Monitoring Metastatic Colorectal Cancer Progression According to Reactive Oxygen Metabolite Derivative Levels
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment and Response Evaluation by CT
2.3. Measurement of d-ROM Levels
2.4. Assessment of d-ROM Levels
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Anticancer Drug Treatment and Effects
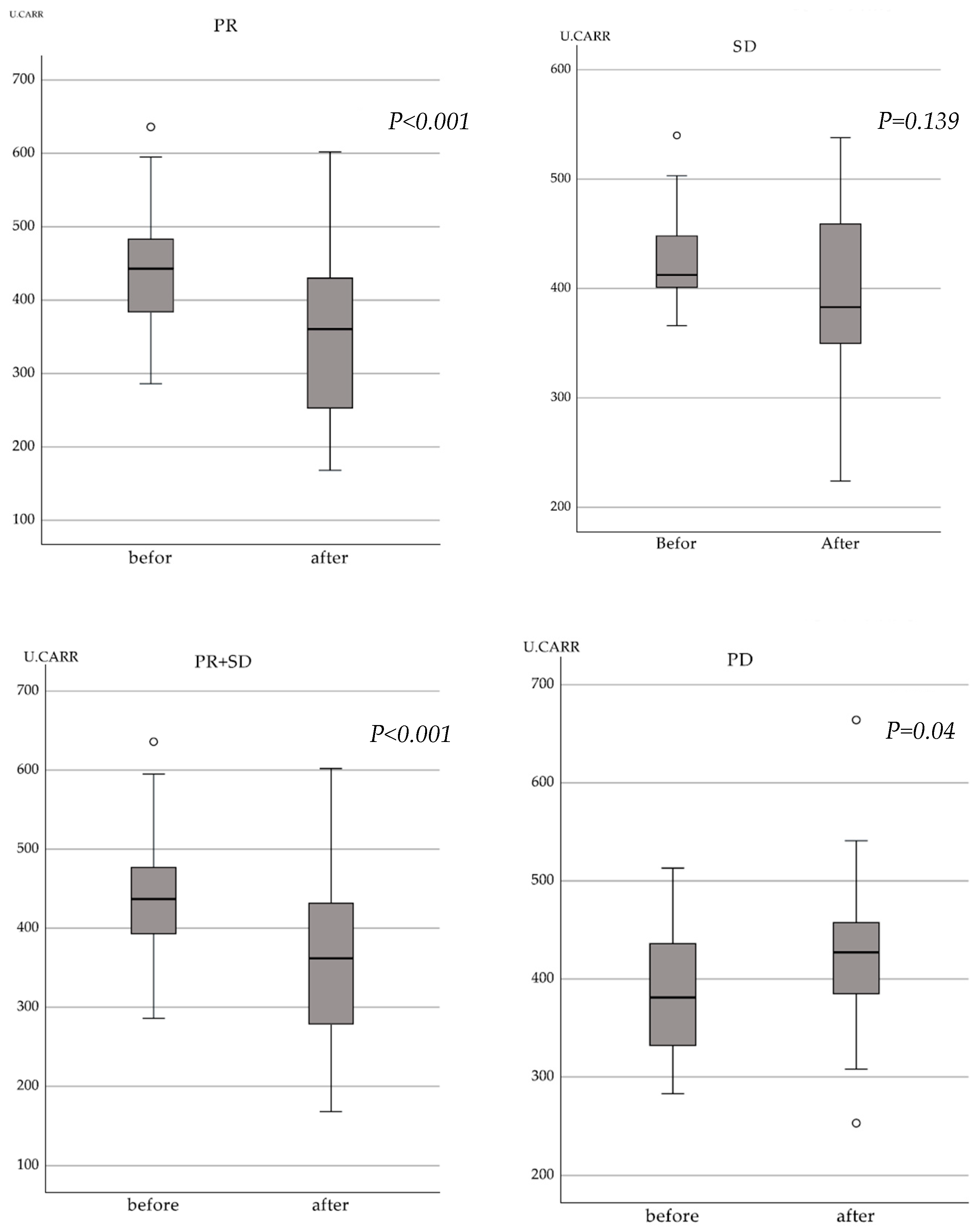
3.3. Relationship between Treatment Response by the RECIST Criteria and Changes in d-ROM Levels
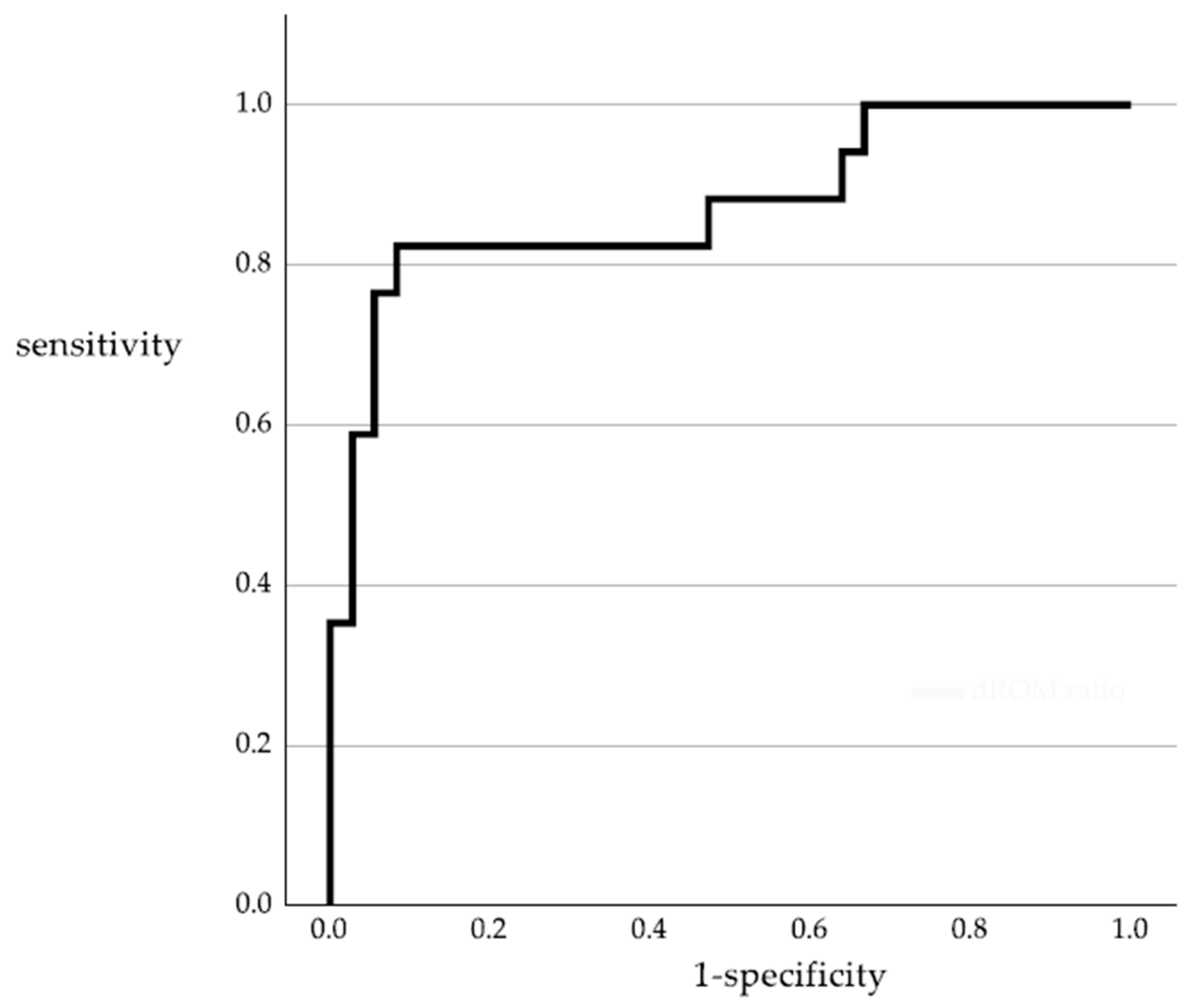
3.4. Accuracy of the d-ROM Ratio in Predicting Disease Progression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- El-Shami, K.; Oeffinger, K.C.; Erb, N.L.; Willis, A.; Bretsch, J.K.; Pratt-Chapman, M.L.; Cannady, R.S.; Wong, S.L.; Rose, J.; Barbour, A.L.; et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J. Clin. 2015, 65, 428–455. [Google Scholar] [CrossRef]
- Meyerhardt, J.A.; Mangu, P.B.; Flynn, P.J.; Korde, L.; Loprinzi, C.L.; Minsky, B.D.; Petrelli, N.J.; Ryan, K.; Schrag, D.H.; Wong, S.L.; et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J. Clin. Oncol. 2013, 31, 4465–4470. [Google Scholar] [CrossRef]
- Duffy, M.J.; van Dalen, A.; Haglund, C.; Hansson, L.; Holinski-Feder, E.; Klapdor, R.; Lamerz, R.; Peltomaki, P.; Sturgeon, C.; Topolcan, O. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur. J. Cancer 2007, 43, 1348–1360. [Google Scholar] [CrossRef]
- Locker, G.Y.; Hamilton, S.; Harris, J.; Jessup, J.M.; Kemeny, N.; Macdonald, J.S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 2006, 24, 5313–5327. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef]
- Kim, G.; Jung, E.J.; Ryu, C.G.; Hwang, D.Y. Usefulness of Carcinoembryonic Antigen for Monitoring Tumor Progression during Palliative Chemotherapy in Metastatic Colorectal Cancer. Yonsei Med. J. 2013, 54, 116–122. [Google Scholar] [CrossRef] [PubMed]
- de Haas, R.J.; Wicherts, D.A.; Flores, E.; Ducreux, M.; Levi, F.; Paule, B.; Azoulay, D.; Castaing, D.; Lemoine, A.; Adam, R. Tumor Marker Evolution: Comparison with Imaging for Assessment of Response to Chemotherapy in Patients with Colorectal Liver Metastases. Ann. Surg. Oncol. 2010, 17, 1010–1023. [Google Scholar] [CrossRef]
- Bardelcíková, A.; Soltys, J.; Mojzis, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Perse, M. Oxidative Stress in the Pathogenesis of Colorectal Cancer: Cause or Consequence? Biomed. Res. Int. 2013, 2013, 725710. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.S.; Mirmiranpour, H.; Rabizadeh, S.; Esteghamati, A.; Tomasello, G.; Alibakhshi, A.; Najafi, N.; Rajab, A.; Nakhjavani, M. Improvement in Redox Homeostasis after Cytoreductive Surgery in Colorectal Adenocarcinoma. Oxid. Med. Cell Longev. 2021, 2021, 8864905. [Google Scholar] [CrossRef]
- Boakye, D.; Jansen, L.; Schottker, B.; Jansen, E.; Schneider, M.; Halama, N.; Gao, X.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Blood markers of oxidative stress are strongly associated with poorer prognosis in colorectal cancer patients. Int. J. Cancer 2020, 147, 2373–2386. [Google Scholar] [CrossRef]
- Inokuma, T.; Haraguchi, M.; Fujita, F.; Tajima, Y.; Kanematsu, T. Oxidative Stress and Tumor Progression in Colorectal Cancer. Hepato Gastroenterol. 2009, 56, 343–347. [Google Scholar]
- Inokuma, T.; Haraguchi, M.; Fujita, F.; Torashima, Y.; Eguchi, S.; Kanematsu, T. Suppression of Reactive Oxygen Species Develops Lymph Node Metastasis in Colorectal Cancer. Hepato Gastroenterol. 2012, 59, 2480–2483. [Google Scholar] [CrossRef]
- Wu, R.; Feng, J.F.; Yang, Y.W.; Dai, C.M.; Lu, A.Y.; Li, J.; Liao, Y.; Xiang, M.; Huang, Q.M.; Wang, D.; et al. Significance of Serum Total Oxidant/Antioxidant Status in Patients with Colorectal Cancer. PLoS ONE 2017, 12, 13. [Google Scholar] [CrossRef]
- Carini, F.; Mazzola, M.; Rappa, F.; Jurjus, A.; Geagea, A.G.; Al Kattar, S.; Bou-Assi, T.; Jurjus, R.; Damiani, P.; Leone, A.; et al. Colorectal Carcinogenesis: Role of Oxidative Stress and Antioxidants. Anticancer. Res. 2017, 37, 4759–4766. [Google Scholar] [CrossRef]
- Kilk, K.; Meitern, R.; Härmson, O.; Soomets, U.; Hõrak, P. Assessment of oxidative stress in serum by d-ROMs test. Free Radic. Res. 2014, 48, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Sawai, K.; Goi, T.; Sakamoto, S.; Matsunaka, T.; Maegawa, N.; Koneri, K. Oxidative stress as a biomarker for predicting the prognosis of patients with colorectal cancer. Oncology 2022, 100, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Trotti, R.; Carratelli, M.; Barbieri, M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Medica 2002, 44, 37–40. [Google Scholar] [PubMed]
- Pasquini, A.; Luchetti, E.; Marchetti, V.; Cardini, G.; Iorio, E.L. Analytical performances of d-ROMs test and BAP test in canine plasma. Definition of the normal range in healthy Labrador dogs. Vet. Res. Commun. 2008, 32, 137–143. [Google Scholar] [CrossRef]
- Yagi, H.; Sumino, H.; Yoshida, K.; Aoki, T.; Tsunekawa, K.; Araki, O.; Kimura, T.; Nara, M.; Nakajima, K.; Murakami, M. Biological Antioxidant Potential Negatively Correlates With Carotid Artery Intima-Media Thickness. Int. Heart J. 2016, 57, 220–225. [Google Scholar] [CrossRef]
- Sugimoto, K.; Sakamoto, K.; Kawai, M.; Kawano, S.; Munakata, S.; Ishiyama, S.; Takahashi, M.; Kojima, Y.; Tomiki, Y. Serum oxidative stress is an independent prognostic marker in colorectal cancer. Transl. Cancer Res. 2019, 8, 1699–1708. [Google Scholar] [CrossRef]
- Wang, D.; Feng, J.F.; Zeng, P.; Yang, Y.H.; Luo, J.; Yang, Y.W. Total oxidant/antioxidant status in sera of patients with thyroid cancers. Endocr. Relat. Cancer 2011, 18, 773–782. [Google Scholar] [CrossRef]
- Feng, J.F.; Lu, L.; Zeng, P.; Yang, Y.H.; Luo, J.; Yang, Y.W.; Wang, D. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int. J. Clin. Oncol. 2012, 17, 575–583. [Google Scholar] [CrossRef]
- Eroglu, M.; Yilmaz, N.; Yalcinkaya, S.; Ay, N.; Aydin, O.; Sezer, C. Enhanced HDL-cholesterol-associated anti-oxidant PON-1 activity in prostate cancer patients. Kaohsiung J. Med. Sci. 2013, 29, 368–373. [Google Scholar] [CrossRef]
- Gào, X.; Wilsgaard, T.; Jansen, E.H.; Holleczek, B.; Zhang, Y.; Xuan, Y.; Anusruti, A.; Brenner, H.; Schöttker, B. Pre-diagnostic derivatives of reactive oxygen metabolites and the occurrence of lung, colorectal, breast and prostate cancer: An individual participant data meta-analysis of two large population-based studies. Int. J. Cancer 2019, 145, 49–57. [Google Scholar] [CrossRef]
- Byström, P.; Berglund, Å.; Nygren, P.; Wernroth, L.; Johansson, B.; Larsson, A.; Glimelius, B. Evaluation of predictive markers for patients with advanced colorectal cancer. Acta Oncol. 2012, 51, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-León, D.; Monzó-Beltrán, L.; Gómez-Abril, S.; Estañ-Capell, N.; Camarasa-Lillo, N.; Pérez-Ebri, M.L.; Escandón-Álvarez, J.; Alonso-Iglesias, E.; Santaolaria-Ayora, M.L.; Carbonell-Moncho, A.; et al. The Effectiveness of Glutathione Redox Status as a Possible Tumor Marker in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6183. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Delimaris, I.; Faviou, E.; Antonakos, G.; Stathopoulou, E.; Zachari, A.; Dionyssiou-Asteriou, A. Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin. Biochem. 2007, 40, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Zabirnyk, O.; Liu, W.; Khalil, S.; Sharma, A.; Phang, J.M. Oxidized low-density lipoproteins upregulate proline oxidase to initiate ROS-dependent autophagy. Carcinogenesis 2010, 31, 446–454. [Google Scholar] [CrossRef]
- Crespo-Sanjuán, J.; Calvo-Nieves, M.D.; Aguirre-Gervás, B.; Herreros-Rodríguez, J.; Velayos-Jiménez, B.; Castro-Alija, M.J.; Muñoz-Moreno, M.F.; Sánchez, D.; Zamora-González, N.; Bajo-Grañeras, R.; et al. Early detection of high oxidative activity in patients with adenomatous intestinal polyps and colorectal adenocarcinoma: Myeloperoxidase and oxidized low-density lipoprotein in serum as new markers of oxidative stress in colorectal cancer. Lab. Med. 2015, 46, 123–135. [Google Scholar] [CrossRef]
- Zettler, M.E.; Prociuk, M.A.; Austria, J.A.; Massaeli, H.; Zhong, G.M.; Pierce, G.N. OxLDL stimulates cell proliferation through a general induction of cell cycle proteins. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H644–H653. [Google Scholar] [CrossRef]
- Bitorina, A.V.; Oligschlaeger, Y.; Shiri-Sverdlov, R.; Theys, J. Low profile high value target: The role of OxLDL in cancer. Biochim. Et. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158518. [Google Scholar] [CrossRef]
- van Heijst, J.W.; Niessen, H.W.; Hoekman, K.; Schalkwijk, C.G. Advanced glycation end products in human cancer tissues: Detection of Nepsilon-(carboxymethyl)lysine and argpyrimidine. Ann. N. Y. Acad. Sci. 2005, 1043, 725–733. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Lopez-Gonzalez, J.S.; Baez-Viveros, J.L.; Aguilar-Cazares, D.; Prado-Garcia, H. Tumor cell metabolism An integral view. Cancer Biol. Ther. 2011, 12, 939–948. [Google Scholar] [CrossRef]
- Sharaf, H.; Matou-Nasri, S.; Wang, Q.; Rabhan, Z.; Al-Eidi, H.; Al Abdulrahman, A.; Ahmed, N. Advanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231. Biochim. Et. Biophys. Acta Mol. Basis Dis. 2015, 1852, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxidative Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed]
- Hristozov, D.; Gadjeva, V.; Vlaykova, T.; Dimitrov, G. Evaluation of oxidative stress in patients with cancer. Arch. Physiol. Biochem. 2001, 109, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Charushila, K.Y.; Subodhini, A.A. Evaluation of Serum Antioxidants during Adjuvant Chemotherapy of Breast Cancer—A Prospective Observational Study. Biochem. Anal. Biochem. 2015, 4, 1. [Google Scholar] [CrossRef]
- Rao, C.S.; Kumari, D.S. Changes in plasma lipid peroxidation and the antioxidant system in women with breast cancer. Int. J. Basic Appl. Sci. 2012, 1, 429–438. [Google Scholar]
- El-Hefny, M.A.; Karimova, S.T.; Afandiev, A.M. Lipid peroxidation and antioxidant status in breast cancer patients before and after therapy. Med. J. Cairo Univ. 2009, 77, 37–42. [Google Scholar]
- Yokoyama, C.; Sueyoshi, Y.; Ema, M.; Mori, Y.; Takaishi, K.; Hisatomi, H. Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncol. Lett. 2017, 14, 6066–6070. [Google Scholar] [CrossRef]
- Wang, W.S.; Lin, J.K.; Lin, T.C.; Chiou, T.J.; Liu, J.H.; Fan, F.S.; Yen, C.C.; Chen, W.S.; Jiang, J.K.; Yang, S.H.; et al. Carcinoembryonic antigen in monitoring of response to systemic chemotherapy in patients with metastatic colorectal cancer. Int. J. Color. Dis. 2001, 16, 96–101. [Google Scholar] [CrossRef]
- Huang, S.C.; Lin, J.K.; Lin, T.C.; Chen, W.S.; Yang, S.H.; Wang, H.S.; Lan, Y.T.; Lin, C.C.; Jiang, J.K.; Chang, S.C. Concordance of Carcinoembryonic Antigen Ratio and Response Evaluation Criteria in Solid Tumors as Prognostic Surrogate Indicators of Metastatic Colorectal Cancer Patients Treated with Chemotherapy. Ann. Surg. Oncol. 2015, 22, 2262–2268. [Google Scholar] [CrossRef]
| Characteristic | Number of Patients (%) |
|---|---|
| Sex (%) | |
| Male/female | 30 (56.6)/23 (43.4) |
| Median age, years (range) | 69 (38–93) |
| Location (%) | |
| Colon/rectum | 30 (56.6)/23 (43.4) |
| Histological type (%) | |
| Well/moderate/poor | 15 (28.3)/30 (56.6)/8 (15.1) |
| Primary tumor status (%) | |
| Resected/not resected | 44 (83.0)/9 (17.0) |
| Metastatic presentation (%) | |
| Metachronous/synchronous | 6 (11.3)/47 (88.7) |
| History of anticancer drug use (%) | |
| No/yes | 37 (69.8)/16 (30.2) |
| Metastatic organs (%) | |
| Single/multiple | 28 (52.8)/25 (47.2) |
| Anticancer Drug | Number of Patients |
|---|---|
| Doublet oxaliplatin-based regimen (targeting agent used) | 33 (23) |
| Doublet irinotecan-based regimen (targeting agent used) | 5 (4) |
| Triplet regimen (targeting agent used) | 4 (3) |
| 5-Fluorouracil-based oral drug (targeting agent used) | 5 (2) |
| Trifluridine/tipiracil hydrochloride (targeting agent) | 2 (2) |
| No treatment | 4 |
| Evaluation by RECIST | Number of Patients (%) |
|---|---|
| Complete response | 0 (0) |
| Partial response | 26 (49.0) |
| Stable disease | 10 (18.9) |
| Progressive disease | 17 (32.1) |
| Total | 53 Cases |
|---|---|
| Levels at the start of treatment (median) | 283–636 (424) |
| Levels after 3 months of treatment (median) | 168–664 (375) |
| d-ROM ratio ≤ 1 | 36 cases |
| (RECIST non-PD: 33 cases; PD: 3 cases) | |
| d-ROM ratio > 1 | 17 cases |
| (RECIST non-PD: 3 cases; PD: 14 cases) |
| Independent Variables | Multivariate Linear Regression | |
|---|---|---|
| β (95% CI) | p-Value | |
| RECIST criteria (Non-PD vs. PD) | 0.416 (0.279–0.555) | <0.001 |
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Diagnostic Accuracy | |
|---|---|---|---|---|---|
| d-ROMs ratio > 1 | 82.4% | 91.7% | 82.4% | 91.7% | 88.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawai, K.; Goi, T.; Kimura, Y.; Koneri, K. Monitoring Metastatic Colorectal Cancer Progression According to Reactive Oxygen Metabolite Derivative Levels. Cancers 2023, 15, 5517. https://doi.org/10.3390/cancers15235517
Sawai K, Goi T, Kimura Y, Koneri K. Monitoring Metastatic Colorectal Cancer Progression According to Reactive Oxygen Metabolite Derivative Levels. Cancers. 2023; 15(23):5517. https://doi.org/10.3390/cancers15235517
Chicago/Turabian StyleSawai, Katsuji, Takanori Goi, Youhei Kimura, and Kenji Koneri. 2023. "Monitoring Metastatic Colorectal Cancer Progression According to Reactive Oxygen Metabolite Derivative Levels" Cancers 15, no. 23: 5517. https://doi.org/10.3390/cancers15235517
APA StyleSawai, K., Goi, T., Kimura, Y., & Koneri, K. (2023). Monitoring Metastatic Colorectal Cancer Progression According to Reactive Oxygen Metabolite Derivative Levels. Cancers, 15(23), 5517. https://doi.org/10.3390/cancers15235517






