Thoracoscopy for Pediatric Thoracic Neurogenic Tumors—A European Multi-Center Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistics
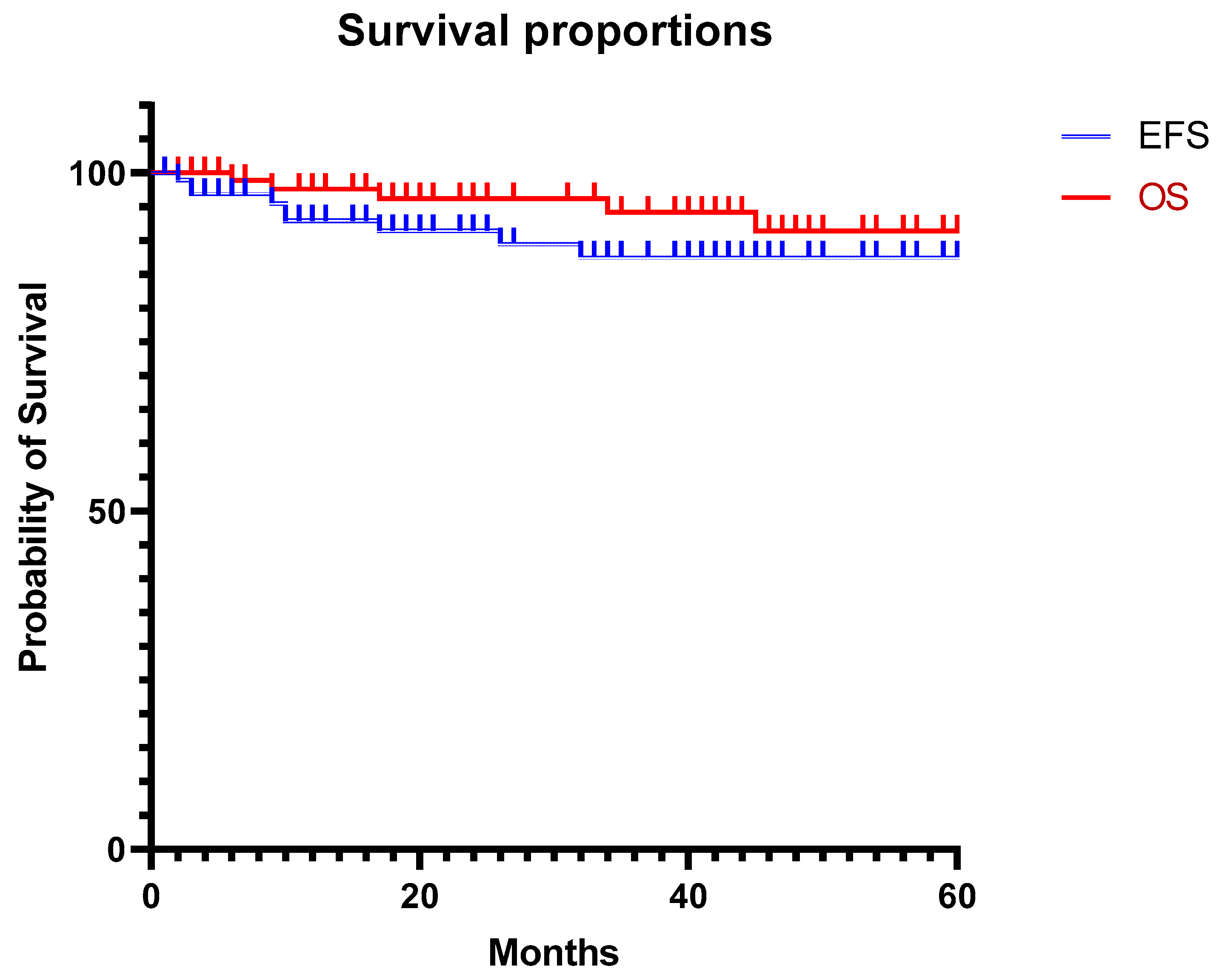
3. Results
3.1. Surgical Procedures
3.2. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suita, S.; Tajiri, T.; Sera, Y.; Takamatsu, H.; Mizote, H.; Ohgami, H.; Kurosaki, N.; Hara, T.; Okamura, J.; Miyazaki, S.; et al. The Characteristics of Mediastinal Neuroblastoma. Eur. J. Pediatr. Surg. 2000, 10, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; La Quaglia, M.P. Advances in the Surgical Treatment of Neuroblastoma: A Review. Eur. J. Pediatr. Surg. 2014, 24, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Decarolis, B.; Simon, T.; Krug, B.; Leuschner, I.; Vokuhl, C.; Kaatsch, P.; von Schweinitz, D.; Klingebiel, T.; Mueller, I.; Schweigerer, L.; et al. Treatment and outcome of Ganglioneuroma and Ganglioneuroblastoma intermixed. BMC Cancer 2016, 16, 542. [Google Scholar]
- Kucukarslan, N.; Kirilmaz, A.; Arslan, Y.; Sanioglu, Y.; Ozal, E.; Tatar, H. Muscle sparing thoracotomy in pediatric age: A comparative study with standard posterolateral thoracotomy. Pediatr. Surg. Int. 2006, 22, 779–783. [Google Scholar] [PubMed]
- Clark, R.A.; Perez, E.A.; Chung, D.H.; Pandya, S.R. Predictive Factors and Outcomes for Successful Thoracoscopic Lung Resection in Pediatric Patients. J. Am. Coll. Surg. 2021, 232, 551–558. [Google Scholar]
- Lam, F.K.; Lau, C.-T.; Yu, M.O.; Wong, K.K. Comparison of thoracoscopy vs. thoracotomy on musculoskeletal outcomes of children with congenital pulmonary airway malformation (CPAM). J. Pediatr. Surg. 2021, 56, 1732–1736. [Google Scholar]
- Lacquet, M.; Moons, J.; Ceulemans, L.J.; De Leyn, P.; Van Raemdonck, D. Surgery for mediastinal neurogenic tumors: A 25-year single-centre retrospective study. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 737–743. [Google Scholar]
- Gabra, H.; Irtan, S.; Cross, K.; Lobos, P.; Froeba-Pohl, A.; Pio, L.; Virgone, C.; Burrieza, G.G.; Villalba, J.G.C.; Riccipetitoni, G.; et al. Minimally invasive surgery for neuroblastic tumours: A SIOPEN multicentre study: Proposal for guidelines. Eur. J. Surg. Oncol. (EJSO) 2021, 48, 283–291. [Google Scholar]
- Lacreuse, I.; Valla, J.S.; de Lagausie, P.; Varlet, F.; Héloury, Y.; Temporal, G.; Bastier, R.; Becmeur, F. Thoracoscopic resection of neurogenic tumors in children. J. Pediatr. Surg. 2007, 42, 1725–1728. [Google Scholar]
- Malek, M.M.; Mollen, K.P.; Kane, T.D.; Shah, S.R.; Irwin, C. Thoracic neuroblastoma: A retrospective review of our institutional experience with comparison of the thoracoscopic and open approaches to resection. J. Pediatr. Surg. 2010, 45, 1622–1626. [Google Scholar] [CrossRef]
- Fraga, J.C.; Aydogdu, B.; Aufieri, R.; Silva, G.V.; Schopf, L.; Takamatu, E.; Brunetto, A.; Kiely, E.; Pierro, A. Surgical Treatment for Pediatric Mediastinal Neurogenic Tumors. Ann. Thorac. Surg. 2010, 90, 413–418. [Google Scholar] [PubMed]
- Irtan, S.; Brisse, H.J.; Minard-Colin, V.; Schleiermacher, G.; Canale, S.; Sarnacki, S. Minimally invasive surgery of neuroblastic tumors in children: Indications depend on anatomical location and image-defined risk factors: MIS in NBL Based on Anatomy and IDRFs. Pediatr. Blood Cancer 2014, 62, 256–261. [Google Scholar]
- Fraga, J.C.; Rothenberg, S.; Kiely, E.; Pierro, A. Video-assisted thoracic surgery resection for pediatric mediastinal neurogenic tumors. J. Pediatr. Surg. 2012, 47, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Petty, J.K.; Bensard, D.D.; Partrick, D.A.; Hendrickson, R.J.; Albano, E.A.; Karrer, F.M. Resection of neurogenic tumors in children: Is thoracoscopy superior to thoracotomy? J. Am. Coll. Surg. 2006, 203, 699–703. [Google Scholar] [CrossRef]
- Kawano, T.; Souzaki, R.; Sumida, W.; Shimojima, N.; Hishiki, T.; Kinoshita, Y.; Uchida, H.; Tajiri, T.; Yoneda, A.; Oue, T.; et al. Current thoracoscopic approach for mediastinal neuroblastoma in Japan–results from nationwide multicenter survey. Pediatr. Surg. Int. 2021, 37, 1651–1658. [Google Scholar] [CrossRef]
- Delforge, X.; De Cambourg, P.; Defachelles, A.; Haffreingue, A.; Rod, J.; Kassite, I.; Chabani, N.; Lauriot-Dit-Prevost, A.; Gourmel, A.; Arnaud, A.; et al. Unresectable thoracic neuroblastic tumors: Changes in image-defined risk factors after chemotherapy and impact on surgical management. Pediatr. Blood Cancer 2021, 68, e29260. [Google Scholar]
- Gurria, J.P.; Malek, M.M.; Heaton, T.E.; Gehred, A.; Lautz, T.B.; Rhee, D.S.; Tracy, E.T.; Grant, C.N.; Baertshiger, R.M.; Bruny, J.; et al. Minimally invasive surgery for abdominal and thoracic neuroblastic tumors: A systematic review by the APSA Cancer committee. J. Pediatr. Surg. 2020, 55, 2260–2272. [Google Scholar] [CrossRef]
- Clark, R.A.; Jacobson, J.C.; Murphy, J.T. Preoperative spinal angiography decreases risk of spinal ischemia in pediatric posterior thoracic tumor resection. Pediatr. Surg. Int. 2022, 38, 1427–1434. [Google Scholar] [CrossRef]
- Leclair, M.-D.; Hartmann, O.; Heloury, Y.; Fourcade, L.; Laprie, A.; Mechinaud, F.; Munzer, C.; Rubie, H. Localized Pelvic Neuroblastoma: Excellent Survival and Low Morbidity with Tailored Therapy—The 10-Year Experience of the French Society of Pediatric Oncology. J. Clin. Oncol. 2004, 22, 1689–1695. [Google Scholar]
- Brisse, H.J.; Blanc, T.; Schleiermacher, G.; Mosseri, V.; Philippe-Chomette, P.; Janoueix-Lerosey, I.; Pierron, G.; Lapouble, E.; Peuchmaur, M.; Fréneaux, P.; et al. Radiogenomics of neuroblastomas: Relationships between imaging phenotypes, tumor genomic profile and survival. PLoS ONE 2017, 12, e0185190. [Google Scholar] [CrossRef]
- Ambros, I.M.; Tonini, G.P.; Pötschger, U.; Gross, N.; Mosseri, V.; Beiske, K.; Berbegall, A.P.; Bénard, J.; Bown, N.; Caron, H.; et al. Age Dependency of the Prognostic Impact of Tumor Genomics in Localized Resectable MYCN-Nonamplified Neuroblastomas. Report From the SIOPEN Biology Group on the LNESG Trials and a COG Validation Group. J. Clin. Oncol. 2020, 38, 3685–3697. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Gou, W.; Cui, B.; Sheng, Y.; Bai, X.; Jin, D.; Lu, Y.; Gou, Y. Analysis of the efficacy of the da Vinci robot in surgery for posterior mediastinal neurogenic tumors. BMC Surg. 2022, 22, 413. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, S.; Coletta, R.; Piccolo, R.L.; Dell’Otto, F.; Voltolini, L.; Gonfiotti, A.; Morabito, A. Uniportal Video-Assisted Thoracic Surgery in a Pediatric Hospital: Early Results and Review of the Literature. J. Laparoendosc. Adv. Surg. Tech. 2022, 32, 713–720. [Google Scholar] [CrossRef] [PubMed]
| Characteristics of the Study Population | Median/n | (Extremes) or (%) |
|---|---|---|
| Age | 4 years | (3 months–17 years) |
| Left location | 58 | (49%) |
| INRG staging | ||
| L1 | 46 | |
| L2 | 56 | |
| M | 12 | |
| MS | 5 | |
| IDRF | 69 | (58%) |
| T9–T12 location as only IDRF | 29 | |
| Extension to the medullary canal | 28 | |
| Encasement of aorta/subclavian artery | 14 | |
| Compression of trachea/bronchus | 6 | |
| Diameter | ||
| At diagnosis | 57.1 mm | (11–150) |
| At surgery | 51.6 mm | (11–123) |
| Histopathology | ||
| Neuroblastoma | 53 | (45%) |
| Ganglioneuroblastoma | 30 | (30%) |
| Ganglioneuroma | 36 | (30%) |
| Tumor biology | ||
| nMyc amplification | 2 | |
| Segmental alteration on CGH | 11 | |
| Numeric alteration on CGH | 13 |
| Patients with preoperative chemotherapy n = 34 | ||
| INRG staging | ||
| L1 | 0 | |
| L2 | 19 | |
| MS | 3 | |
| M | 12 | |
| Pathology | ||
| NBL | 26 | |
| GNBL | 6 | |
| GN | 2 | |
| Pre CT | Post CT | |
| Size | 71 mm (30–50) | 55 mm (11–120) |
| IDRF | 31 | 24 |
| Conversions | n = 14 |
|---|---|
| Vascular attachment | 6 |
| Lack of space | 5 |
| Friable tumor | 2 |
| Need for pulm. resection | 1 |
| Residue | Complication | Conversion | Relapse | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + 14 (11.7%) | − 105 (88.2%) | + 25 (21%) | − 94 (79%) | + 14 (11.7%) | − 105 (88.2%) | + 9 (7.5%) | − 110 (92.4%) | |||
| CGH/nMyc | Fav | 13 | 3 | 9 | 3 | 10 | 2 | 11 | 1 | 12 |
| Unfav | 12 | 3 | 10 | 2 | 10 | 2 | 10 | 5 | 7 | |
| N/A | 94 | p = 0.99 | p = 0.724 | p = 0.930 | p = 0.034 | |||||
| Histology | NB | 53 | 7 | 46 | 9 | 44 | 6 | 47 | 9 | 44 |
| GN/B | 66 | 7 | 59 | 16 | 50 | 8 | 58 | 0 | 66 | |
| p = 0.33 | p = 0.173 | p = 0.903 | p < 0.001 | |||||||
| IDRF * | + | 30 | 8 | 22 | 10 | 20 | 6 | 24 | 4 | 26 |
| − N/A | 88 1 | 6 | 82 p = 0.004 | 15 | 73 p = 0.036 | 8 | 80 p = 0.063 | 5 | 83 p = 0.105 | |
| Pre-op ChemoT | + | 34 | 8 | 26 | 8 | 26 | 7 | 27 | 6 | 28 |
| − | 85 | 6 | 79 p = 0.020 | 17 | 68 p = 0.33 | 7 | 78 p = 0.039 | 3 | 82 p = 0.015 | |
| Post Operative n = 20 | Long Term n = 13 | |
|---|---|---|
| Medical | 4 | |
| Pneumothorax | 4 | |
| Requiring new drainage | 3 | |
| Horner’s syndrome | 4 | 6 |
| Chylothorax | 7 | 1 |
| Requiring surgery | 3 | 0 |
| Harlequin syndrome | 1 | |
| Scoliosis | 4 | |
| Chronic back pain | 3 |
| Vascular or Bronchial IDRF | p | |||
|---|---|---|---|---|
| + 18 (15.1%) | − 101 (84.8%) | |||
| Complication | +15 (12.6%) −104 (87.3%) | 2 16 | 13 88 | p = 0.445 |
| Conversion | +14 (11.7%) −105 (88.2%) | 4 14 | 10 91 | p = 0.087 |
| Residue | +14 (11.7%) −105 (88.2%) | 5 13 | 9 92 | (p = 0.037) |
| Relapse | +9 (7.5%) −110 (92.4%) | 2 16 | 7 94 | p = 0.272 |
| Authors | Type of Study | N | Conversion | Complications | Residue | Recurrence | Remarks |
|---|---|---|---|---|---|---|---|
| Lacreuse et al. 2007 [9] | Multicenter | 21 | 1 (4%) | 6 (28%) | 0 | 0 | Few comments on the outcomes |
| Gabra et al. [8] | Multicenter | 78 | 11 (14%) | NR | 33 (42%) | NR | |
| Malek et al. [10] | Monocentric comparative | 11 | NR | 3 (11%) | NR | 1 (9%) | |
| Fraga et al. [13] | Multicenter | 17 | 0 | 2 (11%) | NR | 0 | |
| Petty et al. [14] | Monocentric comparative | 10 | 0 | 2 (20%) | NR | 1 (10%) | |
| Kawano et al. [15] | Multicenter | 28 | 0 | 1 (5%) | NR | NR | Few outcomes reports |
| Delforge [16] | Multicenter comparative | 9 | NR | NR | NR | NR | Few statistics |
| Present study | Multicenter | 114 | 14 (11.7%) | 25 (21%) | 14 (11.7%) | 9 (7.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecompte, J.F.; Sarnacki, S.; Irtan, S.; Piolat, C.; Scalabre, A.; Talon, I.; Rod, J.; Panait, N.; Rodesch, G.; Luis Huertas, A.L.; et al. Thoracoscopy for Pediatric Thoracic Neurogenic Tumors—A European Multi-Center Study. Cancers 2023, 15, 5467. https://doi.org/10.3390/cancers15225467
Lecompte JF, Sarnacki S, Irtan S, Piolat C, Scalabre A, Talon I, Rod J, Panait N, Rodesch G, Luis Huertas AL, et al. Thoracoscopy for Pediatric Thoracic Neurogenic Tumors—A European Multi-Center Study. Cancers. 2023; 15(22):5467. https://doi.org/10.3390/cancers15225467
Chicago/Turabian StyleLecompte, Jean François, Sabine Sarnacki, Sabine Irtan, Christian Piolat, Aurélien Scalabre, Isabelle Talon, Julien Rod, Nicoleta Panait, Gregory Rodesch, Ana Lourdes Luis Huertas, and et al. 2023. "Thoracoscopy for Pediatric Thoracic Neurogenic Tumors—A European Multi-Center Study" Cancers 15, no. 22: 5467. https://doi.org/10.3390/cancers15225467
APA StyleLecompte, J. F., Sarnacki, S., Irtan, S., Piolat, C., Scalabre, A., Talon, I., Rod, J., Panait, N., Rodesch, G., Luis Huertas, A. L., Abbo, O., Demarche, M., Habonimana, E., Ballouhey, Q., Valteau-Couanet, D., & Guérin, F. (2023). Thoracoscopy for Pediatric Thoracic Neurogenic Tumors—A European Multi-Center Study. Cancers, 15(22), 5467. https://doi.org/10.3390/cancers15225467








