (1.08–1.52) | 0.004 |
| AST, U/L | 1.004 (1.001–1.007) | 0.021 | ||
| Prothrombin, % | 0.98 (0.97–0.99) | 0.004 | ||
| CRP, mg/dL | 1.09 (1.03–1.16) | 0.006 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okumura, T.; Kimura, T.; Iwadare, T.; Wakabayashi, S.-i.; Kobayashi, H.; Yamashita, Y.; Sugiura, A.; Joshita, S.; Fujimori, N.; Kunimoto, H.; et al. Prognostic Significance of C-Reactive Protein in Lenvatinib-Treated Unresectable Hepatocellular Carcinoma: A Multi-Institutional Study. Cancers 2023, 15, 5343. https://doi.org/10.3390/cancers15225343
Okumura T, Kimura T, Iwadare T, Wakabayashi S-i, Kobayashi H, Yamashita Y, Sugiura A, Joshita S, Fujimori N, Kunimoto H, et al. Prognostic Significance of C-Reactive Protein in Lenvatinib-Treated Unresectable Hepatocellular Carcinoma: A Multi-Institutional Study. Cancers. 2023; 15(22):5343. https://doi.org/10.3390/cancers15225343
Chicago/Turabian StyleOkumura, Taiki, Takefumi Kimura, Takanobu Iwadare, Shun-ichi Wakabayashi, Hiroyuki Kobayashi, Yuki Yamashita, Ayumi Sugiura, Satoru Joshita, Naoyuki Fujimori, Hideo Kunimoto, and et al. 2023. "Prognostic Significance of C-Reactive Protein in Lenvatinib-Treated Unresectable Hepatocellular Carcinoma: A Multi-Institutional Study" Cancers 15, no. 22: 5343. https://doi.org/10.3390/cancers15225343
APA StyleOkumura, T., Kimura, T., Iwadare, T., Wakabayashi, S.-i., Kobayashi, H., Yamashita, Y., Sugiura, A., Joshita, S., Fujimori, N., Kunimoto, H., Komatsu, M., Fukushima, H., Mori, H., & Umemura, T. (2023). Prognostic Significance of C-Reactive Protein in Lenvatinib-Treated Unresectable Hepatocellular Carcinoma: A Multi-Institutional Study. Cancers, 15(22), 5343. https://doi.org/10.3390/cancers15225343