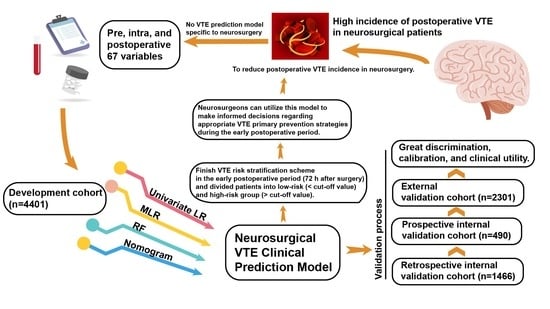
Development and Validation of a Clinical Prediction Model for Venous Thromboembolism Following Neurosurgery: A 6-Year, Multicenter, Retrospective and Prospective Diagnostic Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Defining VTE and Sample Size Considerations
2.3. Collection of Variables
2.4. Statistical Analysis
3. Results
3.1. Entire Retrospective Study Cohort
3.2. Model Development
3.3. Model Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, R.H.; Zhou, H.; Romano, P.S. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb. Haemost. 2003, 90, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, D.; Ariano, C.; Fiacchino, F. Deep vein thrombosis and low-dose heparin prophylaxis in neurosurgical patients. J. Neurosurg. 1978, 49, 378–381. [Google Scholar] [CrossRef]
- Joffe, S.N. Incidence of postoperative deep vein thrombosis in neurosurgical patients. J. Neurosurg. 1975, 42, 201–203. [Google Scholar] [CrossRef]
- Hamidi, S.; Riazi, M. Incidence of venous thromboembolic complications in instrumental spinal surgeries with preoperative chemoprophylaxis. J. Korean Neurosurg. Soc. 2015, 57, 114–118. [Google Scholar] [CrossRef]
- Semrad, T.J.; O’Donnell, R.; Wun, T.; Chew, H.; Harvey, D.; Zhou, H.; White, R.H. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J. Neurosurg. 2007, 106, 601–608. [Google Scholar] [CrossRef]
- Chang, M.T.; Jitaroon, K.; Song, S.; Roozdar, P.; Wangworat, Y.; Ibrahim, N.; Ma, Y.; Rao, V.K.; Chang, S.D.; Fernandez-Miranda, J.C.; et al. Venous thromboembolism rates and risk factors following endoscopic skull base surgery. Int. Forum Allergy Rhinol. 2021, 12, 935–941. [Google Scholar] [CrossRef]
- Spinazzi, E.F.; Pines, M.J.; Fang, C.H.; Raikundalia, M.D.; Baredes, S.; Liu, J.K.; Eloy, J.A. Impact and cost of care of venous thromboembolism following pituitary surgery. Laryngoscope 2015, 125, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Horlander, K.T.; Mannino, D.M.; Leeper, K.V. Pulmonary embolism mortality in the United States, 1979–1998: An analysis using multiple-cause mortality data. Arch. Intern. Med. 2003, 163, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, L.; Brown, D.A.; Bhargav, A.G.; Rusheen, A.E.; Naylor, R.M.; Gilder, H.E.; Monie, D.D.; Youssef, S.J.; Parney, I.F. Venous thromboembolic events in patients undergoing craniotomy for tumor resection: Incidence, predictors, and review of literature. J. Neurosurg. 2019, 132, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Rolston, J.D.; Han, S.J.; Bloch, O.; Parsa, A.T. What clinical factors predict the incidence of deep venous thrombosis and pulmonary embolism in neurosurgical patients? J. Neurosurg. 2014, 121, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Kimmell, K.T.; Jahromi, B.S. Clinical factors associated with venous thromboembolism risk in patients undergoing craniotomy. J. Neurosurg. 2015, 122, 1004–1011. [Google Scholar] [CrossRef]
- Bekelis, K.; Labropoulos, N.; Coy, S. Risk of Venous Thromboembolism and Operative Duration in Patients Undergoing Neurosurgical Procedures. Neurosurgery 2017, 80, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Garcia, D.A.; Wren, S.M.; Karanicolas, P.J.; Arcelus, J.I.; Heit, J.A.; Samama, C.M. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e227S. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, C.J.; Bailey, S.H.; Dreszer, G.; Fisher Wachtman, C.; Zumsteg, J.W.; Jaber, R.M.; Hamill, J.B.; Hume, K.M.; Rubin, J.P.; Neligan, P.C.; et al. Validation of the Caprini risk assessment model in plastic and reconstructive surgery patients. J. Am. Coll. Surg. 2011, 212, 105–112. [Google Scholar] [CrossRef]
- Obi, A.T.; Pannucci, C.J.; Nackashi, A.; Abdullah, N.; Alvarez, R.; Bahl, V.; Wakefield, T.W.; Henke, P.K. Validation of the Caprini Venous Thromboembolism Risk Assessment Model in Critically Ill Surgical Patients. JAMA Surgery 2015, 150, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Caprini, J.A. Thrombosis risk assessment as a guide to quality patient care. Dis. Mon. 2005, 51, 70–78. [Google Scholar] [CrossRef]
- Wells, P.S.; Anderson, D.R.; Bormanis, J.; Guy, F.; Mitchell, M.; Gray, L.; Clement, C.; Robinson, K.S.; Lewandowski, B. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997, 350, 1795–1798. [Google Scholar] [CrossRef]
- Vardi, M.; Ghanem-Zoubi, N.O.; Zidan, R.; Yurin, V.; Bitterman, H. Venous thromboembolism and the utility of the Padua Prediction Score in patients with sepsis admitted to internal medicine departments. J. Thromb. Haemost. 2013, 11, 467–473. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Woller, S.C.; Stevens, S.M.; Jones, J.P.; Lloyd, J.F.; Evans, R.S.; Aston, V.T.; Elliott, C.G. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am. J. Med. 2011, 124, 947–954.e942. [Google Scholar] [CrossRef]
- Collen, J.F.; Jackson, J.L.; Shorr, A.F.; Moores, L.K. Prevention of venous thromboembolism in neurosurgery: A metaanalysis. Chest 2008, 134, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Zhang, Q.; Chen, L.; He, W.; Ma, Z.; Ye, Z.; He, M.; Zhang, Z.; Zhou, X.; Shen, M.; et al. Machine learning prediction of venous thromboembolism after surgeries of major sellar region tumors. Thromb. Res. 2023, 226, 1–8. [Google Scholar] [CrossRef]
- Lim, W.; Le Gal, G.; Bates, S.M.; Righini, M.; Haramati, L.B.; Lang, E.; Kline, J.A.; Chasteen, S.; Snyder, M.; Patel, P.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Diagnosis of venous thromboembolism. Blood Adv. 2018, 2, 3226–3256. [Google Scholar] [CrossRef]
- Wynants, L.; Bouwmeester, W.; Moons, K.G.; Moerbeek, M.; Timmerman, D.; Van Huffel, S.; Van Calster, B.; Vergouwe, Y. A simulation study of sample size demonstrated the importance of the number of events per variable to develop prediction models in clustered data. J. Clin. Epidemiol. 2015, 68, 1406–1414. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E., Jr.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Ogundimu, E.O.; Altman, D.G. Sample size considerations for the external validation of a multivariable prognostic model: A resampling study. Stat. Med. 2016, 35, 214–226. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Kleihues, P.; Louis, D.N.; Scheithauer, B.W.; Rorke, L.B.; Reifenberger, G.; Burger, P.C.; Cavenee, W.K. The WHO classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol. 2002, 61, 215–225; discussion 226–219. [Google Scholar] [CrossRef]
- Pagès, J. Analyse factorielle de données mixtes. Rev. De Stat. Appliquée 2004, 52, 93–111. [Google Scholar]
- Strobl, C.; Boulesteix, A.L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinformatics 2007, 8, 25. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Marras, L.C.; Geerts, W.H.; Perry, J.R. The risk of venous thromboembolism is increased throughout the course of malignant glioma: An evidence-based review. Cancer 2000, 89, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Prell, J.; Schenk, G.; Taute, B.M.; Scheller, C.; Marquart, C.; Strauss, C.; Rampp, S. Reduced risk of venous thromboembolism with the use of intermittent pneumatic compression after craniotomy: A randomized controlled prospective study. J. Neurosurg. 2018, 130, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Portegies, M.L.P.; Koudstaal, P.J.; Ikram, M.A. Chapter 14—Cerebrovascular disease. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 138, pp. 239–261. [Google Scholar]
- Li, J.; Ren, X.; Zhu, X.; Chen, H.; Lin, Z.; Huang, M.; Gu, Z. Clinical Predictive Factors of Lower Extremity Deep Vein Thrombosis in Relative High-Risk Patients after Neurosurgery: A Retrospective Study. Dis. Markers 2020, 2020, 5820749. [Google Scholar] [CrossRef] [PubMed]
- Righini, M.; Le Gal, G.; De Lucia, S.; Roy, P.M.; Meyer, G.; Aujesky, D.; Bounameaux, H.; Perrier, A. Clinical usefulness of D-dimer testing in cancer patients with suspected pulmonary embolism. Thromb. Haemost. 2006, 95, 715–719. [Google Scholar]
- Francalanci, I.; Comeglio, P.; Liotta, A.A.; Cellai, A.P.; Fedi, S.; Parretti, E.; Mello, G.; Prisco, D.; Abbate, R. D-dimer concentrations during normal pregnancy, as measured by ELISA. Thromb. Res. 1995, 78, 399–405. [Google Scholar] [CrossRef]
- Di Nisio, M.; Sohne, M.; Kamphuisen, P.W.; Büller, H.R. D-Dimer test in cancer patients with suspected acute pulmonary embolism. J. Thromb. Haemost. 2005, 3, 1239–1242. [Google Scholar] [CrossRef]
- Bagot, C.N.; Arya, R. Virchow and his triad: A question of attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef]
- Nomani, A.Z.; Nabi, Z.; Rashid, H.; Janjua, J.; Nomani, H.; Majeed, A.; Chaudry, S.R.; Mazhar, A.S. Osmotic nephrosis with mannitol: Review article. Ren. Fail. 2014, 36, 1169–1176. [Google Scholar] [CrossRef]
- Roberts, B.E.; Smith, P.H. Hazards of Mannitol Infusions. Lancet 1966, 288, 421–422. [Google Scholar] [CrossRef]
- Robinson, G.S.; Wiese, W.H. Pulmonary embolism during mannitol therapy. Chest 1980, 77, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Magnus, N.; D’Asti, E.; Garnier, D.; Meehan, B.; Rak, J. Brain neoplasms and coagulation. Semin. Thromb. Hemost. 2013, 39, 881–895. [Google Scholar] [CrossRef]
- Jenkins, E.O.; Schiff, D.; Mackman, N.; Key, N.S. Venous thromboembolism in malignant gliomas. J. Thromb. Haemost. 2010, 8, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.T.; Della Puppa, A.; Ballin, A.; Saggiorato, G.; Bernardi, D.; Padoan, A.; Scienza, R.; d’Avella, D.; Cella, G. Prothrombotic state in glioblastoma multiforme: An evaluation of the procoagulant activity of circulating microparticles. J. Neurooncol. 2011, 104, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.; Ay, C.; Mackman, N.; Bertina, R.M.; Kaider, A.; Marosi, C.; Key, N.S.; Barcel, D.A.; Scheithauer, W.; Kornek, G.; et al. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J. Thromb. Haemost. 2012, 10, 1363–1370. [Google Scholar] [CrossRef]
- Thaler, J.; Preusser, M.; Ay, C.; Kaider, A.; Marosi, C.; Zielinski, C.; Pabinger, I.; Hainfellner, J.A. Intratumoral tissue factor expression and risk of venous thromboembolism in brain tumor patients. Thromb. Res. 2013, 131, 162–165. [Google Scholar] [CrossRef]
- Müller, H.L.; Tauber, M.; Lawson, E.A.; Özyurt, J.; Bison, B.; Martinez-Barbera, J.-P.; Puget, S.; Merchant, T.E.; van Santen, H.M. Hypothalamic syndrome. Nat. Rev. Dis. Primers 2022, 8, 24. [Google Scholar] [CrossRef]
- Eichinger, S.; Hron, G.; Bialonczyk, C.; Hirschl, M.; Minar, E.; Wagner, O.; Heinze, G.; Kyrle, P.A. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch. Intern. Med. 2008, 168, 1678–1683. [Google Scholar] [CrossRef]
- Lidegaard, Ø.; Løkkegaard, E.; Svendsen, A.L.; Agger, C. Hormonal contraception and risk of venous thromboembolism: National follow-up study. BMJ 2009, 339, b2890. [Google Scholar] [CrossRef]
- Chandrakasan, S.; Sood, S.; Ham, S.; Moltz, K.; Frey, M.J.; Rajpurkar, M. Risk factors and management of deep venous thrombosis in children following post-surgical hypopituitarism in craniopharyngioma. Pediatr. Blood Cancer 2011, 57, 175–177. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Carlsson, S.; Harris, A.S. Desmopressin, surgery and thrombosis. Thromb. Haemost. 1994, 71, 154–155. [Google Scholar] [CrossRef] [PubMed]
- McLeod, B.C. Myocardial infarction in a blood donor after administration of desmopressin. Lancet 1990, 336, 1137–1138. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Yasaka, M.; Wakugawa, Y.; Inoue, T.; Ibayashi, S.; Okada, Y. Deep venous thrombosis after acute intracerebral hemorrhage. J. Neurol. Sci. 2008, 272, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Faraoni, D.; Comes, R.F.; Geerts, W.; Wiles, M.D. European guidelines on perioperative venous thromboembolism prophylaxis: Neurosurgery. Eur. J. Anaesthesiol.|EJA 2018, 35, 90–95. [Google Scholar] [CrossRef]
- Anderson, D.R.; Morgano, G.P.; Bennett, C.; Dentali, F.; Francis, C.W.; Garcia, D.A.; Kahn, S.R.; Rahman, M.; Rajasekhar, A.; Rogers, F.B.; et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: Prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019, 3, 3898–3944. [Google Scholar] [CrossRef]
- Miranda, S.; Benhamou, Y.; Wells, P.; Carrier, M. Safety of Primary Thromboprophylaxis Using Apixaban in Ambulatory Cancer Patients with Intracranial Metastatic Disease or Primary Brain Tumors. Thromb. Haemost. 2019, 119, 1886–1887. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wan, Y.D.; Liu, X.Z.; Wang, H.; Jiang, G.Y.; Yang, B. A Single-Center, Randomized, Double-Blind Study of 94 Patients Undergoing Surgery for Cerebral Glioma to Compare Postoperative Thromboprophylaxis with and without Rivaroxaban. Med. Sci. Monit. 2022, 28, e934341. [Google Scholar] [CrossRef] [PubMed]






| Baseline Characteristics and Variables | Development Cohort (n = 4401) † | Retrospective Internal Validation Cohort (n = 1466) † | Prospective Internal Validation Cohort (n = 490) | External Validation Cohort (n = 2301) |
|---|---|---|---|---|
| VTE events | 1167 (26.52%) | 390 (26.60%) | 40 (8.16%) | 104 (4.52%) |
| Preoperative | ||||
| Age (years), mean (SD) | 50.42 (13.85) | 50.08 (14.06) | 48.64 (15.02) | 49.08 (13.33) |
| Sex | ||||
| Male | 2156 (49%) | 753 (51.4%) | 270 (55.7%) | 1064 (46.2%) |
| Female | 2245 (51%) | 713 (48.6%) | 215 (44.3%) | 1237 (53.8%) |
| BMI (kg/m2), mean (SD) | 24.72 (3.91) | 24.62 (3.84) | 25.04 (4.46) | 24.59 (3.76) |
| KPS (score), mean (SD) | 84.96 (18.9) | 84.6 (19.95) | NA | NA |
| ASA | ||||
| 1 or 2 level | 4293 (97.5%) | 1431 (97.6%) | NA | NA |
| 3 or 4 or 5 level | 465 (11.3%) | 182 (13.3%) | NA | NA |
| Preoperative hospital stays (days), mean (SD) | 5.85 (3.84) | 5.78 (3.66) | NA | NA |
| Wheelchair or bedridden | 108 (2.5%) | 35 (2.4%) | NA | NA |
| Medical history | ||||
| Hypertension | 841 (19.1%) | 275 (18.8%) | NA | NA |
| Diabetes | 388 (8.8%) | 118 (8%) | NA | NA |
| Hyperlipidemia | 170 (3.9%) | 60 (4.1%) | NA | NA |
| Nephropathy | 6 (0.1%) | 3 (0.2%) | NA | NA |
| Hepatopathy | 33 (0.7%) | 16 (1.1%) | NA | NA |
| Varicosity | 9 (0.2%) | 4 (0.3%) | NA | NA |
| Preoperative diagnosis | ||||
| Intracranial aneurysm | 158 (3.6%) | 54 (3.7%) | NA | NA |
| Carotid artery stenosis | 66 (1.5%) | 26 (1.8%) | NA | NA |
| Trauma | 35 (0.8%) | 10 (0.7%) | NA | NA |
| Hydrocephalus | 84 (1.9%) | 24 (1.6%) | NA | NA |
| Spinal vascular malformation | 11 (0.2%) | 4 (0.3%) | NA | NA |
| Epilepsy | 27 (0.6%) | 8 (0.5%) | NA | NA |
| Trigeminal neuralgia | 48 (1.1%) | 14 (1%) | NA | NA |
| Hemifacial spasm | 7 (0.2%) | 1 (0.1%) | NA | NA |
| Brain abscess | 39 (0.9%) | 15 (1%) | NA | NA |
| Laboratory test results | ||||
| D-dimer (µg/mL), mean (SD) | 0.66 (1.33) | 0.84 (2.87) | 0.94 (1.62) | 2.89 (3.78) |
| Prothrombin time (s), mean (SD) | 11.46 (1.11) | 11.51 (0.92) | NA | NA |
| APTT (s), mean (SD) | 25.57 (3.22) | 25.41 (3.17) | 27.81 (3.88) | 23.9 (3.53) |
| Thrombin time (s), mean (SD) | 17.75 (1.47) | 17.74 (1.51) | NA | NA |
| Fibrinogen (g/L), mean (SD) | 2.8 (0.87) | 2.82 (0.97) | NA | NA |
| Prothrombin activity (%), mean (SD) | 109.65 (22.52) | 108.64 (21.44) | NA | NA |
| Hemoglobin (g/L), mean (SD) | 133.15 (17.12) | 133.2 (17.11) | NA | NA |
| Platelets (109/L), mean (SD) | 227.11 (65.63) | 224.17 (62.82) | NA | NA |
| White blood cells (109/L), mean (SD) | 7.83 (4.8) | 7.91 (4.73) | NA | NA |
| LDL (mmol/L), mean (SD) | 3.06 (5.45) | 2.94 (0.89) | NA | NA |
| Triglycerides (mmol/L), mean (SD) | 1.62 (1.13) | 1.6 (1.1) | NA | NA |
| Total cholesterol (mmol/L), mean (SD) | 4.67 (1.12) | 4.67 (1.15) | NA | NA |
| Uric acid (μmol/L), mean (SD) | 313.55 (97.9) | 315.79 (101.72) | NA | NA |
| ALT (U/L), mean (SD) | 24.41 (25.04) | 24.07 (21.92) | NA | NA |
| Na (mmol/L), mean (SD) | 139.38 (3.08) | 139.34 (3.22) | NA | NA |
| K (mmol/L), mean (SD) | 3.98 (0.35) | 3.98 (0.36) | NA | NA |
| Cl (mmol/L), mean (SD) | 104.56 (3.54) | 104.48 (3.66) | NA | NA |
| Serum homocysteine (umol/L), mean (SD) | 14.94 (8.37) | 14.96 (8.42) | NA | NA |
| Intraoperative | ||||
| Duration of operation (min), mean (SD) | 263.73 (144.03) | 265.41 (141.15) | 230.22 (120.83) | 202.88 (110.26) |
| Bleeding volume (mL), mean (SD) | 444.49 (573.07) | 449.6 (567.06) | NA | NA |
| Operation position (prone position) | 258 (6.4%) | 86 (6.5%) | NA | NA |
| Operation level | ||||
| 3 level | 519 (11.8%) | 141 (9.6%) | NA | NA |
| 4 level | 3662 (83.5%) | 1243 (84.9%) | NA | NA |
| Anesthesia method (general anesthesia) | 4101 (99.2%) | 1362 (99.3%) | NA | NA |
| Operative site | ||||
| Cerebellar hemisphere | 119 (2.7%) | 38 (2.6%) | NA | NA |
| Lateral ventricle | 21 (0.5%) | 7 (0.5%) | NA | NA |
| Fourth ventricle | 27 (0.6%) | 5 (0.3%) | NA | NA |
| Third ventricle | 15 (0.3%) | 2 (0.1%) | NA | NA |
| Cavernous sinus | 109 (2.5%) | 46 (3.1%) | NA | NA |
| Cranial base | 109 (2.5%) | 46 (3.1%) | NA | NA |
| Intraspinal | 183 (4.2%) | 51 (3.5%) | NA | NA |
| Intramedullary | 116 (2.6%) | 36 (2.5%) | NA | NA |
| Postoperative | ||||
| Highest D-dimer within 72 h ‖, mean (SD) | 3.58 (4.47) | 3.95 (5.35) | 2.63 (3.86) | 3.9 (5.83) |
| Disturbance of consciousness ‡ | 241 (8.8%) | 92 (10%) | 39 (8%) | 21 (0.9%) |
| High dose of mannitol § | 1230 (27.9%) | 424 (28.9%) | 151 (31.1%) | 117 (5.1%) |
| CVC | 1294 (29.4%) | 399 (27.2%) | NA | NA |
| Lumbar cisterna drainage | 481 (10.9%) | 144 (9.8%) | NA | NA |
| Hemiplegia or Paraplegia | 49 (1.1%) | 21 (1.4%) | NA | NA |
| Malignant tumor ¶ | 1421 (34.7%) | 483 (35.6%) | 88 (18.1%) | NA |
| Secondary tumor ¶ | 259 (5.9%) | 97 (6.6%) | 19 (3.9%) | NA |
| Pituitary tumor ¶ | 423 (9.6%) | 135 (9.2%) | NA | NA |
| Germinoma ¶ | 21 (0.5%) | 14 (1%) | NA | NA |
| Acoustic neuromas ¶ | 257 (5.8%) | 100 (6.8%) | NA | NA |
| Craniopharyngioma ¶ | 251 (5.7%) | 71 (4.8%) | 16 (3.3%) | 216 (9.4%) |
| Variables | Univariable LR | MLR | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value * | OR (95% CI) | p-Value * | |
| Preoperative variables | ||||
| Age (years) | 1.047 (1.041–1.052) | <0.001 | 1.04 (1.033–1.047) | <0.001 |
| Sex (Female) | 1.104 (0.965–1.262) | 0.149 | NA | |
| BMI (kg/m2) | 1.006 (0.989–1.024) | 0.468 | NA | |
| KPS (score) | 0.985 (0.982–0.988) | <0.001 | 0.991 (0.987–0.996) | <0.001 |
| ASA (3 or 4 or 5 level) | 2.706 (2.238–3.271) | <0.001 | 1.411 (1.113–1.787) | 0.004 |
| Preoperative hospital stays (days) | 1.041 (1.023–1.059) | <0.001 | NA | |
| Wheelchair or bedridden | 1.91 (1.283–2.842) | 0.001 | 1.06 (0.649–1.712) | 0.814 |
| Medical history | ||||
| Hypertension | 1.699 (1.446–1.996) | <0.001 | 1.221 (1.013–1.469) | 0.036 |
| Diabetes | 1.349 (1.071–1.697) | 0.011 | NA | |
| Hyperlipidemia | 1.152 (0.823–1.612) | 0.411 | NA | |
| Nephropathy | 1.109 (0.215–5.722) | 0.902 | NA | |
| Hepatopathy | 0.923 (0.45–1.894) | 0.827 | NA | |
| Varicosity | 3.361 (1.024–11.034) | 0.046 | 2.768 (0.619–11.567) | 0.162 |
| Preoperative diagnosis | ||||
| Intracranial AVM | 1.414 (1.007–1.986) | 0.046 | 0.83 (0.395–1.685) | 0.614 |
| Intracranial aneurysm | 1.98 (1.442–2.718) | <0.001 | 1.879 (0.971–3.765) | 0.067 |
| Carotid artery stenosis | 0.362 (0.164–0.799) | 0.012 | NA | |
| Trauma | 1.517 (0.748–3.075) | 0.248 | NA | |
| Hydrocephalus | 0.907 (0.545–1.51) | 0.707 | NA | |
| Spinal vascular malformation | 1.188 (0.307–4.602) | 0.803 | NA | |
| Epilepsy | 0.628 (0.237–1.663) | 0.349 | NA | |
| Trigeminal neuralgia | 1.345 (0.724–2.501) | 0.348 | NA | |
| Hemifacial spasm | 0.00 (0.00–1.57 × 10133) | 0.943 | NA | |
| Brain abscess | 2.03 (1.062–3.878) | 0.032 | NA | |
| Laboratory test results | ||||
| D-dimer (µg/mL) | 1.428 (1.332–1.531) | <0.001 | 1.12 (1.044–1.209) | 0.003 |
| Prothrombin time (s) | 1.007 (0.948–1.07) | 0.826 | NA | |
| APTT (s) | 0.919 (0.898–0.94) | <0.001 | 0.945 (0.921–0.969) | <0.001 |
| Thrombin time (s) | 0.982 (0.937–1.029) | 0.439 | NA | |
| Fibrinogen (g/L) | 1.2 (1.116–1.29) | <0.001 | 0.989 (0.904–1.08) | 0.801 |
| Prothrombin activity (%) | 1.001 (0.998–1.004) | 0.415 | NA | |
| Hemoglobin (g/L) | 0.996 (0.992–1) | 0.053 | NA | |
| Platelets (109/L) | 1 (0.999–1.001) | 0.596 | NA | |
| White blood cells (109/L) | 1.025 (1.011–1.04) | 0.001 | 1.012 (0.995–1.029) | 0.165 |
| LDL (mmol/L) | 1.008 (0.995–1.022) | 0.207 | NA | |
| Triglycerides (mmol/L) | 0.941 (0.88–1.007) | 0.078 | NA | |
| Total cholesterol (mmol/L) | 1.114 (1.048–1.185) | 0.001 | 0.992 (0.923–1.065) | 0.822 |
| Uric acid (μmol/L) | 0.998 (0.997–0.998) | <0.001 | NA | |
| ALT (U/L) | 1.002 (0.999–1.005) | 0.211 | NA | |
| Na (mmol/L) | 0.983 (0.962–1.005) | 0.13 | NA | |
| K (mmol/L) | 0.784 (0.645–0.952) | 0.014 | NA | |
| Cl (mmol/L) | 0.981 (0.962–1) | 0.048 | NA | |
| Serum homocysteine (umol/L) | 1.003 (0.995–1.011) | 0.483 | NA | |
| Intraoperative variables | ||||
| Duration of operation (min) | 1.003 (1.002–1.003) | <0.001 | 1.002 (1.002–1.003) | <0.001 |
| Bleeding volume (mL) | 1 (1–1) | <0.001 | 1 (1–1) | 0.343 |
| Operation position (prone position) | 0.682 (0.498–0.935) | 0.017 | NA | |
| The operation level | ||||
| 3 level | 0.83 (0.67–1.03) | 0.035 | NA | |
| 4 level | 1.295 (1.069–1.569) | 0.008 | 1.122 (0.882–1.435) | 0.353 |
| Anesthesia method (general anesthesia) | 3.089 (1.094–8.724) | 0.033 | NA | |
| Operative site | ||||
| Cerebellar hemisphere | 0.541 (0.337–0.868) | 0.011 | NA | |
| Lateral ventricle | 1.214 (0.498–2.958) | 0.67 | NA | |
| Fourth ventricle | 0.691 (0.259–1.847) | 0.462 | NA | |
| Third ventricle | 0.923 (0.297–2.869) | 0.891 | NA | |
| Cavernous sinus | 1.057 (0.7–1.598) | 0.791 | NA | |
| Cranial base | 1.057 (0.7–1.598) | 0.791 | NA | |
| Intraspinal | 0.891 (0.622–1.275) | 0.527 | NA | |
| Intramedullary | 1.204 (0.791–1.833) | 0.387 | NA | |
| Postoperative variables | ||||
| Highest_D_dimer_within_72_hours ‖ | 1.208 (1.184–1.233) | <0.001 | 1.124 (1.101–1.148) | <0.001 |
| Disturbance of consciousness † | 3.363 (2.617–4.321) | <0.001 | 1.619 (1.195–2.192) | 0.002 |
| High dose of mannitol ‡ | 1.447 (1.253–1.67) | <0.001 | 1.79 (1.39–2.30) | <0.001 |
| CVC | 1.346 (1.166–1.554) | <0.001 | 0.969 (0.817–1.146) | 0.712 |
| Lumbar cisterna drainage | 1.472 (1.2–1.806) | <0.001 | NA | |
| Hemiplegia or Paraplegia | 1.351 (0.754–2.421) | 0.312 | NA | |
| Malignant tumor § | 1.287 (1.119–1.48) | <0.001 | NA | |
| Secondary tumor § | 1.042 (0.792–1.371) | 0.769 | NA | |
| Pituitary tumor § | 0.467 (0.353–0.617) | <0.001 | NA | |
| Germinoma § | 0.24 (0.056–1.017) | 0.053 | NA | |
| Acoustic neuromas § | 0.709 (0.522–0.965) | 0.029 | NA | |
| Craniopharyngioma § | 1.948 (1.499–2.531) | <0.001 | 2.348 (1.709–3.219) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Song, D.; Ning, W.; Guo, Y.; Lei, T.; Qu, Y.; Zhang, M.; Gu, C.; Wang, H.; Ji, J.; et al. Development and Validation of a Clinical Prediction Model for Venous Thromboembolism Following Neurosurgery: A 6-Year, Multicenter, Retrospective and Prospective Diagnostic Cohort Study. Cancers 2023, 15, 5483. https://doi.org/10.3390/cancers15225483
Liu D, Song D, Ning W, Guo Y, Lei T, Qu Y, Zhang M, Gu C, Wang H, Ji J, et al. Development and Validation of a Clinical Prediction Model for Venous Thromboembolism Following Neurosurgery: A 6-Year, Multicenter, Retrospective and Prospective Diagnostic Cohort Study. Cancers. 2023; 15(22):5483. https://doi.org/10.3390/cancers15225483
Chicago/Turabian StyleLiu, Deshan, Dixiang Song, Weihai Ning, Yuduo Guo, Ting Lei, Yanming Qu, Mingshan Zhang, Chunyu Gu, Haoran Wang, Junpeng Ji, and et al. 2023. "Development and Validation of a Clinical Prediction Model for Venous Thromboembolism Following Neurosurgery: A 6-Year, Multicenter, Retrospective and Prospective Diagnostic Cohort Study" Cancers 15, no. 22: 5483. https://doi.org/10.3390/cancers15225483
APA StyleLiu, D., Song, D., Ning, W., Guo, Y., Lei, T., Qu, Y., Zhang, M., Gu, C., Wang, H., Ji, J., Wang, Y., Zhao, Y., Qiao, N., & Zhang, H. (2023). Development and Validation of a Clinical Prediction Model for Venous Thromboembolism Following Neurosurgery: A 6-Year, Multicenter, Retrospective and Prospective Diagnostic Cohort Study. Cancers, 15(22), 5483. https://doi.org/10.3390/cancers15225483