Financial Toxicity in Swiss Cancer Patients Treated with Proton Therapy: An Observational Cross-Sectional Study on Self-Reported Outcome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Setting
2.3. Participants
2.4. Outcomes and Independent Variables
2.5. Data Sources/Measurement
2.6. Study Size
2.7. Statistical Analysis
3. Results
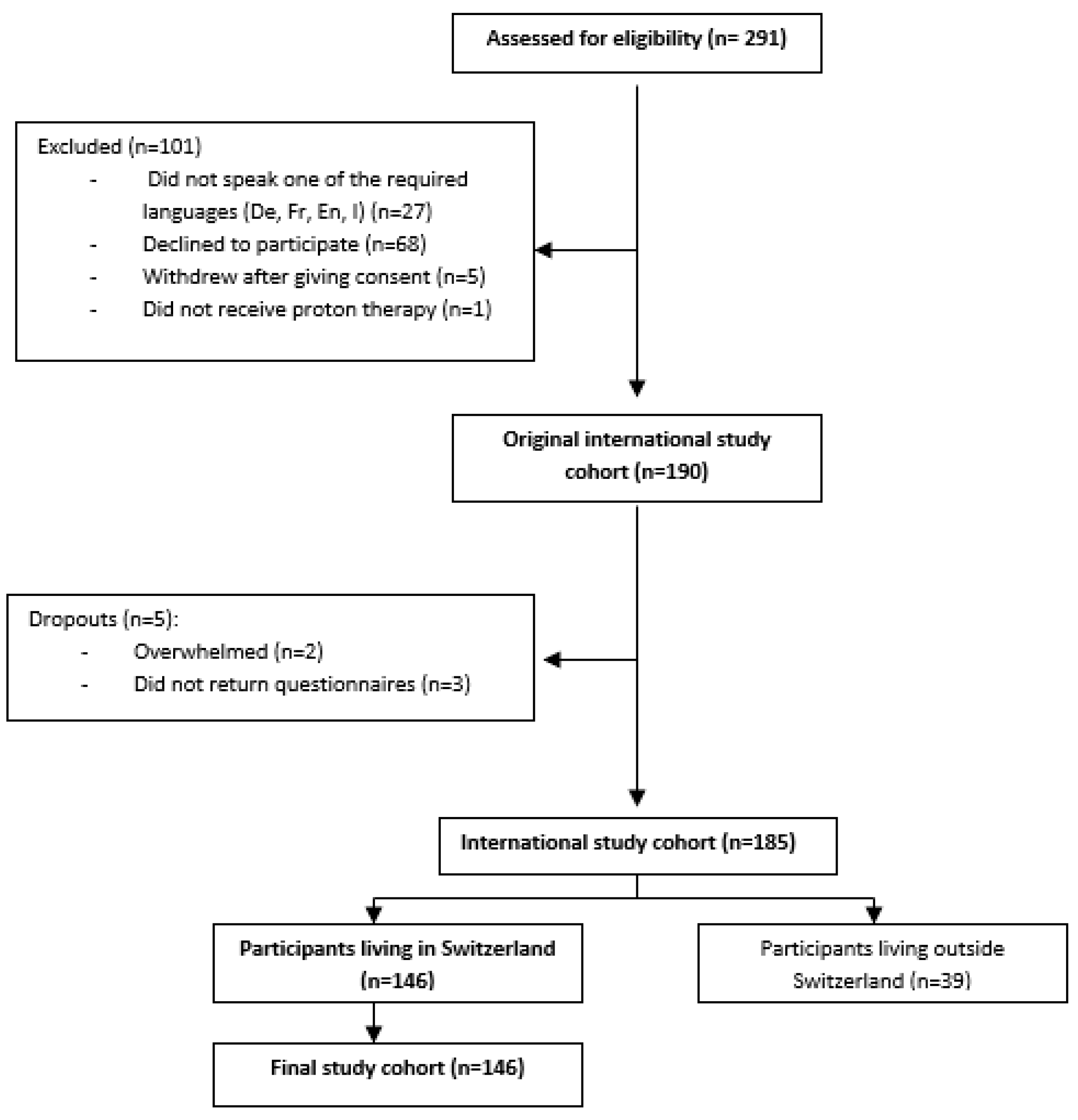
3.1. Participants
3.2. Primary Outcome
3.3. Coping Strategies
3.4. Out-of-Pocket Costs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernard, D.S.; Farr, S.L.; Fang, Z. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. J. Clin. Oncol. 2011, 29, 2821–2826. [Google Scholar] [CrossRef]
- Langa, K.M.; Fendrick, A.M.; Chernew, M.E.; Kabeto, M.U.; Paisley, K.L.; Hayman, J.A. Out-of-pocket health-care expenditures among older Americans with cancer. Value Health 2004, 7, 186–194. [Google Scholar] [CrossRef]
- Zafar, S.Y.; Peppercorn, J.M.; Schrag, D.; Taylor, D.H.; Goetzinger, A.M.; Zhong, X.; Abernethy, A.P. The financial toxicity of cancer treatment: A pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 2013, 18, 381–390. [Google Scholar] [CrossRef]
- Zafar, S.Y. Financial Toxicity of Cancer Care: It’s Time to Intervene. J. Natl. Cancer Inst. 2016, 108, djv370. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Bansal, A.; Fedorenko, C.R.; Blough, D.K.; Overstreet, K.A.; Shankaran, V.; Newcomb, P. Financial Insolvency as a Risk Factor for Early Mortality Among Patients with Cancer. J. Clin. Oncol. 2016, 34, 980–986. [Google Scholar] [CrossRef]
- PDQ Adult Treatment Editorial Board. Financial Toxicity and Cancer Treatment (PDQ(R)): Health Professional Version. In PDQ Cancer Information Summaries; Bethesda: Rockville, ML, USA, 2002. [Google Scholar]
- Jagsi, R.; Ward, K.C.; Abrahamse, P.H.; Wallner, L.P.; Kurian, A.W.; Hamilton, A.S.; Katz, S.J.; Hawley, S.T. Unmet need for clinician engagement regarding financial toxicity after diagnosis of breast cancer. Cancer 2018, 124, 3668–3676. [Google Scholar] [CrossRef]
- de Souza, J.A.; Yap, B.J.; Hlubocky, F.J.; Wroblewski, K.; Ratain, M.J.; Cella, D.; Daugherty, C.K. The development of a financial toxicity patient-reported outcome in cancer: The COST measure. Cancer 2014, 120, 3245–3253. [Google Scholar] [CrossRef]
- de Souza, J.A.; Yap, B.J.; Wroblewski, K.; Blinder, V.; Araujo, F.S.; Hlubocky, F.J.; Nicholas, L.H.; O’Connor, J.M.; Brockstein, B.; Ratain, M.J.; et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer 2017, 123, 476–484. [Google Scholar] [CrossRef]
- Huntington, S.F.; Weiss, B.M.; Vogl, D.T.; Cohen, A.D.; Garfall, A.L.; Mangan, P.A.; Doshi, J.A.; Stadtmauer, E.A. Financial toxicity in insured patients with multiple myeloma: A cross-sectional pilot study. Lancet Haematol. 2015, 2, e408–e416. [Google Scholar] [CrossRef]
- Eremenco, S.L.; Cella, D.; Arnold, B.J. A comprehensive method for the translation and cross-cultural validation of health status questionnaires. Eval. Health Prof. 2005, 28, 212–232. [Google Scholar] [CrossRef]
- Raumgliederung am 01.01.2022, Basierend auf Offiziellem Gemeindestand vom 01.01.2022. Available online: https://www.agvchapp.bfs.admin.ch/de/typologies/results?SnapshotDate=01.01.2022&SelectedTypologies%5B0%5D=HR_GDETYP2012&SelectedTypologies%5B1%5D=HR_SPRGEB2016 (accessed on 20 June 2023).
- Bland, J.M. The tyranny of power: Is there a better way to calculate sample size? BMJ 2009, 339, b3985. [Google Scholar] [CrossRef] [PubMed]
- Haynes, A.; Lenz, A.; Stalder, O.; Limacher, A. presize: An R-package for precision-based sample size calculation in clinical research. J. Open Source Softw. 2021, 6, 3118. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Haushalte. Available online: https://www.bfs.admin.ch/bfs/de/home/statistiken/bevoelkerung/stand-entwicklung/haushalte.html (accessed on 19 December 2022).
- Verteilung des Verfügbaren Äquivalenzeinkommens und das Quintilverhältnis S80/S20, Nach Verschiedenen Soziodemographischen Merkmalen. Available online: https://www.bfs.admin.ch/bfs/de/home/statistiken/wirtschaftliche-soziale-situation-bevoelkerung/soziale-situation-wohlbefinden-und-armut/ungleichheit-der-einkommensverteilung/einkommensverteilung.assetdetail.21084136.html (accessed on 19 December 2022).
- OECD. Out of pocket medical expenditure as a share of final household expenditures, 2014. Gov. Glance 2017, 2017, 229–235. [Google Scholar] [CrossRef]
- Huey, R.W.; George, G.C.; Phillips, P.; White, R.; Fu, S.; Janku, F.; Karp, D.D.; Naing, A.; Piha-Paul, S.; Subbiah, V.; et al. Patient-Reported Out-of-Pocket Costs and Financial Toxicity During Early-Phase Oncology Clinical Trials. Oncologist 2021, 26, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Gardner, U., Jr.; McClelland, S., 3rd; Deville, C., Jr. Disparities in the Utilization of Radiation Therapy for Prostate Cancer in the United States: A Comprehensive Review. Adv. Radiat. Oncol. 2022, 7, 100943. [Google Scholar] [CrossRef]
- Weil, C.R.; Lew, F.H.; Williams, V.M.; Burt, L.M.; Ermoian, R.P.; Poppe, M.M. Patterns of Care and Utilization Disparities in Proton Radiation Therapy for Pediatric Central Nervous System Malignancies. Adv. Radiat. Oncol. 2022, 7, 100868. [Google Scholar] [CrossRef]
- Shen, C.J.; Hu, C.; Ladra, M.M.; Narang, A.K.; Pollack, C.E.; Terezakis, S.A. Socioeconomic factors affect the selection of proton radiation therapy for children. Cancer 2017, 123, 4048–4056. [Google Scholar] [CrossRef]
- Woodhouse, K.D.; Hwang, W.T.; Vapiwala, N.; Jain, A.; Wang, X.; Both, S.; Shah, M.; Frazier, M.; Gabriel, P.; Christodouleas, J.P.; et al. Sociodemographic disparities in the utilization of proton therapy for prostate cancer at an urban academic center. Adv. Radiat. Oncol. 2017, 2, 132–139. [Google Scholar] [CrossRef]
- Aviki, E.M.; Thom, B.; Braxton, K.; Chi, A.J.; Manning-Geist, B.; Chino, F.; Brown, C.L.; Abu-Rustum, N.R.; Gany, F.M. Patient-reported benefit from proposed interventions to reduce financial toxicity during cancer treatment. Support. Care Cancer 2022, 30, 2713–2721. [Google Scholar] [CrossRef]
- Baddour, K.; Fadel, M.; Zhao, M.; Corcoran, M.; Owoc, M.S.; Thomas, T.H.; Sabik, L.M.; Nilsen, M.L.; Ferris, R.L.; Mady, L.J. The cost of cure: Examining objective and subjective financial toxicity in head and neck cancer survivors. Head Neck 2021, 43, 3062–3075. [Google Scholar] [CrossRef]
- Fabian, A.; Domschikowski, J.; Greiner, W.; Bockelmann, G.; Karsten, E.; Ruhle, A.; Nicolay, N.H.; Grosu, A.L.; Dunst, J.; Krug, D. Financial toxicity in cancer patients treated with radiotherapy in Germany-a cross-sectional study. Strahlenther Onkol. 2022, 198, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Offodile, A.C.; Asaad, M.; Boukovalas, S.; Bailey, C.; Lin, Y.L.; Teshome, M.; Greenup, R.A.; Butler, C. Financial Toxicity Following Surgical Treatment for Breast Cancer: A Cross-sectional Pilot Study. Ann. Surg. Oncol. 2021, 28, 2451–2462. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.J.; Fitch, M.I.; Loree, J.M.; Carlson, L.E.; Turner, D.; Cheung, W.Y.; Gopaul, D.; Ellis, J.; Ringash, J.; Mathews, M.; et al. Patient and family financial burden associated with cancer treatment in Canada: A national study. Support. Care Cancer 2021, 29, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.G.; Merollini, K.M.D.; Lowe, A.; Chan, R.J. A Systematic Review of Financial Toxicity Among Cancer Survivors: We Can’t Pay the Co-Pay. Patient 2017, 10, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Gyawali, B.; Ando, M.; Kumanishi, R.; Kato, K.; Sugiyama, K.; Mitani, S.; Masuishi, T.; Narita, Y.; Bando, H.; et al. Prospective Survey of Financial Toxicity Measured by the Comprehensive Score for Financial Toxicity in Japanese Patients With Cancer. J. Glob. Oncol. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Banegas, M.P.; Acquati, C.; Chang, S.; Chino, F.; Conti, R.M.; Greenup, R.A.; Kroll, J.L.; Liang, M.I.; Pisu, M.; et al. Navigating financial toxicity in patients with cancer: A multidisciplinary management approach. CA Cancer J. Clin. 2022, 72, 437–453. [Google Scholar] [CrossRef]

| Demographics | Cohort (n = 146) | Adults (n = 90) | Child Caregivers (n = 56) | |
|---|---|---|---|---|
| Age, years (median, IQR) | 30.5 [11.0; 52.0] | 48.5 [37.3; 61.8] | 9.5 [5.8; 13.0] | |
| Gender | ||||
| 57 (39.0%) | 36 (40.0%) | 21 (37.5%) | |
| 89 (61.0%) | 54 (60.0%) | 35 (62.5%) | |
| Marital status (n, %) | ||||
| 24 (26.7) | |||
| 13 (14.4) | |||
| 47 (52.2) | |||
| 6 (6.7) | |||
| Net household income per year (Swiss francs, CHF) | ||||
| Low (<65 kCHF) | 35(24.0%) | 28 (31.1%) | 7 (12.5%) | |
| Medium (65–<125 kCHF) | 71 (48.6%) | 37 (41.1%) | 34 (60.7%) | |
| High (≥125 kCHF) | 37 (25.3%) | 22 (24.5%) | 15 (26.8%) | |
| not reported | 3 (2.1%) | 3 (3.3%) | 0 (0.0%) | |
| Level of Education | M = maternal P = paternal | |||
| Secondary school | 14 (15.6%) | M: 4 (7.1%) | P: 4 (7.1%) | |
| Vocational training | 43 (47.8%) | M: 26 (46.4%) | P: 29 (51.8%) | |
| High school | 9 (10.0%) | M: 5 (8.9%) | P: 8 (14.3%) | |
| University | 24 (26.7%) | M: 20 (35.7%) | P: 14 (25.0%) | |
| Not reported | 0 (0.0%) | M: 1 (1.8%) | P: 1 (1.8%) | |
| Employment status | M = maternal, P = paternal | |||
| Employed | 53 (58.9%) | M: 39 (69.6%) | P: 45 (80.4%) | |
| Self-reliant | 5 (5.6%) | M: 4 (7.1%) | P: 6 (10.7%) | |
| Not gainfully employed | 8 (8.9%) | M: 9 (16.1%) | P: 0 (0.0%) | |
| Unemployed | 3 (3.4%) | M: 3 (5.4%) | P: 3 (5.4%) | |
| Sickness benefit recipient | 1 (1.1%) | M: 0 (0.0%) | P: 1 (1.8%) | |
| Retired | 20 (22.2%) | M: 0 (0.0%) | F: 0 (0.0%) | |
| Not reported | 0 (0.0%) | M: 1 (1.8%) | F: 1 (1.8%) | |
| Language region | ||||
| German-speaking | 112 (76.7%) | 72 (80.0%) | 40 (71.4%) | |
| French-speaking | 25 (17.1%) | 13 (14.4%) | 12 (21.4%) | |
| Italian-speaking | 9 (6.2%) | 5 (5.6%) | 4 (7.1%) | |
| Place of residence | ||||
| Urban region | 91 (62.3%) | 59 (65.6%) | 32 (57.1%) | |
| Suburban region | 24 (16.4%) | 9 (10.0%) | 15 (26.8%) | |
| Rural region | 31 (21.2%) | 22 (24.4%) | 9 (16.1%) | |
| Distance from home (km) to proton therapy centre, median (IQR) | 83 (52; 153.0) | 83.5 (55.0; 147.5) | 82 (46.5; 187.0) | |
| Diagnosis | ||||
| Primary cancer site | ||||
| 86 (58.9%) | 48 (53.3%) | 38 (67.9%) | |
| 24 (16.4%) | 21 (23.3%) | 3 (5.4%) | |
| 36 (24.7%) | 21 (23.3%) | 15 (26.8%) | |
| Recurrent tumour, n (%) | 25 (17.1%) | 19 (21.1%) | 6 (10.7%) | |
| Time since diagnosis to proton therapy months (IQR) | 2.9 (1.7; 4.8) | 3.0 (1.9; 6.7) | 2.9 (1.5; 4.4) | |
| Treatment | ||||
| Duration of proton therapy (days), median (IQR) | 43 (41; 49) | 44 (42; 51), | 43 (37; 44) | |
| Proton dose Gy (RBE), median (IQR) | 54 (54; 65) | 60 (54;70) | 54 (50.4; 54.7) | |
| Chemotherapy concomitant to proton therapy, n (%) | 29 (19.9%) | 9 (10.0%) | 20 (35.7%) | |
| COST Score | Median | IQR |
|---|---|---|
| Full cohort (n = 146) | 29.9 | 21.0, 36.0 |
| Adults (n = 90) | 30.0 | 21.3, 37.9 |
| Female adults | 29.0 | 21.0, 34.5 |
| Male adults | 32.0 | 22.5, 38.9 |
| Paediatric patients (<18 years) (n = 56) | 28.0 | 20.5, 34.0 |
| Estimate | Lower 95% CI | Upper 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Intercept | 27.98 | 22.8 | 33.2 | <0.0001 | |
| Income | |||||
| Medium vs. low | 2.8 | −1.0 | 6.6 | 0.15 | |
| High vs. low | 8.1 | 3.7 | 12.4 | 0.0003 | |
| Residence | |||||
| Urban vs. rural | 0.7 | −3.2 | 4.6 | 0.72 | |
| Suburban vs. rural | 2.1 | −2.9 | 7.2 | 0.40 | |
| Distance from home to the proton centre (per 100 km) | −3.8 | −5.69 | −1.9 | 0.0002 | |
| Time since diagnosis (months) | 0.1 | −0.2 | 0.5 | 0.44 | |
| Treatment of recurrent tumour or progression vs. first diagnosis | −1.6 | −5.7 | 2.5 | 0.44 |
| Estimate | Lower 95% CI | Upper 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Intercept | 22.5 | 12.08 | 32.93 | <0.0001 | |
| Male vs. female | 3.4 | −1.22 | 8.03 | 0.15 | |
| Relationship status | |||||
| Married vs. single | −9.1 | −14.8 | −3.4 | 0.002 | |
| Permanent partnership vs. single | −9.4 | −16.2 | −2.6 | 0.008 | |
| Educational level | |||||
| Occupational training vs. secondary school | 2.3 | −3.8 | 8.4 | 0.46 | |
| High school vs. secondary school | 4.3 | −4.6 | 13.3 | 0.34 | |
| College, university vs. secondary school | 3.8 | −2.9 | 10.6 | 0.26 | |
| Employment | |||||
| Unemployed vs. employed | −0.0 | −6.7 | 6.6 | 0.99 | |
| Retired vs. employed | −2.0 | −9.5 | 5.6 | 0.60 | |
| Age (in years) | 0.2 | −0.0 | 0.4 | 0.08 |
| Coping Strategies | Whole Cohort (n = 146) | Adult (n= 90) | Caregivers (n = 56) | p-Value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| I/we need to reduce our spending on leisure activities. | 54 | 37.0 | 27 | 30.0 | 27 | 48.2 | 0.041 |
| I/we need to reduce our spending on the basics of life. | 21 | 14.4 | 12 | 13.3 | 9 | 16.1 | 0.83 |
| I/we need to use our savings or part of the savings. | 62 | 42.5 | 33 | 36.7 | 29 | 51.8 | 0.10 |
| I/we need to borrow money. | 14 | 9.6 | 6 | 6.7 | 8 | 14.3 | 0.22 |
| I/we need to sell our property or part of it. | 4 | 2.7 | 3 | 3.3 | 1 | 1.8 | 1 |
| My partner or I need to work more. | 9 | 6.2 | 5 | 5.6 | 4 | 7.1 | 0.73 |
| Estimate | Lower 95% CI | Upper 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Intercept | 34.06 | 31.38 | 36.73 | <0.0001 | |
| Income | |||||
| Medium vs. low | 0.8 | −2. 00 | 3.5 | 0.56 | |
| High vs. low | 0.9 | −2.3 | 4.2 | 0.58 | |
| Coping strategies | |||||
| Spending less on leisure activities | −9.5 | −12.4 | −6.6 | <0.0001 | |
| Spending less on basics | −0.7 | −4.7 | 3.2 | 0.72 | |
| Spending savings | −3.9 | −6.3 | −1.4 | 0.002 | |
| Borrowing money | −6.3 | −10.4 | −2.2 | 0.003 | |
| Selling property | −6.5 | −13.3 | 0.4 | 0.063 | |
| Working more | −5.5 | −10.5 | −0.4 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachtiary, B.; Grawehr, L.; Grillo Ruggieri, F.; Held, U.; Weber, D.C. Financial Toxicity in Swiss Cancer Patients Treated with Proton Therapy: An Observational Cross-Sectional Study on Self-Reported Outcome. Cancers 2023, 15, 5498. https://doi.org/10.3390/cancers15235498
Bachtiary B, Grawehr L, Grillo Ruggieri F, Held U, Weber DC. Financial Toxicity in Swiss Cancer Patients Treated with Proton Therapy: An Observational Cross-Sectional Study on Self-Reported Outcome. Cancers. 2023; 15(23):5498. https://doi.org/10.3390/cancers15235498
Chicago/Turabian StyleBachtiary, Barbara, Leonie Grawehr, Filippo Grillo Ruggieri, Ulrike Held, and Damien C. Weber. 2023. "Financial Toxicity in Swiss Cancer Patients Treated with Proton Therapy: An Observational Cross-Sectional Study on Self-Reported Outcome" Cancers 15, no. 23: 5498. https://doi.org/10.3390/cancers15235498





