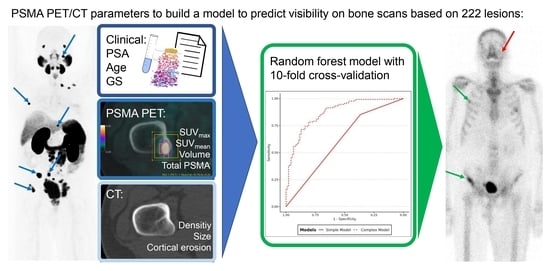
Can We Predict Skeletal Lesion on Bone Scan Based on Quantitative PSMA PET/CT Features?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. PSMA PET/CT and Bone Scan
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
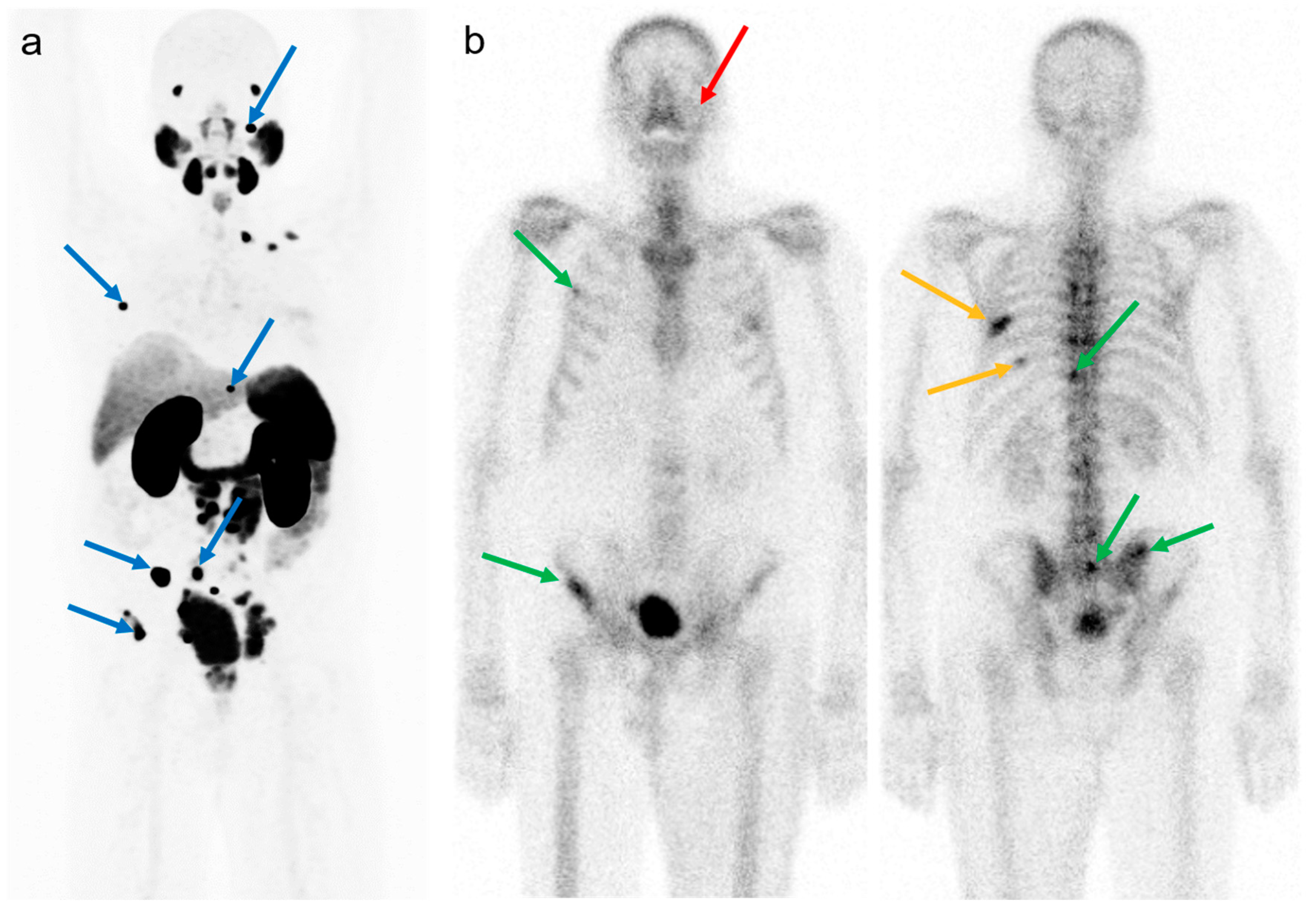
3.1. Visible Versus Non-Visible Lesions on BS
3.1.1. Univariate Analysis
3.1.2. Inference Study
3.1.3. Prediction Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Fassbind, S.; Ferraro, D.A.; Stelmes, J.J.; Fankhauser, C.D.; Guckenberger, M.; Kaufmann, P.A.; Eberli, D.; Burger, I.A.; Kranzbühler, B. 68Ga-PSMA-11 PET imaging in patients with ongoing androgen deprivation therapy for advanced prostate cancer. Ann. Nucl. Med. 2021, 35, 1109–1116. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Fendler, W.P.; Weber, M.; Iravani, A.; Hofman, M.S.; Calais, J.; Czernin, J.; Ilhan, H.; Saad, F.; Small, E.J.; Smith, M.R.; et al. Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 7448–7454. [Google Scholar] [CrossRef]
- Kroeze, S.G.C.; Henkenberens, C.; Schmidt-Hegemann, N.S.; Vogel, M.M.E.; Kirste, S.; Becker, J.; Burger, I.A.; Derlin, T.; Bartenstein, P.; Eiber, M.; et al. Prostate-specific Membrane Antigen Positron Emission Tomography-detected Oligorecurrent Prostate Cancer Treated with Metastases-directed Radiotherapy: Role of Addition and Duration of Androgen Deprivation. Eur. Urol. Focus 2021, 7, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Lengana, T.; Lawal, I.O.; Boshomane, T.G.; Popoola, G.O.; Mokoala, K.M.G.; Moshokoa, E.; Maes, A.; Mokgoro, N.P.; Van de Wiele, C.; Vorster, M.; et al. 68Ga-PSMA PET/CT Replacing Bone Scan in the Initial Staging of Skeletal Metastasis in Prostate Cancer: A Fait Accompli? Clin. Genitourin. Cancer 2018, 16, 392–401. [Google Scholar] [CrossRef]
- Pyka, T.; Okamoto, S.; Dahlbender, M.; Tauber, R.; Retz, M.; Heck, M.; Tamaki, N.; Schwaiger, M.; Maurer, T.; Eiber, M. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Zacho, H.D.; Ravn, S.; Afshar-Oromieh, A.; Fledelius, J.; Ejlersen, J.A.; Petersen, L.J. Added value of 68Ga-PSMA PET/CT for the detection of bone metastases in patients with newly diagnosed prostate cancer and a previous 99mTc bone scintigraphy. EJNMMI Res. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Simsek, D.H.; Sanli, Y.; Civan, C.; Engin, M.N.; Isik, E.G.; Ozkan, Z.G.; Kuyumcu, S. Does bone scintigraphy still have a role in the era of 68 Ga-PSMA PET/CT in prostate cancer? Ann. Nucl. Med. 2020, 34, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Hescheler, D.A.; Stegger, L.; Schlack, K.; Bögemann, M.; Rahbar, K. Discrepancy of PSMA PET imaging and bone scan showing limited value of bone scan and CT in evaluation of extent of bone involvement. Nuklearmedizin 2022, 61, 414–415. [Google Scholar] [CrossRef]
- Moreira, D.M.; Howard, L.E.; Sourbeer, K.N.; Amarasekara, H.S.; Chow, L.C.; Cockrell, D.C.; Hanyok, B.T.; Pratson, C.L.; Aronson, W.J.; Kane, C.J.; et al. Predicting bone scan positivity in non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2015, 18, 333–337. [Google Scholar] [CrossRef]
- Van den Wyngaert, T.; Strobel, K.; Kampen, W.U.; Kuwert, T.; van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; Beheshti, M.; et al. The EANM practice guidelines for bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef]
- Woythal, N.; Arsenic, R.; Kempkensteffen, C.; Miller, K.; Janssen, J.C.; Huang, K.; Makowski, M.R.; Brenner, W.; Prasad, V. Immunohistochemical Validation of PSMA Expression Measured by 68Ga-PSMA PET/CT in Primary Prostate Cancer. J. Nucl. Med. 2018, 59, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Laudicella, R.; Skawran, S.; Ferraro, D.A.; Mühlematter, U.J.; Maurer, A.; Grünig, H.; Rüschoff, H.J.; Rupp, N.; Donati, O.; Eberli, D.; et al. Quantitative imaging parameters to predict the local staging of prostate cancer in intermediate- to high-risk patients. Insights Imaging 2022, 13, 75. [Google Scholar] [CrossRef]
- Muehlematter, U.J.; Schweiger, L.; Ferraro, D.A.; Hermanns, T.; Maurer, T.; Heck, M.M.; Rupp, N.J.; Eiber, M.; Rauscher, I.; Burger, I.A. Development and external validation of a multivariable [68Ga]Ga-PSMA-11 PET-based prediction model for lymph node involvement in men with intermediate or high-risk prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Lim Fat, D.; Kennedy, J.; Galvin, R.; O’Brien, F.; Mc Grath, F.; Mullett, H. The Hounsfield value for cortical bone geometry in the proximal humerus—An in vitro study. Skelet. Radiol. 2012, 41, 557–568. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Voeten, C.C. Buildmer: Stepwise Elimination and Term Reordering for Mixed-Effects Regression. R Package Version 2.3. 2022. Available online: https://CRAN.R-project.org/package=buildmer (accessed on 20 April 2022).
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.46.0. 2022. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 20 April 2022).
- R Core Team. R: A Language and Environment for Statistical Computing 2022; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 20 April 2022).
- Ross, J.S.; Sheehan, C.E.; Fisher, H.A.; Kaufman, R.P., Jr.; Kaur, P.; Gray, K.; Webb, I.; Gray, G.S.; Mosher, R.; Kallakury, B.V. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin. Cancer Res. 2003, 9, 6357–6362. [Google Scholar]
- Laudicella, R.; Rüschoff, J.H.; Ferraro, D.A.; Brada, M.D.; Hausmann, D.; Mebert, I.; Maurer, A.; Hermanns, T.; Eberli, D.; Rupp, N.J.; et al. Infiltrative growth pattern of prostate cancer is associated with lower uptake on PSMA PET and reduced diffusion restriction on mpMRI. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3917–3928. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- Kuten, J.; Fahoum, I.; Savin, Z.; Shamni, O.; Gitstein, G.; Hershkovitz, D.; Mabjeesh, N.J.; Yossepowitch, O.; Mishani, E.; Even-Sapir, E. Head-to-Head Comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in Staging Prostate Cancer Using Histopathology and Immunohistochemical Analysis as a Reference Standard. J. Nucl. Med. 2020, 61, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Grünig, H.; Maurer, A.; Thali, Y.; Kovacs, Z.; Strobel, K.; Burger, I.A.; Müller, J. Focal unspecific bone uptake on [18F]-PSMA-1007 PET: A multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- Vollnberg, B.; Alberts, I.; Genitsch, V.; Rominger, A.; Afshar-Oromieh, A. Assessment of malignancy and PSMA expression of uncertain bone foci in [18F]PSMA-1007 PET/CT for prostate cancer-a single-centre experience of PET-guided biopsies. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3910–3916. [Google Scholar] [CrossRef]
- Arnfield, E.G.; Thomas, P.A.; Roberts, M.J.; Pelecanos, A.M.; Ramsay, S.C.; Lin, C.Y.; Latter, M.J.; Garcia, P.L.; Pattison, D.A. Clinical insignificance of [18F]PSMA-1007 avid non-specific bone lesions: A retrospective evaluation. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4495–4507. [Google Scholar] [CrossRef] [PubMed]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouvière, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Gravis, G.; Boher, J.M.; Joly, F.; Soulié, M.; Albiges, L.; Priou, F.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel Versus ADT Alone in Metastatic Non castrate Prostate Cancer: Impact of Metastatic Burden and Long-term Survival Analysis of the Randomized Phase 3 GETUG-AFU15 Trial. Eur. Urol. 2016, 70, 256–262. [Google Scholar] [CrossRef]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Bauckneht, M.; Checcucci, E.; Cisero, E.; Rizzo, A.; Racca, M.; De Cillis, S.; Amparore, D.; De Luca, S.; Fiori, C.; Rescigno, P.; et al. The prognostic role of next-generation imaging-driven upstaging in newly diagnosed prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2023. ahead of print. [Google Scholar] [CrossRef]
- Barbato, F.; Fendler, W.P.; Rauscher, I.; Herrmann, K.; Wetter, A.; Ferdinandus, J.; Seifert, R.; Nader, M.; Rahbar, K.; Hadaschik, B.; et al. PSMA-PET for the assessment of metastatic hormone-sensitive prostate cancer volume of disease. J. Nucl. Med. 2021, 62, 1747–1750. [Google Scholar] [CrossRef] [PubMed]




| Patients’ Number | 43 |
| [68Ga]Ga-PSMA-11—[18F]PSMA-1007 | 17–26 |
| Mean age at first scan± SD | 73.2 ± 8.5 years |
| Median PSA at first scan (n = 42) | 13.15 ng/mL (0.34–2189) |
| Mean days between PSMA PET and bone scan | 55 ± 49.3 days |
| Stage of disease | |
| Staging | 5/43 (11.5%) |
| HSPCa | 3/43 (7%) |
| EBR | 3/43 (7%) |
| CRPCa | 32/43 (74.5%) |
| Ongoing main therapy | |
| None | 10/43 |
| ADT | 10/43 |
| Enzalutamide | 3/43 |
| Chemotherapy | 2/43 |
| 223Ra | 4/43 |
| Leuprorelin | 2/43 |
| Abiraterone | 6/43 |
| Abiraterone + Leuprorelin | 2/43 |
| ADT + Abiraterone | 1/43 |
| ADT + 223Ra | 1/43 |
| ADT + Enzalutamide | 2/43 |
| Lesion Number (BS+; BS−) | 222 (129;93) |
|---|---|
| Sternum | 7 (3%) (5;2) |
| Skull | 0 (4.5%) (4;6) |
| Rib | 42 (19%) (22;20) |
| Pelvis | 44 (20%) (26;18) |
| Extremities | 50 (22.5%) (35;15) |
| Spine | 69 (31%) (37;32) |
| BS Non-Visible | BS Visible | p | Test | |
|---|---|---|---|---|
| Number of lesions | 93 | 129 | ||
| [68Ga]Ga-PSMA-11 | 43 (46.2%) | 46 (35.7%) | 0.148 | Chi-Squared |
| [18F]PSMA-1007 | 50 (53.8%) | 83 (64.3%) | ||
| PSMA Parameters | ||||
| SUVmax | 7.94 (5.67) | 17.08 (16.19) | <0.001 | t-test |
| SUVmean | 4.60 (2.78) | 8.85 (8.03) | <0.001 | t-test |
| PSMAvol | 1.65 (6.11) | 8.35 (16.44) | <0.001 | t-test |
| PSMAtot | 12.62 (54.05) | 90.94 (258.98) | 0.005 | t-test |
| Max-diameter PET | 2.32 (1.12) | 3.71 (2.13) | <0.001 | t-test |
| CT Parameters | ||||
| Max-diameter CT | 0.93 (1.15) | 2.32 (1.95) | <0.001 | t-test |
| Density (HU) | 440.90 (282.84) | 532.44 (283.05) | 0.018 | t-test |
| No cortical erosion | 90 (96.8) | 115 (89.1) | 0.064 | Chi-Squared |
| With cortical erosion | 3 (3.2) | 14 (10.9) | ||
| Clinical Parameters Age (y) | 74.11 (6.88) | 72.64 (9.86) | 0.217 | t-test |
| PSA (ng/mL) | 216.39 (500.14) | 165.85 (506.89) | 0.457 | t-test |
| PSA change (ng/mL) | 75.89 (282.19) | 29.25 (138.52) | 0.487 | t-test |
| Days between scans | 26.21 (71.05) | 27.18 (65.82) | 0.917 | t-test |
| ISUP 1 | 2 (2.2) | 2 (1.5) | 0.71 | Chi-Squared |
| 2 | 12 (12.9) | 17 (13.2) | ||
| 3 | 3 (3.2) | 10 (7.8) | ||
| 4 | 38 (40.9) | 49 (38.0) | ||
| 5 | 38 (40.9) | 51 (39.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laudicella, R.; Bauckneht, M.; Maurer, A.; Heimer, J.; Gennari, A.G.; Di Raimondo, T.; Paone, G.; Cuzzocrea, M.; Messerli, M.; Eberli, D.; et al. Can We Predict Skeletal Lesion on Bone Scan Based on Quantitative PSMA PET/CT Features? Cancers 2023, 15, 5471. https://doi.org/10.3390/cancers15225471
Laudicella R, Bauckneht M, Maurer A, Heimer J, Gennari AG, Di Raimondo T, Paone G, Cuzzocrea M, Messerli M, Eberli D, et al. Can We Predict Skeletal Lesion on Bone Scan Based on Quantitative PSMA PET/CT Features? Cancers. 2023; 15(22):5471. https://doi.org/10.3390/cancers15225471
Chicago/Turabian StyleLaudicella, Riccardo, Matteo Bauckneht, Alexander Maurer, Jakob Heimer, Antonio G. Gennari, Tania Di Raimondo, Gaetano Paone, Marco Cuzzocrea, Michael Messerli, Daniel Eberli, and et al. 2023. "Can We Predict Skeletal Lesion on Bone Scan Based on Quantitative PSMA PET/CT Features?" Cancers 15, no. 22: 5471. https://doi.org/10.3390/cancers15225471
APA StyleLaudicella, R., Bauckneht, M., Maurer, A., Heimer, J., Gennari, A. G., Di Raimondo, T., Paone, G., Cuzzocrea, M., Messerli, M., Eberli, D., & Burger, I. A. (2023). Can We Predict Skeletal Lesion on Bone Scan Based on Quantitative PSMA PET/CT Features? Cancers, 15(22), 5471. https://doi.org/10.3390/cancers15225471