Simple Summary
Cancer treatment, in particular with gonadotoxic potential, may affect the fertility of cancer patients and cause temporary or permanent damage to the reproductive organs and glands that control fertility. Taking into account that some patients ultimately do not lose fertility during treatment, some do not survive cancer therapy, and some do not decide to use cryopreserved reproductive material, the review analysed the percentage of usage of cryopreserved reproductive material collected before treatment to preserve the fertility of patients after cancer treatment. The obtained review results indicate a low return/usage rate of cryopreserved reproductive material among both women and men. This review highlights potential organizational issues related to storage costs, space needed, and the use or disposal of stored material. Considering the increase in the number of cancer patients, the scale of this problem may turn out to be significant in the coming years.
Abstract
Background: Many cancer treatment methods can affect fertility by damaging the reproductive organs and glands that control fertility. Changes can be temporary or permanent. In order to preserve the fertility of cancer patients and protect the genital organs against gonadotoxicity, methods of fertility preservation are increasingly used. Considering that some patients ultimately decide not to use cryopreserved reproductive material, this review analysed the percentage of post-cancer patients using cryopreserved reproductive material, collected before treatment as part of fertility preservation. Methods: A systematic search of studies was carried out in accordance with the Cochrane Collaboration guidelines, based on a previously prepared research protocol. The search was conducted in Medline (via PubMed), Embase (via OVID), and the Cochrane Library. In addition, a manual search was performed for recommendations/clinical practice guidelines regarding fertility preservation in cancer patients. Results: Twenty-six studies met the inclusion criteria. The studies included in the review discussed the results of cryopreservation of oocytes, embryos, ovarian tissue, and semen. In 10 studies, the usage rate of cryopreserved semen ranged from 2.6% to 21.5%. In the case of cryopreserved female reproductive material, the return/usage rate ranged from 3.1% to 8.7% for oocytes, approx. 9% to 22.4% for embryos, and 6.9% to 30.3% for ovarian tissue. In studies analysing patients’ decisions about unused reproductive material, continuation of material storage was most often indicated. Recovering fertility or death of the patient were the main reasons for rejecting cryopreserved semen in the case of men. Conclusion: Fertility preservation before gonadotoxic treatment is widely recommended and increasingly used in cancer patients. The usage rate is an important indicator for monitoring the efficacy of these methods. In all of the methods described in the literature, this indicator did not exceed 31%. It is necessary to create legal and organizational solutions regulating material collection and storage and to create clear paths for its usage in the future, including by other recipients.
1. Introduction
According to the definition by the International Classification of Diseases 11th Revision, female infertility (ICD-11: GA31) is a “disease of the reproductive system defined by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse” [1]. Male infertility (ICD-11: GB04) is defined as “any disorder of the reproductive system affecting males, characterized by dysfunctionalities in the ejection of semen or an abnormal absence in the measurable level of sperm in semen” [2].
According to the data of the International Agency for Research on Cancer (based on the GLOBOCAN estimates), in 2020 there were approximately 3.1 million new cases of cancer among people of reproductive age (aged 15–49) [3]. Many cancer treatment methods can affect fertility by damaging the reproductive organs and glands that control fertility. Changes can be temporary or permanent. For some patients, infertility can be one of the most difficult and distressing long-term effects of cancer treatment. It is important to assess how the planned therapy may affect fertility before starting treatment [4,5,6,7,8].
In 2006, Dr. Teresa K. Woodruff of Northwestern University launched a new field of medicine that combines oncology with reproductive health, calling it “oncofertility”. The interdisciplinary initiative “Oncofertility Consortium”, established by her, focuses on problems related to healthcare and the quality of life of young cancer patients, in particular on issues related to fertility after cancer [9].
In order to preserve the fertility of cancer patients and protect the genitals against gonadotoxic effects (chemotherapy and/or radiotherapy), the following are used in women: oocyte cryopreservation, embryo cryopreservation, ovarian tissue cryopreservation, gonadal shielding, ovarian transposition (oophoropexy), surgical techniques (trachelectomy), and treatment with gonadotropin-releasing hormone agonist (GnRHa) [10,11,12,13,14,15]. In men, fertility preservation techniques may include semen cryopreservation, gonadal shielding, testicular sperm extraction, partial ovariectomy, and testicular tissue cryopreservation [16,17,18]. For the purposes of our article, reproductive material is defined as human gametes, embryos, and tissues.
Factors affecting the patient’s decision regarding the usage of the above-mentioned methods may include personal beliefs, religious and cultural limitations, prognosis, the patient’s knowledge of fertility preservation methods, as well as the method of disseminating information about the possibility of fertility preservation by medical personnel [19,20,21,22,23].
Due to the increasing usage of fertility preservation methods and emerging discussions on the ethics of the above-mentioned procedures (in terms of embryo freezing) and subsequent usage of cryopreserved materials, a review of scientific evidence describing the reproductive results obtained as a result of using fertility preservation procedures among cancer patients was performed. Considering that some cancer patients do not lose fertility during treatment, some do not survive cancer therapy, and some ultimately do not decide to use cryopreserved reproductive material, this review analyses the percentage of patients using cryopreserved reproductive material collected before treatment to preserve fertility.
Bearing in mind the above, the main objective of the article is to analyse the usage of cryopreserved reproductive material collected from patients before cancer treatment as part of fertility preservation.
2. Materials and Methods
The analysis was carried out based on the results of available studies. The search was based on a detailed protocol developed prior to the commencement of this work. It takes into consideration the criteria for including studies in the review, the search strategy, the method of selecting studies, and the planned methodology for conducting the analysis. The review was performed according to the Cochrane Collaboration guidelines [24].
The analysis included clinical trials that met the criteria regarding:
- Population: people diagnosed with cancer;
- Interventions: fertility preservation (cryopreservation of oocytes, embryos, ovarian tissues, semen, and testicular tissues);
- Alternative technologies (comparators): not restricted;
- Outcome: return/usage rate, decisions/disposal of unused reproductive material;
- Types of studies: systematic reviews, experimental or observational studies.
The review did not include studies that analysed fertility preservation methods in patients undergoing cancer treatment that did not require the collection and storage of reproductive material, e.g., gonadal protection, hormone therapy, conserving treatment, and ovarian transposition.
The following sources of medical information were searched for studies published in the last 10 years (i.e., since 2013): Medline (via PubMed), Embase (via Ovid), and The Cochrane Library. The search of the databases was carried out on 12 June 2023 in accordance with the search strategies presented in the Supplementary Materials. In addition, publications with clearly defined endpoints (specified in the PICO scheme), included in the bibliography of studies found in the review, were also included in the analysis. In addition, manual searches of clinical practice recommendations/guidelines regarding fertility preservation in cancer patients and grey literature were performed (searches included TRIP Database and Google Scholar).
At all stages of the review, studies were selected by two analysts working independently (K.W. and J.Ś.). Inconsistencies were resolved by consensus, with the participation of a third independent analyst (W.M.).
The review included publications that clarified the presented methodology, were of high quality, and had a low risk of error.
Based on the included publications, data from each publication were summarized in terms of two main outcomes (return/usage rate, and decisions/disposal of unused reproductive material). In terms of the usage of cryopreserved reproductive material in cancer patients, the results were presented in tabular and descriptive form (separately for women and men). Reproductive material that was cryopreserved was analysed and the number of used materials/return for materials, number of patients who cryopreserved material, and the return/usage rate were indicated. In the case of the decisions/disposal of unused reproductive material outcome, information was presented regarding the number of patients who cryopreserved material, reproductive material, disposal of unused cryopreserved material, and, additionally, usage rate.
3. Results
The study selection stages are presented in Figure 1. The list of publications included and excluded, along with the reasons for exclusions from the review, can be found in the Supplementary Materials Table S1.
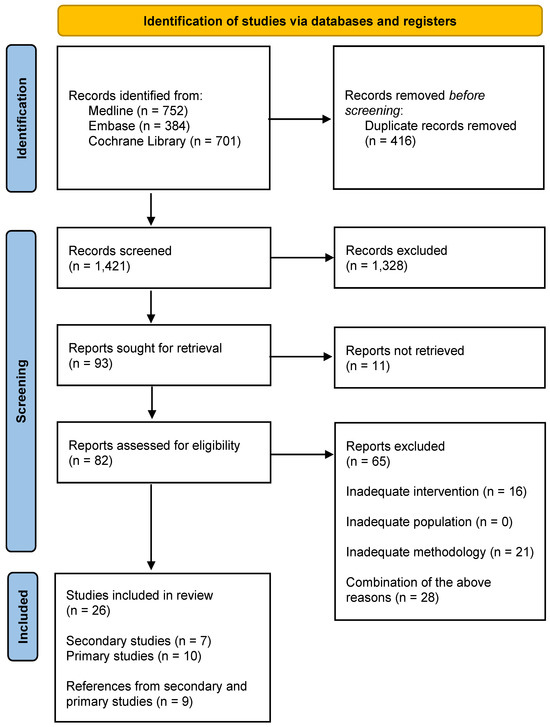
Figure 1.
PRISMA flow diagram.
The most common reasons for the exclusion of studies from the analysis were issues related to methodology (lack of proper description of the materials and methods, incorrect synthesis of review results, misinterpretation of statistical results) and intervention (preserving fertility among people without cancer).
3.1. The Usage of Cryopreserved Reproductive Material in Cancer Patients
3.1.1. Women
As part of this review, sixteen publications were found indicating the percentage of female cancer patients who underwent fertility preservation procedures, who after their treatment decided to use cryopreserved reproductive material [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40].
According to the results of the Fraison 2023 meta-analysis, 4.6% of women used cryopreserved oocytes for fertilization under assisted reproductive technology (ART), and approx. 9% of women decided to get pregnant using cryopreserved embryos [25]. In turn, the Xu 2023 meta-analysis, based on twenty-six observational studies, analysing the reproductive results of female cancer patients undergoing FP procedures, indicated a return rate of 7.9% in total for cryopreserved oocytes, embryos, and ovarian tissues [26]. On the other hand, in the Sheshpari 2019 systematic review assessing the efficacy of fertility preservation as part of the ovarian tissue collection and cryopreservation procedure, it was indicated that 30.3% of women returned to have the OTT procedure after the completion of cancer treatment [34].
As part of the studies analysed in the found reviews, the return rate was 3.1% to 7.5% for cryopreserved oocytes [32,35,38], 18.75% for cryopreserved embryos [27,37], and 12% for cryopreserved oocytes and embryos together [30,40].
In a 25-year prospective cohort study, 8.7% of women returned to attempt pregnancy using their stored oocytes [28]. Moreover, in other studies, the return rate for cryopreserved reproductive material ranged from 6.9% (for ovarian tissue) [33] to 22.4% (for cryopreserved embryos) [39].
Below are the individual results of the studies regarding the percentage of women who used reproductive material after completing cancer treatment as part of fertility preservation (Table 1).

Table 1.
The usage of cryopreserved reproductive material by women after completing cancer treatment.
3.1.2. Men
As part of the analysis of publications found in the review, studies were also indicated that determined the percentage of men undergoing the FP procedure who, after completing cancer treatment, returned and used cryopreserved reproductive material (semen) for fertilization under ART (n = 10) [41,42,43,44,45,46,47,48,49,50].
According to the results of individual studies, the return/usage rate did not exceed 22%, and it ranged from 6.5% [49] to 21.5% [48]. However, in a meta-analysis of 30 observational studies covering a population of 11,798 men, the percentage of people who used cryopreserved semen was 8.5% [45].
Below are the individual results of the studies on the number of men undergoing cancer treatment who underwent cryopreservation as part of FP and the return/usage rate of the material under assisted reproduction after the completion of cancer treatment (Table 2).

Table 2.
The usage of cryopreserved semen by men after the completion of cancer treatment.
3.1.3. Decisions and Disposal of Unused Reproductive Material
On the basis of five studies, the results regarding the decision to transfer or reject unused cryopreserved reproductive material were presented [36,43,45,46,50].
Depending on the observation period, cancer patients most often continued to store the cryopreserved material (from 34.3% to 55.4% of the cryopreserved material) [36,46,50]. In turn, the Moravek 2018 retrospective cohort study indicated that female patients who stored unused cryopreserved oocytes or embryos decided to donate their reproductive material for research purposes or decided to donate oocytes and/or embryos to another couple or family members [36].
In turn, the main reasons for the rejection of the cryopreserved material (semen) were regaining fertility through the return of reproductive functions after cancer treatment [43,46] and the patient’s death [43,46,51].
The individual results of the studies concerning the decisions and disposal related to the unused reproductive material of cancer patients are presented below (Table 3).

Table 3.
Disposal of unused reproductive material as part of fertility preservation in cancer patients.
In the Sankara-Narayana 2019, Muller 2016, and Johnson 2013 studies, the results concerning the disposal of unused cryopreserved material and the usage rate refer to all banked samples (100%). In the Ferrari 2016 study, the discard rate included a meta-analysis of eleven studies (n/N = 691/4291), and the usage rate refers to the number of all patients included in the meta-analysis (n/N = 974/11,798). In the case of the Moravek 2018 study, the results refer to the material disposal selected at the time of cryopreservation.
4. Discussion
Based on the results of studies found as part of the systematic search, the usage of cryopreserved reproductive material, collected from patients before cancer treatment as part of fertility preservation, was analysed. The publications included in the review covered various fertility preservation methods (including cryopreservation of eggs, embryos, ovarian tissue, and semen).
The usage rate varied depending on the study and the type of cryopreserved material. The usage rate of cryopreserved sperm ranged from 2.6% to 21.5% [41,42,43,44,45,46,47,48,49,50]. Considering cryopreserved reproductive material in women, some studies analysed the use of oocytes, embryos, and ovarian tissues together. Taking into account this type of study, the return/usage rate ranged from 7.9% to 21.2% [26,29,31,33]. Bearing in mind the studies that showed the results for individual types of material, it can be seen that the return/usage rate ranged from 3.1% to 8.7% in the case of oocytes [25,28,32,35,38], from approx. 9% to 22.4% in the case of embryos [25,27,37,39], and from 6.9% to 30.3% in the case of ovarian tissues [33,34].
Considering the above results, attention should be paid to potential organizational problems related to the costs of storage, the space needed, and the usage or disposal of material (which may be particularly problematic from an ethical point of view in the case of cryopreserved embryos) [52]. Considering the increase in the number of cancer patients, the scale of this problem may turn out to be significant in the coming years. The small number of national oncofertility registries is also problematic and translates into potential organizational problems and limited possibilities for using cryopreserved materials [51,52].
In two studies included in this review, the control groups were non-oncological women [28] and women who decided to undergo elective fertility preservation [35]. According to the results of the Porcu 2022 study, the return rate was lower in the group of women with cancer compared to the group of women who had not been diagnosed with cancer—8.7% vs. 83.5% (44/508 vs. 870/1042) [28]. In the second study, the return rate was statistically significantly higher in the elective fertility preservation group compared to the oncological women intervention group (12.1% vs. 7.4%; p < 0.05). Therefore, there is a visible difference in the return rate in the case of healthy women and women suffering from cancer. The above situation may be related primarily to the death of oncological patients and the lack of loss or recovery of the functions of reproductive organs after recovery from the disease. These situations reduce the level of use of frozen reproductive materials. In the case of women not suffering from cancer, it should be borne in mind that freezing reproductive materials is most often associated with diagnosed infertility and planned in vitro fertilization procedures. The use of frozen eggs/embryos will therefore be more frequent (including due to the sometimes necessary repetition of in vitro fertilization procedures). To complete the analysis, databases and websites of scientific societies were manually searched for clinical practice guidelines on fertility preservation against gonadotoxic therapy. Recommendations from the last 10 years were sought. The main conclusions are presented below.
The vast majority of recommendations indicate the need to inform all people diagnosed with cancer about the potentially harmful impact of the planned treatment on fertility. Before the commencement of treatment, the available fertility preservation methods should be discussed with the patient [53,54,55,56,57,58,59,60,61,62,63,64,65,66] and information and educational materials provided should be age-appropriate [53,59,60]. Patients interested in fertility preservation should be referred to fertility specialists [53,54,55,56,57,60,62,67]. The recommendations also emphasise that comprehensive care for cancer patients in terms of fertility preservation should include psychosocial support [53,59,62,64].
Almost all documents found indicate cryopreservation of oocytes and/or embryos as a method of fertility preservation in women of reproductive age, which should be implemented before commencing cancer treatment with gonadotoxic potential [53,54,55,56,57,59,60,61,62,63,64,65,66,67,68]. Most of the recommendations are consistent in that if the implementation of the above-mentioned methods is not possible (e.g., in pre-pubescent patients or when the commencement of cancer therapy should not be delayed), cryopreservation and subsequent auto-graft of ovarian tissue are recommended [53,54,55,56,57,60,61,62]. In some guidelines, this method was considered experimental [55,58,66,67] and the need to consider the individual risk associated with cancer reimplantation must be taken into account [53,60,63].
In the population of men and boys after puberty, in whom cancer treatment with gonadotoxic potential is planned, the basic method of preserving fertility is semen cryopreservation [53,54,55,56,57,58,60,61,62,63,64,65,66,67,68]. In a situation where they are unable to provide a semen sample themselves, testicular sperm extraction or electroejaculation is recommended [56,57,63].
Cryopreservation of testicular tissue is an option for pre-pubertal fertility preservation in boys. However, the societies emphasize that this is an invasive and experimental method, and should only be considered in clinical trials [53,54,55,56,57,61,62,63,66].
When analysing the issue of cryopreservation of reproductive materials, the costs associated with this procedure should also be taken into account. In one publication, the authors attempted to estimate the costs of fertility preservation activities among women undergoing cancer treatment. Cost estimates in this case referred to oocyte cryopreservation. The publication did not indicate the general cost-effectiveness threshold for the implementation of the discussed intervention; however, based on the collected data from the systematic search, it was estimated that the total cost of one cycle (including collection, cryopreservation, storage, and fertilization with implementation) ranges between USD 7000 and USD 14,000. In addition, pharmaceuticals used in the collection of oocytes from women also play an important role. In this case, the cost of drugs for one egg cell ranges from USD 2000 to USD 7000. An additional element significantly affecting such high costs of oocyte cryopreservation involves the costs associated with long-term storage of the collected cells. In this case, annual fees for stored cells range from USD 350 to USD 600 [58].
In another publication, the authors attempted to estimate the cost-effectiveness of semen cryopreservation as a method of preserving the fertility of men with testicular cancer. The publication did not indicate the general cost-effectiveness threshold for semen cryopreservation; however, according to estimates, the average cost of the procedure using a single vial was USD 754 in the first year of intervention. In turn, each subsequent year of storage of the collected reproductive material costs an average of USD 343 [69].
Taking into account the high level of non-use of frozen reproductive materials among people with cancer, attention should be paid to the validity of the distinction between gametes and embryos in legal acts, with particular emphasis on the handling of each of these reproductive materials. For example, in the Polish Act on the Treatment of Infertility, a reproductive cell is defined as “a human male reproductive cell (human sperm) or a human female reproductive cell (human egg cell) intended for use in a medically assisted procreation procedure”. In turn, an embryo is defined as “a group of cells resulting from the extracorporeal fusion of female and male reproductive cells, from the completion of the process of fusion of the germ cell nuclei (karyogamy) to the moment of implantation in the uterine mucosa” [70].
Pursuant to the provisions of the Act, reproductive cells may be destroyed or donated for research purposes at the express request of the donor at any time. In turn, embryos created from the collected reproductive cells are transferred for embryo donation (“transfer of an embryo for the purpose of using it in a medically assisted procreation procedure in a recipient who is not a donor of female reproductive cells and is not married or in cohabitation with a donor of male reproductive cells reproductive cells from which the embryo was created”) after the expiry of the deadline specified in the agreement concluded between the reproductive cell and embryo bank and the donors. However, this deadline cannot be longer than 20 years from the date on which the embryos were transferred to the bank of reproductive cells and embryos for their storage. Embryo donation may also take place in the event of the death of both embryo donors or—if the embryo was created as a result of non-partner donation—the death of the recipient and her husband or a person in cohabitation with her [70]. However, the problem in this case may be the increasing number of unused and stored embryos.
A very important issue is the conscious decision of people with cancer and their partners regarding the method of future fertility protection they choose. In potential legal cases described in the literature, there may be situations where the partner does not consent to the woman’s use of embryos after the couple breaks up [71,72]. For some women, egg freezing might be a better choice, which would avoid the situation described above.
Another aspect is the need to carry out the in vitro fertilization procedure for people who decide to freeze and later use eggs, embryos, or sperm. For some people, carrying out such a procedure may be unacceptable due to their beliefs. The solution for these people may be collecting and freezing ovarian tissue and its subsequent autotransplantation. In the case of men, the solution of freezing testicular tissue is not a procedure with proven effectiveness, therefore other procedures are performed in their case (mainly sperm freezing), which consequently lead to in vitro fertilization.
5. Conclusions
Fertility preservation in the case of gonadotoxic treatment is widely recommended and increasingly used in cancer patients. The basic methods include cryopreservation of oocytes and embryos in women and semen in men. The usage rate is an important indicator for monitoring the efficacy of these methods. In all of the methods described in the literature, this indicator did not exceed 31%.
Unused cryopreserved reproductive material was usually stored further. Some of the material was donated for scientific purposes or to other people in need. Important factors that prevent the usage of cryopreserved material include the patient’s death or regaining fertility through the return of reproductive functions after cancer treatment.
It is necessary to create legal and organizational solutions regulating the collection and storage of material and to create clear paths for its usage in the future, including by other recipients.
6. Review Limitations
Only publications in English were included in the review. The studies were characterised by high heterogeneity (e.g., different methods of presenting the analysed data or differences in the scope of the applied interventions). In the context of the analysed endpoints, individual studies covered a varied follow-up period, which ranged from several months to several decades (in the case of retrospective studies).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15225348/s1, Table S1. List of studies included and excluded after full-text analysis.
Author Contributions
Conceptualisation: K.W. and W.M.; methodology: K.W. and W.M.; formal analysis: K.W., J.Ś. and W.M.; writing—original draft preparation: K.W., J.Ś. and W.M.; writing—review and editing: K.W., J.Ś., W.M., T.T., U.R., P.O. and A.A.; supervision: T.T., U.R., P.O. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
The study was conducted as part of the Project “Treatment of infertility in local government health policy programs” No. 05/Z/GW/N/21, implemented from 2021 to 2023, financed by a subsidy for science, obtained by the Medical University of Warsaw.
Data Availability Statement
All data are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ART | assisted reproductive technology |
| FP | fertility preservation |
| GnRHa | gonadotropin-releasing hormone agonist |
| ICD-11: GA31 | International Classification of Diseases 11th Revision, female infertility |
| OTT | ovarian tissue transplantation |
| PR | primary research |
| SR | systematic review |
References
- World Health Organization. ICD-11 for Mortality and Morbidity Statistics (Version: 01/2023). Female Infertility. 2023. Available online: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/1237004558 (accessed on 13 June 2023).
- International Agency for Research on Cancer (World Health Organization). ICD-11 for Mortality and Morbidity Statistics (Version: 01/2023). Male Infertility. 2023. Available online: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/753457327 (accessed on 13 June 2023).
- Estimated Number of New Cases in 2020, World, Both Sexes, Ages 15–49. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=3&ages_group%5B%5D=9&group_cancer=1&include_nmsc=1&include_nmsc_other=1 (accessed on 13 June 2023).
- Hao, X.; Anastácio, A.; Liu, K.; Rodriguez-Wallberg, K.A. Ovarian Follicle Depletion Induced by Chemotherapy and the Investigational Stages of Potential Fertility-Protective Treatments—A Review. Int. J. Mol. Sci. 2019, 20, 4720. [Google Scholar] [CrossRef] [PubMed]
- Demeestere, I.; Moffa, F.; Peccatori, F.; Poirot, C.; Shalom-Paz, E. Multiple Approaches for Individualized Fertility Protective Therapy in Cancer Patients. Obstet. Gynecol. Int. 2012, 2012, 961232. [Google Scholar] [CrossRef] [PubMed]
- Poorvu, P.D.; Frazier, A.L.; Feraco, A.M.; Manley, P.E.; Ginsburg, E.S.; Laufer, M.R.; LaCasce, A.S.; Diller, L.R.; Partridge, A.H. Cancer Treatment-Related Infertility: A Critical Review of the Evidence. JNCI Cancer Spectr. 2019, 3, pkz008. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.; Johnstone, E.; Dorais, J.; Silver, B.; Peterson, C.M.; Hotaling, J. Female infertility, infertility-associated diagnoses, and comorbidities: A review. J. Assist. Reprod. Genet. 2017, 34, 167–177. [Google Scholar] [CrossRef]
- Mehedintu, C.; Frincu, F.; Carp-Veliscu, A.; Barac, R.; Badiu, D.C.; Zgura, A. A Warning Call for Fertility Preservation Methods for Women Undergoing Gonadotoxic Cancer Treatment. Medicina 2021, 57, 1340. [Google Scholar] [CrossRef]
- National Cancer Institute. Oncofertility: Creating a Bridge between Cancer Care and Reproductive Health. 2019. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2019/woodruff-oncofertility (accessed on 13 June 2023).
- Khattak, H.; Amorim, C.A. What are my options? Fertility preservation methods for young girls and women. Fertil. Steril. 2022, 117, 1277–1278. [Google Scholar] [CrossRef]
- Arecco, L.; Blondeaux, E.; Bruzzone, M.; Ceppi, M.; Latocca, M.M.; Marrocco, C.; Boutros, A.; Spagnolo, F.; Razeti, M.G.; Favero, D.; et al. Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: A systematic review and meta-analysis. Hum. Reprod. 2022, 37, 954–968. [Google Scholar] [CrossRef]
- Santos, M.L.; Pais, A.S.; Almeida Santos, T. Fertility preservation in ovarian cancer patients. Gynecol. Endocrinol. 2021, 37, 483–489. [Google Scholar] [CrossRef]
- Chen, C.N.; Chang, L.T.; Chen, C.H.; Tam, K.W. Fertility preservation for women with breast cancer before chemotherapy: A systematic review and meta-analysis. Reprod. BioMed. Online. 2022, 44, 357–369. [Google Scholar] [CrossRef]
- Hussein, R.S.; Khan, Z.; Zhao, Y. Fertility Preservation in Women: Indications and Options for Therapy. Mayo Clin. Proc. 2020, 95, 770–783. [Google Scholar] [CrossRef]
- Henry, L.; Labied, S.; Jouan, C.; Nisolle, M. Preservation of female fertility: The current therapeutic strategy. Int. J. Gynaecol. Obstet. 2022, 156, 3–9. [Google Scholar] [CrossRef]
- Brannigan, R.E.; Fantus, R.J.; Halpern, J.A. Fertility preservation in men: A contemporary overview and a look toward emerging technologies. Fertil. Steril. 2021, 115, 1126–1139. [Google Scholar] [CrossRef]
- Grin, L.; Girsh, E.; Harlev, A. Male fertility preservation-Methods, indications and challenges. Andrologia 2021, 53, e13635. [Google Scholar] [CrossRef] [PubMed]
- Barak, S. Fertility preservation in male patients with cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 55, 59–66. [Google Scholar] [CrossRef]
- Daly, C.; Micic, S.; Facey, M.; Speller, B.; Yee, S.; Kennedy, E.D.; Corter, A.L.; Baxter, N.N. A review of factors affecting patient fertility preservation discussions & decision-making from the perspectives of patients and providers. Eur. J. Cancer Care 2019, 28, e12945. [Google Scholar]
- Peddie, V.L.; Porter, M.A.; Barbour, R.; Culligan, D.; MacDonald, G.; King, D.; Horn, J.; Bhattacharya, S. Factors affecting decision making about fertility preservation after cancer diagnosis: A qualitative study. BJOG 2012, 119, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Vogt, K.S.; Hughes, J.; Wilkinson, A.; Mahmoodi, N.; Skull, J.; Wood, H.; McDougall, S.; Slade, P.; Greenfield, D.M.; Pacey, A.; et al. Preserving fertility in women with cancer (PreFer): Decision-making and patient-reported outcomes in women offered egg and embryo freezing prior to cancer treatment. Psychooncology 2018, 27, 2725–2732. [Google Scholar] [CrossRef]
- Han, J.; Park, S.; Kim, S.H. Supporting decision-making regarding fertility preservation in patients with cancer: An integrative review. Eur. J. Cancer Care 2022, 31, e13748. [Google Scholar] [CrossRef]
- Ayensu-Coker, L.; Essig, E.; Breech, L.L.; Lindheim, S. Ethical quandaries in gamete-embryo cryopreservation related to oncofertility. J. Law Med. Ethics 2013, 41, 711–719. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2; The Cochrane Collaboration in London: London, UK, 2021. [Google Scholar]
- Fraison, E.; Huberlant, S.; Labrune, E.; Cavalieri, M.; Montagut, M.; Brugnon, F.; Courbiere, B. Live birth rate after female fertility preservation for cancer or haematopoietic stem cell transplantation: A systematic review and meta-analysis of the three main techniques; embryo, oocyte and ovarian tissue cryopreservation. Hum. Reprod. 2023, 38, 489–502. [Google Scholar] [CrossRef]
- Xu, Z.; Ibrahim, S.; Burdett, S.; Rydzewska, L.; Al Wattar, B.H.; Davies, M.C. Long term pregnancy outcomes of women with cancer following fertility preservation: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 281, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M.C.; Snegovskikh, V.; Adamson, G.D. Oocyte and embryo cryopreservation before gonadotoxic treatments: Principles of safe ovarian stimulation, a systematic review. Womens Health 2022, 18, 17455065221074886. [Google Scholar] [CrossRef] [PubMed]
- Porcu, E.; Cipriani, L.; Dirodi, M.; De Iaco, P.; Perrone, A.M.; Zinzani, P.L.; Taffurelli, M.; Zamagni, C.; Ciotti, P.M.; Notarangelo, L.; et al. Successful Pregnancies, Births, and Children Development Following Oocyte Cryostorage in Female Cancer Patients During 25 Years of Fertility Preservation. Cancers 2022, 14, 1429. [Google Scholar] [CrossRef] [PubMed]
- Marklund, A.; Lundberg, F.E.; Eloranta, S.; Hedayati, E.; Pettersson, K.; Rodriguez-Wallberg, K.A. Reproductive Outcomes After Breast Cancer in Women with vs without Fertility Preservation. JAMA Oncol. 2021, 7, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, B.; Massarotti, C.; Bruzzone, M.; Goldrat, O.; Mangili, G.; Anserini, P.; Spinaci, S.; Arecco, L.; Del Mastro, L.; Ceppi, M.; et al. Efficacy and Safety of Controlled Ovarian Stimulation with or without Letrozole Co-administration for Fertility Preservation: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 574669. [Google Scholar] [CrossRef] [PubMed]
- Marklund, A.; Eloranta, S.; Wikander, I.; Kitlinski, M.L.; Lood, M.; Nedstrand, E.; Thurin-Kjellberg, A.; Zhang, P.; Bergh, J.; Rodriguez-Wallberg, K.A. Efficacy and safety of controlled ovarian stimulation using GnRH antagonist protocols for emergency fertility preservation in young women with breast cancer-a prospective nationwide Swedish multicenter study. Hum. Reprod. 2022, 35, 929–938. [Google Scholar] [CrossRef]
- Wang, S.S.Y.; Loong, H.; Chung, J.P.W.; Yeo, W. Preservation of fertility in premenopausal patients with breast cancer. Hong Kong Med. J. 2020, 26, 216–226. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Marklund, A.; Lundberg, F.; Wikander, I.; Milenkovic, M.; Anastacio, A.; Sergouniotis, F.; Wånggren, K.; Ekengren, J.; Lind, T.; et al. A prospective study of women and girls undergoing fertility preservation due to oncologic and non-oncologic indications in Sweden-Trends in patients’ choices and benefit of the chosen methods after long-term follow up. Acta Obstet. Gynecol. Scand. 2019, 98, 604–615. [Google Scholar] [CrossRef]
- Ahmadian, S.; Sheshpari, S.; Pazhang, M.; Bedate, A.M.; Beheshti, R.; Abbasi, M.M.; Nouri, M.; Rahbarghazi, R.; Mahdipour, M. Ovarian function and reproductive outcome after ovarian tissue transplantation: A systematic review. J. Transl. Med. 2019, 17, 396. [Google Scholar]
- Cobo, A.; García-Velasco, J.; Domingo, J.; Pellicer, A.; Remohí, J. Elective and Onco-fertility preservation: Factors related to IVF outcomes. Hum. Reprod. 2018, 33, 2222–2231. [Google Scholar] [CrossRef]
- Moravek, M.B.; Confino, R.; Smith, K.N.; Kazer, R.R.; Klock, S.C.; Lawson, A.K.; Gradishar, W.J.; Pavone, M.E. Long-term outcomes in cancer patients who did or did not pursue fertility preservation. Fertil. Steril. 2018, 109, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Hollanders de Ouderaen, S.; Demylle, D.; Pirard, C. Utilization rates and results of long-term embryo cryopreservation before gonadotoxic treatment. J. Assist. Reprod. Genet. 2015, 32, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Rabadan, S.; Domingo, J.; Cobo, A.; Pellicer, A.; Garcia-Velasco, J.A. Obstetric outcome after oocyte vitrification and warming for fertility preservation in women with cancer. Reprod. BioMed. Online 2014, 29, 722–728. [Google Scholar] [CrossRef]
- Courbiere, B.; Decanter, C.; Bringer-Deutsch, S.; Rives, N.; Mirallié, S.; Pech, J.C.; De Ziegler, D.; Carré-Pigeon, F.; May-Panloup, P.; Sifer, C.; et al. Emergency IVF for embryo freezing to preserve female fertility: A French multicentre cohort study. Hum. Reprod. 2013, 28, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.N.; Dillon, K.E.; Sammel, M.D.; Efymow, B.L.; Mainigi, M.A.; Dokras, A.; Gracia, C.R. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod. BioMed. Online 2013, 26, 337–344. [Google Scholar] [CrossRef][Green Version]
- Vomstein, K.; Reiser, E.; Pinggera, G.M.; Toerzsoek, P.; Deininger, S.; Kriesche, T.; Biasio, W.; Lusuardi, L.; Toth, B. Sperm banking before gonadotoxic treatment: Is it worth the effort? Asian J. Androl. 2021, 23, 490–494. [Google Scholar]
- Yamashita, S.; Kakimoto, K.; Uemura, M.; Kishida, T.; Kawai, K.; Nakamura, T.; Goto, T.; Osawa, T.; Yamada, S.; Nishimura, K.; et al. Fertility and reproductive technology use in testicular cancer survivors in Japan: A multi-institutional, cross-sectional study. Int. J. Urol. 2021, 28, 1047–1052. [Google Scholar]
- Liu, X.; Wang, Q.; Zhu, W.; Zhang, Z.; Tang, W.; Sheng, H.; Yang, J.; Li, Y.; Liang, X.; Meng, T.; et al. Sperm cryopreservation prior to gonadotoxic treatment: Experience of a single academic centre over 4 decades. Hum. Reprod. 2019, 34, 795–803. [Google Scholar]
- Depalo, R.; Falagario, D.; Masciandaro, P.; Nardelli, C.; Vacca, M.P.; Capuano, P.; Specchia, G.; Battaglia, M. Fertility preservation in males with cancer: 16-year monocentric experience of sperm banking and post-thaw reproductive outcomes. Ther. Adv. Med. Oncol. 2016, 8, 412–420. [Google Scholar] [CrossRef]
- Ferrari, S.; Paffoni, A.; Filippi, F.; Busnelli, A.; Vegetti, W.; Somigliana, E. Sperm cryopreservation and reproductive outcome in male cancer patients: A systematic review. Reprod. BioMed. Online 2016, 33, 29–38. [Google Scholar] [CrossRef]
- Muller, I.; Oude Ophuis, R.J.; Broekmans, F.J.; Lock, T.M. Semen cryopreservation and usage rate for assisted reproductive technology in 898 men with cancer. Reprod. BioMed. Online 2016, 32, 147–153. [Google Scholar] [CrossRef]
- Sonnenburg, D.W.; Brames, M.J.; Case-Eads, S.; Einhorn, L.H. Utilization of sperm banking and barriers to its use in testicular cancer patients. Support. Care Cancer 2015, 23, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Van der Kaaij, M.A.; Heutte, N.; Meijnders, P.; Abeilard-Lemoisson, E.; Spina, M.; Moser, E.C.; Allgeier, A.; Meulemans, B.; Simons, A.H.; Lugtenburg, P.J.; et al. Cryopreservation, semen use and the likelihood of fatherhood in male Hodgkin lymphoma survivors: An EORTC-GELA Lymphoma Group cohort study. Hum. Reprod. 2014, 29, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Záková, J.; Lousová, E.; Ventruba, P.; Crha, I.; Pochopová, H.; Vinklárková, J.; Tesařová, E.; Nussir, M. Sperm cryopreservation before testicular cancer treatment and its subsequent utilization for the treatment of infertility. Sci. World J. 2014, 2014, 575978. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Cooper, A.R.; Jungheim, E.S.; Lanzendorf, S.E.; Odem, R.R.; Ratts, V.S. Sperm banking for fertility preservation: A 20-year experience. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 177–182. [Google Scholar] [CrossRef]
- Ozimek, N.; Salama, M.; Woodruff, T.K. National oncofertility registries around the globe: A pilot survey. Front. Endocrinol. 2023, 14, 1148314. [Google Scholar] [CrossRef]
- Khorshid, A.; Alvero, R. Consenting and ethical considerations in embryo cryopreservation. Curr. Opin. Obstet. Gynecol. 2020, 32, 380–384. [Google Scholar] [CrossRef]
- Cancer Council Australia. Fertility Preservation for People with Cancer. COSA Guidelines for Fertility Preservation for People with Cancer. 2022. Available online: https://www.cancer.org.au/clinical-guidelines/cancer-fertility-preservation (accessed on 13 June 2023).
- Rives, N.; Courbière, B.; Almont, T.; Kassab, D.; Berger, C.; Grynberg, M.; Papaxanthos, A.; Decanter, C.; Elefant, E.; Dhedin, N.; et al. What should be done in terms of fertility preservation for patients with cancer? The French 2021 guidelines. Eur. J. Cancer 2022, 173, 146–166. [Google Scholar] [CrossRef]
- Harada, M.; Kimura, F.; Takai, Y.; Nakajima, T.; Ushijima, K.; Kobayashi, H.; Satoh, T.; Tozawa, A.; Sugimoto, K.; Saji, S.; et al. Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 for fertility preservation in childhood, adolescent, and young adult cancer patients: Part 1. Int. J. Clin. Oncol. 2022, 27, 265–280. [Google Scholar] [CrossRef]
- Mulder, R.L.; Font-Gonzalez, A.; Hudson, M.M.; van Santen, H.M.; Loeffen, E.A.H.; Burns, K.C.; Quinn, G.P.; van Dulmen-den Broeder, E.; Byrne, J.; Haupt, R.; et al. Fertility preservation for female patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e45–e56. [Google Scholar] [CrossRef]
- Mulder, R.L.; Font-Gonzalez, A.; Green, D.M.; Loeffen, E.A.H.; Hudson, M.M.; Loonen, J.; Yu, R.; Ginsberg, J.P.; Mitchell, R.T.; Byrne, J.; et al. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: Recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021, 22, e57–e67. [Google Scholar] [CrossRef]
- Klipstein, S.; Fallat, M.E.; Savelli, S. Committee On Bioethics; Section On Hematology/Oncology; Section On Surgery. Fertility Preservation for Pediatric and Adolescent Patients with Cancer: Medical and Ethical Considerations. Pediatrics 2020, 145, e20193994. [Google Scholar] [CrossRef] [PubMed]
- European Society of Human Reproduction and Embryology. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 4, hoaa052. [Google Scholar]
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fretis. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef]
- American Society of Clinical Oncology. Fertility Preservation in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Dittrich, R.A.; Kliesch, S.C.; Schüring, A.D.; Balcerek, M.E.; Baston-Büst, D.M.; Beck, R.F.; Beckmann, M.W.; Behringer, K.G.; Borgmann-Staudt, A.H.; Cremer, W.; et al. Fertility Preservation for Patients with Malignant Disease. Guideline of the DGGG, DGU and DGRM (S2k-Level, AWMF Registry No. 015/082, November 2017)—Recommendations and Statements for Girls and Women. Geburtshilfe Frauenheilkd 2018, 78, 567–584. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Adolescent and Young Adult Oncology, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 66–97. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Fertility Problems: Assessment and Treatment. 1.16 People with Cancer Who Wish to Preserve Fertility. 2017. Available online: https://www.nice.org.uk/guidance/cg156 (accessed on 18 June 2023).
- Mintziori, G.; Lambrinoudaki, I.; Goulis, D.G.; Ceausu, I.; Depypere, H.; Erel, C.T.; Pérez-López, F.R.; Schenck-Gustafsson, K.; Simoncini, T.; Tremollieres, F.; et al. EMAS position statement: Fertility preservation. Maturitas 2014, 77, 85–89. [Google Scholar] [CrossRef]
- Roberts, J.; Ronn, R.; Tallon, N.; Holzer, H. Fertility preservation in reproductive-age women facing gonadotoxic treatments. Curr. Oncol. 2015, 22, e294–e304. [Google Scholar] [CrossRef][Green Version]
- Lambertini, M.; Del Mastro, L.; Pescio, M.C.; Andersen, C.Y.; Azim, H.A., Jr.; Peccatori, F.A.; Costa, M.; Revelli, A.; Salvagno, F.; Gennari, A.; et al. Cancer and fertility preservation: International recommendations from an expert meeting. BMC Med. 2016, 14, 1. [Google Scholar] [CrossRef]
- Gilbert, K.; Nangia, A.K.; Dupree, J.M.; Smith, J.F.; Mehta, A. Fertility preservation for men with testicular cancer: Is sperm cryopreservation cost effective in the era of assisted reproductive technology? Urol. Oncol. 2018, 36, e1–e92. [Google Scholar] [CrossRef] [PubMed]
- Republic of Poland. Act of 25 June 2015 on Infertility Treatment. J. Laws 2020. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150001087 (accessed on 18 June 2023).
- Fournier, E.M. Oncofertility and the Rights to Future Fertility. JAMA Oncol. 2016, 2, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.; Crockin, S.L. Legal Battles over Embryos After In Vitro Fertilization: Is There a Way to Avoid Them? JAMA Oncol. 2016, 2, 182–184. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).