Detection of Liver Lesions in Colorectal Cancer Patients Using 18F-FDG PET/CT Dual-Time-Point Scan Imaging
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Reference Standard
2.3. Imaging Protocol
2.4. Imaging Data Analysis
2.5. Statistical Analysis
2.6. Ethics Approval
3. Results
3.1. Patient Characteristics
3.2. Per-Patient Performance of Early and Delayed Imaging
3.3. Semi-Quantitative and Per-Lesion Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversane, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Chen, W.; Wen, Y. Colorectal liver metastasis: Molecular mechanism and interventional therapy. Signal Transduct. Target Ther. 2022, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Kow, A.W.C. Hepatic metastasis from colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 1274–1298. [Google Scholar] [CrossRef] [PubMed]
- Zarour, L.R.; Anand, S.; Bilingsley, K.G.; Bisson, W.H.; Cercek, A.; Clarke, M.F.; Coussens, L.M.; Gast, C.E.; Geltzeiller, C.B.; Hansen, L.; et al. Colorectal Cancer Liver Metastasis: Evolving Paradigms and Future Directions. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 163–173. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 19, 1467–1480. [Google Scholar] [CrossRef]
- Ludwig, D.R.; Mintz, A.J.; Sanders, V.R.; Fowler, K.J. Liver Imaging for Colorectal Cancer Metastases. Curr. Color. Cancer Rep. 2017, 13, 470–480. [Google Scholar] [CrossRef]
- Watanabe, A.; Harimoto, N.; Araki, K.; Yoshizumi, T.; Arima, K.; Yamashita, Y.; Baba, H.; Tetsuya, H.; Kuwano, H.; Shirabe, K. A new strategy based on fluorodeoxyglucose-positron emission tomography for managing liver metastasis from colorectal cancer. J. Surg. Oncol. 2018, 118, 1088–1095. [Google Scholar] [CrossRef]
- Hazhirkarzar, B.; Khoshpuri, P.; Shaghaghi, M.; Ghasabeh, M.A.; Pawlik, T.M.; Kamel, I.R. Current state of the art imaging approaches for colorectal liver metastasis. Hepatobiliary Surg. Nutr. 2020, 9, 35–48. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, J.; Wu, C.; Song, S.; Tong, L.; Huang, G.; Feng, Y.; Jiang, Y.; Liu, Y.; Yin, T.; et al. Prognostic significance of 18FDG PET/CT in colorectal cancer patients with liver metastases: A meta-analysis. Cancer Imaging 2015, 15, 19. [Google Scholar] [CrossRef]
- Agarwal, A.; Marcus, C.; Xiao, J.; Nene, P.; Kachnic, L.A.; Subramaniam, R.M. FDG PET/CT in the management of colorectal and anal cancers. AJR Am. J. Roentgenol. 2014, 203, 1109–1119. [Google Scholar] [CrossRef]
- Bijlstra, O.D.; Boreel, M.M.E.; van Mossel, S.; Burgmans, M.C.; Kapiteijin, E.H.W.; Oprea-Larger, D.E.; Rietbergen, D.D.D.; van Velden, F.H.P.; Vahrmeijer, A.L.; Swijnenburg, R.-J.; et al. The Value of 18F-FDG-PET-CT Imaging in Treatment Evaluation of Colorectal Liver Metastases: A Systematic Review. Diagnostics 2022, 12, 715. [Google Scholar] [CrossRef]
- Viganò, L.; Lopci, E.; Costa, G.; Rodari, M.; Poretti, D.; Pedicini, V.; Solbiati, L.; Chiti, A.; Torzilli, G. Positron Emission Tomography-Computed Tomography for Patients with Recurrent Colorectal Liver Metastases: Impact on Restaging and Treatment Planning. Ann. Surg. Oncol. 2017, 24, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Metser, U.; Even-Sapir, E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): Accumulated data from four years of experience with PET/CT. Semin. Nucl. Med. 2007, 37, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Torigian, D.A.; Zhuang, H.; Alavi, A. When should we recommend use of dual time-point and delayed time-point imaging techniques in FDG PET? Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 779–787. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [PubMed]
- Erdi, Y.E.; Mawlawi, O.; Larson, S.M.; Imbriaco, M.; Yeung, H.; Finn, R.; Humm, J.L. Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer 1997, 15, 2505–2509. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 327–354. [Google Scholar] [CrossRef]
- Maffione, A.M.; Lopci, E.; Bluemel, C.; Giammarile, F.; Herrmann, K.; Rubello, D. Diagnostic accuracy and impact on management of (18)F-FDG PET and PET/CT in colorectal liver metastasis: A meta-analysis and systematic review. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 152–163. [Google Scholar] [CrossRef]
- Amakusa, S.; Marsuoka, K.; Kawano, M.; Hasegawa, K.; Ouchida, M.; Date, A.; Yoshida, T.; Sasaki, M. Influence of region-of-interest determination on measurement of signal-to-noise ratio in liver on PET images. Ann. Nucl. Med. 2018, 32, 1–6. [Google Scholar] [CrossRef]
- Mao, W.; Zhou, J.; Qiu, L.; Yin, H.; Tan, H.; Shi, H. The added value of dual-time-point 18F-FDG PET/CT imaging in the diagnosis of colorectal cancer liver metastases. Abdom. Radiol. 2020, 45, 1075–1081. [Google Scholar] [CrossRef]
- Dirisamer, A.; Halpern, B.S.; Schima, W.; Heinisch, M.; Wolf, F.; Beheshti, M.; Dirisamer, F.; Weber, M.; Langsteger, W. Dual-time-point FDG-PET/CT for the detection of hepatic metastases. Mol. Imaging Biol. 2008, 10, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.-A.; Huang, W.-S.; Chiu, C.-H.; Tyan, Y.-C.; Wang, J.-Y.; Wu, L.-C.; Feng, I.J.; Lee, C.H. Does Routine Triple-Time-Point FDG PET/CT Imaging Improve the Detection of Liver Metastases? Diagnostics 2020, 10, 609. [Google Scholar] [CrossRef]
- Kubota, K.; Itoh, M.; Ono, S.; Tashiro, M.; Yamaguchi, K.; Akaizawa, T.; Yamada, K.; Fukuda, H. Advantage of delayed whole-body FDG-PET imaging for tumour detection. Eur. J. Nucl. Med. 2001, 28, 696–703. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.K.; Lee, S.M.; Moon, S.H.; Kim, T.S. Detection of hepatic metastases using dual-time-point FDG PET/CT scans in patients with colorectal cancer. Mol. Imaging Biol. 2011, 13, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Saga, T.; Nakamoto, Y.; Ishimori, T.; Mamede, M.H.; Wada, M.; Doi, R.; Hosotani, R.; Imamura, M.; Konishi, J. Relationship Between Retention Index in Dual-Phase 18 F-FDG PET, and Hexokinase-II and Glucose Transporter-1 Expression in Pancreatic Cancer. J. Nucl. Med. 2002, 43, 173–180. [Google Scholar] [PubMed]
- Te Riet, J.; Rijnsdorp, S.; Roef, M.J.; Arends, A.J. Evaluation of a Bayesian penalized likelihood reconstruction algorithm for low-count clinical 18F-FDG PET/CT. EJNMMI Phys. 2019, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Rogasch, J.M.M.; Hofheinz, F.; van Heek, L.; Voltin, C.-A.; Boellaard, R.; Kobe, C. Influences on PET quantification and interpretation. Diagnostics 2022, 12, 451. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, M.-J.; Zhou, J.; Du, F.; Chen, L.; Huang, Z.-K.; Hu, J.-B.; Lou, C. Changes of [18F]FDG-PET/CT quantitative parameters in tumor lesions by the Bayesian penalized-likelihood PET reconstruction algorithm and its influencing factors. BMC Med. Imaging 2021, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, N.; Franklin, J.M.; McGowan, D.R.; Teoh, E.J.; Bradley, K.M.; Gleeson, F.V. Does a novel penalized likelihood reconstruction of 18F-FDG PET-CT improve signal-to-background in colorectal liver metastases? Eur. J. Radiol. 2015, 84, 1873–1878. [Google Scholar] [CrossRef]
- Van der Vos, C.S.; Koopman, D.; Rijnsdorp, S.; Arends, A.J.; Boellaard, R.; van Dalen, J.A.; Lubberink, M.; Willemsen, A.T.M. Quantification, improvement, and harmonization of small lesion detection with state-of-the-art PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 4–16. [Google Scholar] [CrossRef]
- Devriese, J.; Beels, L.; Maes, A.; Van de Wiele, C.; Pottel, H. Impact of PET reconstruction protocols on quantification of lesions that fulfil the PERCIST lesion inclusion criteria. EJNMMI Phys. 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Munk, O.L.; Tolbod, L.P.; Hansen, S.B.; Bogsrud, T.V. Point-spread function reconstructed PET images of sub- centimeter lesions are not quantitative. EJNMMI Phys. 2017, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, H.; Alavi, A.; Naqa, I.E. Novel Quantitative PET Techniques for Clinical Decision Support in Oncology. Semin. Nucl. Med. 2018, 48, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Meikle, S.R.; Sossi, V.; Roncali, E.; Cherry, S.R.; Banati, R.; Mankoff, D.; Jones, T.; James, M.; Stutcliffe, J.; Ouyang, J.; et al. Quantitative PET in the 2020s: A roadmap. Phys. Med. Biol. 2021, 66, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Tian, A.; Lin, R.; Yu, J.; Zhang, F.; Zheng, Q.; Yuan, X.; Sun, Z.; Zhong, Z. The differential diagnostic value of dual-phase 18F-DCFPyL PET/CT in prostate carcinoma. Prostate Cancer Prostatic Dis. 2022, 25, 351–358. [Google Scholar] [CrossRef]
- Zaidi, H.; Karakatsanis, N. Towards enhanced PET quantification in clinical oncology. Br. J. Radiol. 2018, 91, 20170508. [Google Scholar] [CrossRef]
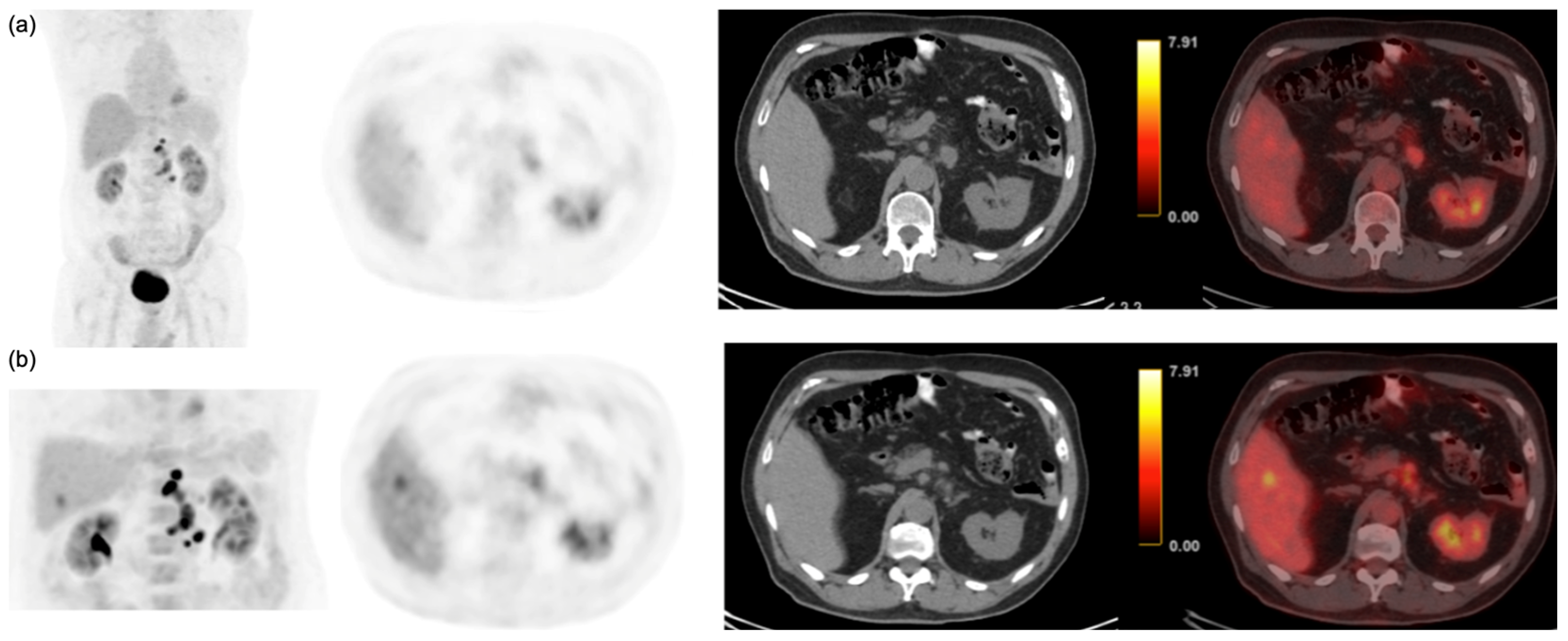
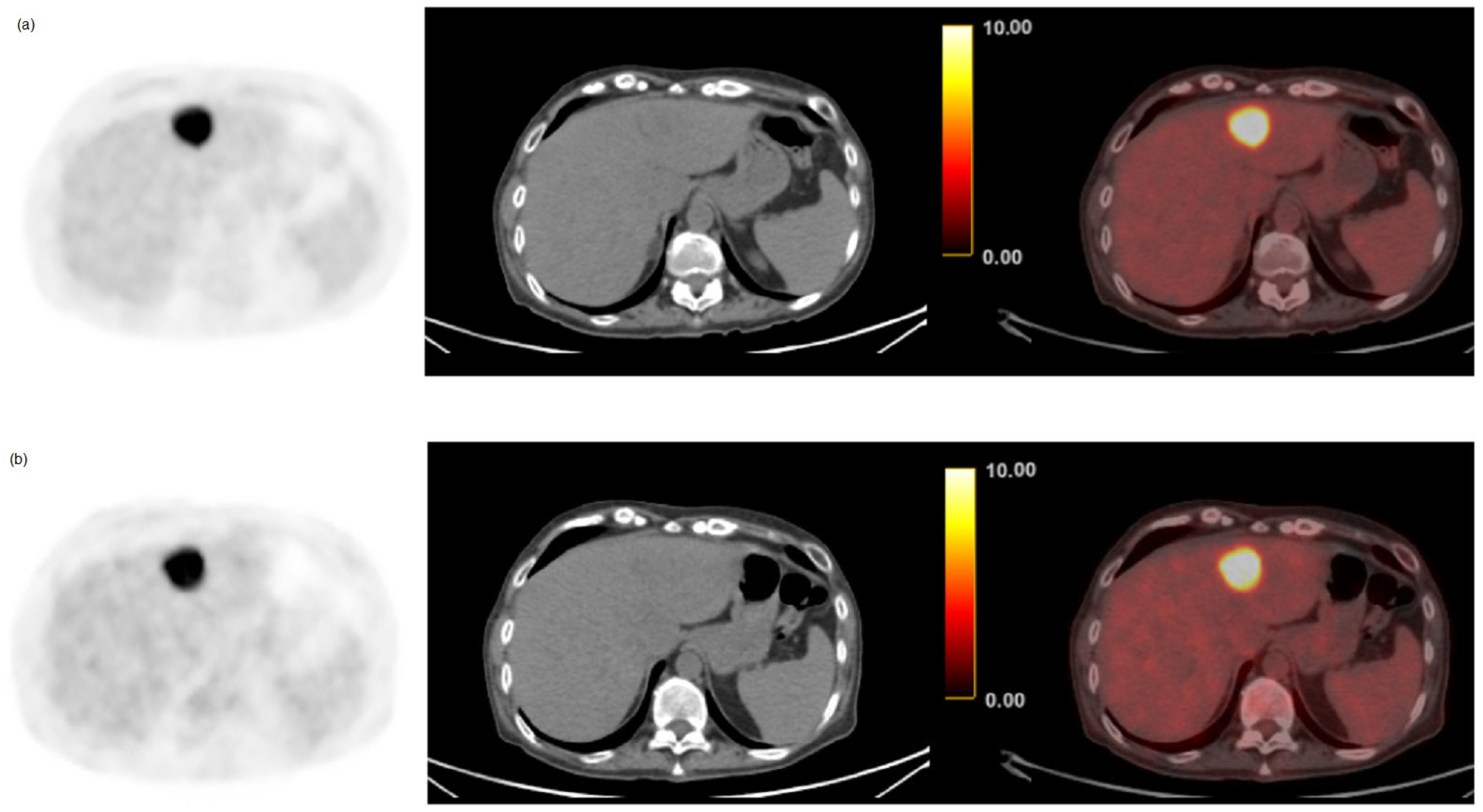
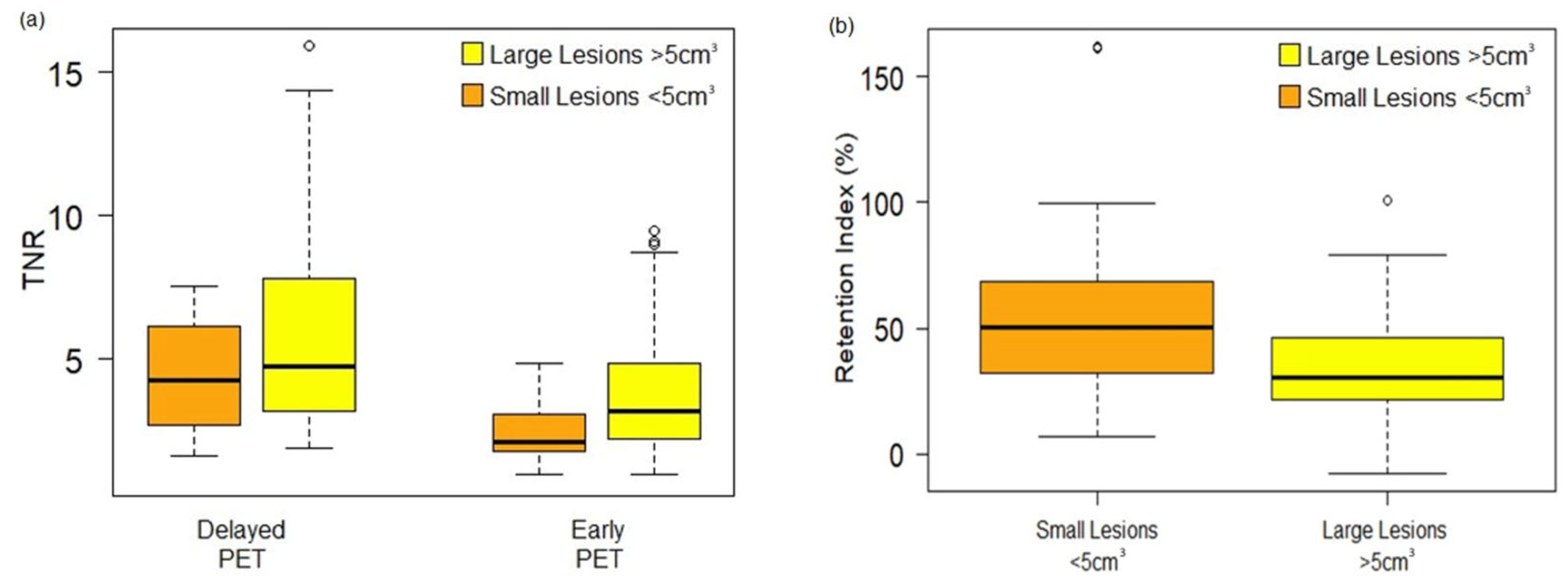
| Characteristics | n = 124 |
|---|---|
| Age | 62.4 ± 12.8 |
| Female | 53 (42.8%) |
| Diabetic Patients | 26 (20.1%) |
| Histological grading of CRC | |
| Moderately differentiated | 90 (72.6%) |
| Well-differentiated | 23 (18.5%) |
| Poorly differentiated | 11 (8.9%) |
| CRLM | 57 (46%) |
| Benign lesions | 6 (4.8%) |
| TP | TN | FP | FN | Sensitivity | Specificity | p-Value * | Accuracy | |
|---|---|---|---|---|---|---|---|---|
| Early | 50 | 61 | 6 | 7 | 87.7% | 91.0% | 0.04 | 88.1% |
| Delayed | 57 | 63 | 4 | 0 | 100.0% | 94.0% | 96.2% |
| Early | Delayed | p | ||
|---|---|---|---|---|
| Normal liver | SUVmean | 2.5 ± 0.4 | 2.3 ± 0.4 | <0.001 |
| SUVmax | 3.1 ± 0.5 | 2.8 ± 0.4 | <0.001 | |
| CRLM | SUVmean | 5.3 ± 2.2 | 5.5 ± 3.0 | <0.001 |
| (n = 168) | SUVmax | 8.6 ± 5.4 | 9.7 ± 7.1 | <0.001 |
| TNR | 3.5 ± 2.5 | 5.4 ± 3.8 | <0.001 | |
| CRLM > 5 cm3 | SUVmean | 5.9 ± 2.6 | 7.0 ± 3.7 | <0.05 |
| (n = 82) | SUVmax | 10.0 ± 6.4 | 13.4 ± 8.8 | <0.01 |
| TNR | 4.1 ± 2.9 | 6.1 ± 4.5 | <0.001 | |
| CRLM < 5 cm3 | SUVmean | 4.4 ± 0.9 | 5.5 ± 1.5 | <0.001 |
| (n = 86) | SUVmax | 6.1 ± 1.7 | 9.6 ± 3.6 | <0.001 |
| TNR | 2.4 ± 0.8 | 4.3 ± 1.8 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boanova, L.G.; Altmayer, S.; Watte, G.; Raupp, A.A.; Francisco, M.Z.; De Oliveira, G.S.; Hochhegger, B.; Andrade, R.G.F. Detection of Liver Lesions in Colorectal Cancer Patients Using 18F-FDG PET/CT Dual-Time-Point Scan Imaging. Cancers 2023, 15, 5403. https://doi.org/10.3390/cancers15225403
Boanova LG, Altmayer S, Watte G, Raupp AA, Francisco MZ, De Oliveira GS, Hochhegger B, Andrade RGF. Detection of Liver Lesions in Colorectal Cancer Patients Using 18F-FDG PET/CT Dual-Time-Point Scan Imaging. Cancers. 2023; 15(22):5403. https://doi.org/10.3390/cancers15225403
Chicago/Turabian StyleBoanova, Luciane G., Stephan Altmayer, Guilherme Watte, Ana Amelia Raupp, Martina Zaguini Francisco, Guilherme Strieder De Oliveira, Bruno Hochhegger, and Rubens G. F. Andrade. 2023. "Detection of Liver Lesions in Colorectal Cancer Patients Using 18F-FDG PET/CT Dual-Time-Point Scan Imaging" Cancers 15, no. 22: 5403. https://doi.org/10.3390/cancers15225403




