Endoscopic Ultrasound-Guided Fiducial Placement for Stereotactic Body Radiation Therapy in Patients with Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
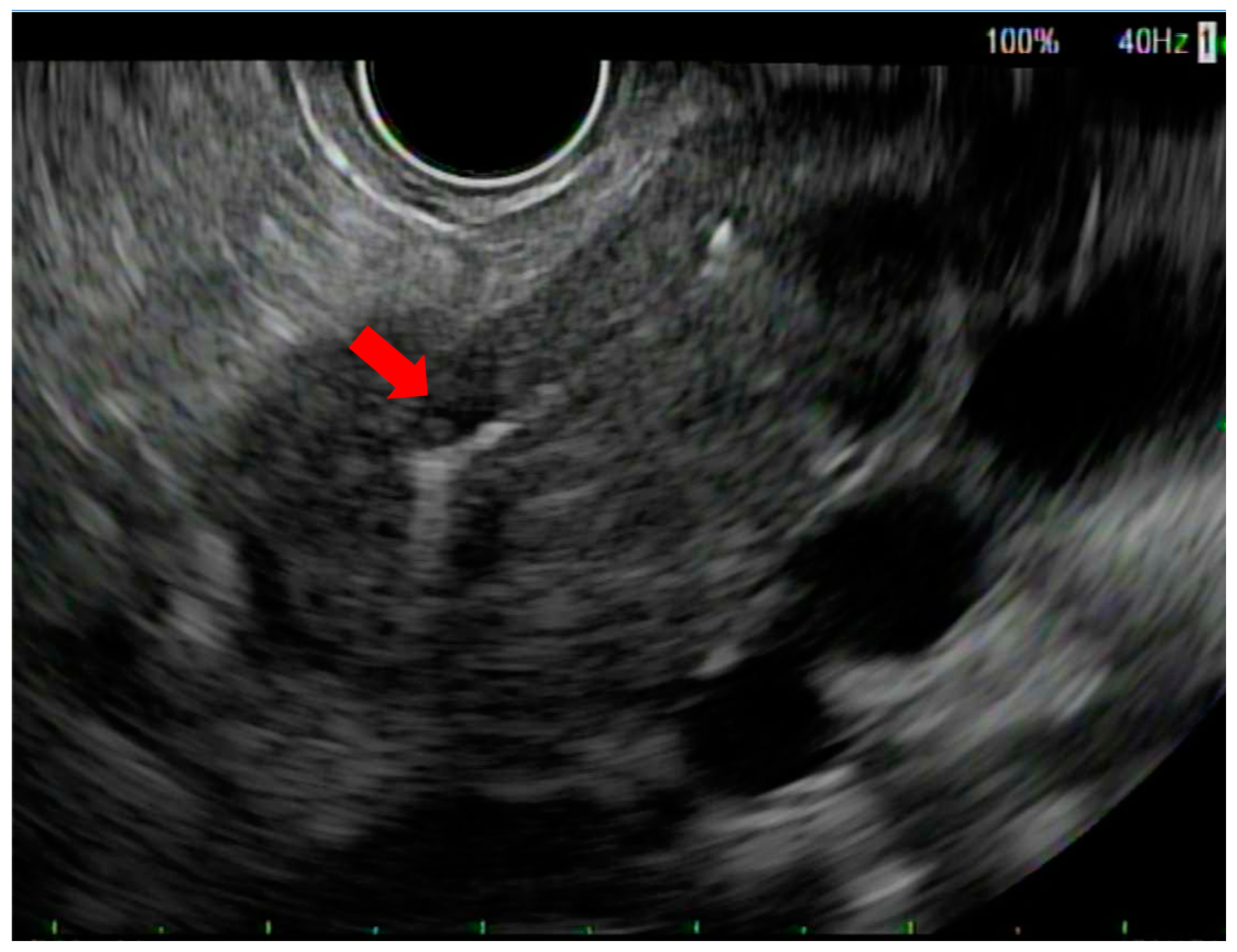
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoffel, E.M.; Brand, R.E.; Goggins, M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology 2023, 164, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; Suker, M.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; Creemers, G.-J.M.; van Dam, R.M. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: Results of the Dutch randomized phae III PREOPANC trial. J. Clin. Oncol. 2020, 38, 1763. [Google Scholar] [CrossRef]
- Katz, M.H.; Crane, C.H.; Varadhachary, G. Management of borderline resectable pancreatic cancer. Semin. Radiat. Oncol. 2014, 24, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: A phase 2 clinical trial. JAMA Oncol. 2018, 4, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A. NCCN guidelines updates: Pancreatic cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 603–605. [Google Scholar]
- Hammel, P.; Huguet, F.; van Laethem, J.-L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouché, O.; Shannon, J.; André, T. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 randomized clinical trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef]
- Ng, S.P.; Kantor, M.E.; Beddar, S.; Koay, E.; Herman, J.; Taniguchi, C.M. Locally Advanced/Unresectable Pancreatic Cancer. In Gastrointestinal Malignancies: A Practical Guide on Treatment Techniques; Russo, S., Hoffe, S., Kim, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 231–256. [Google Scholar] [CrossRef]
- Slagowski, J.M.; Colbert, L.E.; Cazacu, I.M.; Singh, B.S.; Martin, R.; Koay, E.J.; Taniguchi, C.M.; Koong, A.C.; Bhutani, M.S.; Herman, J.M.; et al. Evaluation of the Visibility and Artifacts of 11 Common Fiducial Markers for Image Guided Stereotactic Body Radiation Therapy in the Abdomen. Pract. Radiat. Oncol. 2020, 10, 434–442. [Google Scholar] [CrossRef]
- Kothary, N.; Heit, J.J.; Louie, J.D.; Kuo, W.T.; Loo, B.W., Jr.; Koong, A.; Chang, D.T.; Hovsepian, D.; Sze, D.Y.; Hofmann, L.V. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J. Vasc. Interv. Radiol. 2009, 20, 235–239. [Google Scholar] [CrossRef]
- Micames, C.; Jowell, P.S.; White, R.; Paulson, E.; Nelson, R.; Morse, M.; Hurwitz, H.; Pappas, T.; Tyler, D.; McGrath, K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest. Endosc. 2003, 58, 690–695. [Google Scholar] [CrossRef]
- Coronel, E.; Cazacu, I.M.; Sakuraba, A.; Luzuriaga Chavez, A.A.; Uberoi, A.; Geng, Y.; Tomizawa, Y.; Saftoiu, A.; Shin, E.J.; Taniguchi, C.M.; et al. EUS-guided fiducial placement for GI malignancies: A systematic review and meta-analysis. Gastrointest. Endosc. 2019, 89, 659–670.e618. [Google Scholar] [CrossRef]
- Timmerman, R.D.; Kavanagh, B.D.; Cho, L.C.; Papiez, L.; Xing, L. Stereotactic body radiation therapy in multiple organ sites. J Clin. Oncol. 2007, 25, 947–952. [Google Scholar] [CrossRef]
- Milano, M.T.; Chmura, S.J.; Garofalo, M.C.; Rash, C.; Roeske, J.C.; Connell, P.P.; Kwon, O.H.; Jani, A.B.; Heimann, R. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: Toxicity and clinical outcome. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Pishvaian, A.C.; Collins, B.; Gagnon, G.; Ahlawat, S.; Haddad, N.G. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest. Endosc. 2006, 64, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Bouchart, C.; Moretti, L.; Mans, L.; Engelholm, J.-L.; Bali, M.-A.; Van Laethem, J.-L.; Eisendrath, P. EUS-guided placement of fiducial markers for stereotactic body radiation therapy in pancreatic cancer: Feasibility, security and a new quality score. Endosc. Int. Open 2021, 9, E253–E257. [Google Scholar] [PubMed]
- Patel, J.B.; Revanur, V.; Forcione, D.G.; Bechtold, M.L.; Puli, S.R. Endoscopic ultrasound-guided fiducial marker placement in pancreatic cancer: A systematic review and meta-analysis. World J. Gastrointest. Endosc. 2020, 12, 231–240. [Google Scholar] [CrossRef]
- Machicado, J.D.; Obuch, J.C.; Goodman, K.A.; Schefter, T.E.; Frakes, J.; Hoffe, S.; Latifi, K.; Simon, V.C.; Santangelo, T.; Ezekwe, E. Endoscopic ultrasound placement of preloaded fiducial markers shortens procedure time compared to back-loaded markers. Clin. Gastroenterol. Hepatol. 2019, 17, 2749–2758.e2742. [Google Scholar] [CrossRef] [PubMed]
- Chandnani, M.; Faisal, M.F.; Glissen-Brown, J.; Sawhney, M.; Pleskow, D.; Cohen, J.; Berzin, T.M. EUS-guided fiducial placement for pancreatobiliary malignancies: Safety, infection risk, and use of peri-procedural antibiotics. Endosc. Int. Open 2020, 8, E179–E185. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, S.; Prados, S.; Rubio, M.D.C.; de la Morena, F.; López, M.; Sánchez, E. Endoscopic ultrasound-guided fiducial placement in pancreatic tumors: Safety and technical feasibility. Rev. Esp. Enferm. Dig. 2019, 111, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Park, W.G.; Yan, B.M.; Schellenberg, D.; Kim, J.; Chang, D.T.; Koong, A.; Patalano, C.; Van Dam, J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest. Endosc. 2010, 71, 513–518. [Google Scholar] [CrossRef]
- Khashab, M.A.; Kim, K.J.; Tryggestad, E.J.; Wild, A.T.; Roland, T.; Singh, V.K.; Lennon, A.M.; Shin, E.J.; Ziegler, M.A.; Sharaiha, R.Z. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest. Endosc. 2012, 76, 962–971. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Hoffe, S.E.; Barthel, J.S.; Vignesh, S.; Klapman, J.B.; Harris, C.; Almhanna, K.; Biagioli, M.C.; Meredith, K.L.; Feygelman, V. Stability of endoscopic ultrasound-guided fiducial marker placement for esophageal cancer target delineation and image-guided radiation therapy. Pract. Radiat. Oncol. 2013, 3, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.K.; Moser, A.J.; Khalid, A.; Fasanella, K.E.; Zeh, H.J.; Burton, S.; McGrath, K. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest. Endosc. 2010, 71, 1178–1184. [Google Scholar] [CrossRef]
- Choi, J.H.; Seo, D.W.; Park, D.H.; Lee, S.K.; Kim, M.-H. Fiducial placement for stereotactic body radiation therapy under only endoscopic ultrasonography guidance in pancreatic and hepatic malignancy: Practical feasibility and safety. Gut Liver 2014, 8, 88–93. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, C.J.; Nagula, S.; Goodman, K.A.; Ho, A.Y.; Markowitz, A.J.; Schattner, M.A.; Gerdes, H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with). Gastrointest. Endosc. 2010, 71, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Dhadham, G.C.; Hoffe, S.; Harris, C.L.; Klapman, J.B. Endoscopic ultrasound-guided fiducial marker placement for image-guided radiation therapy without fluoroscopy: Safety and technical feasibility. Endosc. Int. Open 2016, 4, E378–E382. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, E.J. Endoscopic Ultrasound-Guided Fiducial Placement for Stereotactic Body Radiation Therapy in Pancreatic Malignancy. Clin. Endosc. 2021, 54, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.M.; Chang, D.T.; Goodman, K.A.; Dholakia, A.S.; Raman, S.P.; Hacker-Prietz, A.; Iacobuzio-Donahue, C.A.; Griffith, M.E.; Pawlik, T.M.; Pai, J.S. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015, 121, 1128–1137. [Google Scholar] [CrossRef]
- Koong, A.C.; Christofferson, E.; Le, Q.-T.; Goodman, K.A.; Ho, A.; Kuo, T.; Ford, J.M.; Fisher, G.A.; Greco, R.; Norton, J. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 320–323. [Google Scholar] [CrossRef]
- Schellenberg, D.; Kim, J.; Christman-Skieller, C.; Chun, C.L.; Columbo, L.A.; Ford, J.M.; Fisher, G.A.; Kunz, P.L.; Van Dam, J.; Quon, A. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 181–188. [Google Scholar] [CrossRef]
- Palta, M.; Godfrey, D.; Goodman, K.A.; Hoffe, S.; Dawson, L.A.; Dessert, D.; Hall, W.A.; Herman, J.M.; Khorana, A.A.; Merchant, N. Radiation therapy for pancreatic cancer: Executive summary of an ASTRO clinical practice guideline. Pract. Radiat. Oncol. 2019, 9, 322–332. [Google Scholar] [CrossRef]
- Moningi, S.; Abi Jaoude, J.; Kouzy, R.; Lin, D.; Nguyen, N.D.; Garcia, C.J.G.; Phan, J.L.; Avila, S.; Smani, D.; Cazacu, I.M. Impact of fiducial marker placement before stereotactic body radiation therapy on clinical outcomes in patients with pancreatic cancer. Adv. Radiat. Oncol. 2021, 6, 100621. [Google Scholar] [CrossRef] [PubMed]

| Gender | No. Patients (N = 82) |
|---|---|
| Female | 36 (44%) |
| Male | 46 (56%) |
| Age, Mean Year (range) | 69 (23–86) |
| PDAC Diagnosis | |
| Locally Advanced | 32 (39%) |
| Resectable | 13 (16%) |
| Borderline Resectable | 29 (35%) |
| Metastatic | 8 (10%) |
| Tumor Location in Pancreas | |
| Head | 27 (33%) |
| Neck | 16 (20%) |
| Body | 20 (24%) |
| Tail | 12 (15%) |
| Uncinate | 7 (8%) |
| Average Tumor Size, Largest Diameter (mm) | 27 |
| Fiducial Marker Loading System Type (N = 230) | |
|---|---|
| Backloaded | 166 (72%) |
| Preloaded | 64 (28%) |
| Fiducial Marker Type Used in Patients (N = 82) | |
| Gold Anchor 20 mm Fiducial | 64 (78%) |
| Beacon 5 mm Gold Fiducial | 17 (21%) |
| LumiCoil Platinum Fiducial | 1 (1%) |
| Same-Session EUS-FNA | 30 (37%) |
| Technical Success | 98% |
| Median Radiation Dosage over 5 Fractions | 40 cGy |
|---|---|
| Visible Fiducials on Cone Beam CT (N = 216) | 202 (94%) |
| Adverse Event/Symptoms Post-Radiation | |
| Fatigue | 27 (35%) |
| Nausea | 27 (35%) |
| Abdominal pain | 17 (22%) |
| Constipation | 6 (8%) |
| Vomiting | 4 (5%) |
| None | 32 (41%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazacu, I.M.; Singh, B.S.; Martin-Paulpeter, R.M.; Beddar, S.; Chun, S.; Holliday, E.B.; Koong, A.C.; Das, P.; Koay, E.J.; Taniguchi, C.; et al. Endoscopic Ultrasound-Guided Fiducial Placement for Stereotactic Body Radiation Therapy in Patients with Pancreatic Cancer. Cancers 2023, 15, 5355. https://doi.org/10.3390/cancers15225355
Cazacu IM, Singh BS, Martin-Paulpeter RM, Beddar S, Chun S, Holliday EB, Koong AC, Das P, Koay EJ, Taniguchi C, et al. Endoscopic Ultrasound-Guided Fiducial Placement for Stereotactic Body Radiation Therapy in Patients with Pancreatic Cancer. Cancers. 2023; 15(22):5355. https://doi.org/10.3390/cancers15225355
Chicago/Turabian StyleCazacu, Irina M., Ben S. Singh, Rachael M. Martin-Paulpeter, Sam Beddar, Stephen Chun, Emma B. Holliday, Albert C. Koong, Prajnan Das, Eugene J. Koay, Cullen Taniguchi, and et al. 2023. "Endoscopic Ultrasound-Guided Fiducial Placement for Stereotactic Body Radiation Therapy in Patients with Pancreatic Cancer" Cancers 15, no. 22: 5355. https://doi.org/10.3390/cancers15225355
APA StyleCazacu, I. M., Singh, B. S., Martin-Paulpeter, R. M., Beddar, S., Chun, S., Holliday, E. B., Koong, A. C., Das, P., Koay, E. J., Taniguchi, C., Herman, J. M., & Bhutani, M. S. (2023). Endoscopic Ultrasound-Guided Fiducial Placement for Stereotactic Body Radiation Therapy in Patients with Pancreatic Cancer. Cancers, 15(22), 5355. https://doi.org/10.3390/cancers15225355










