Impact of KIF4A on Cancer Stem Cells and EMT in Lung Cancer and Glioma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Sphere-Forming Media Treatment
2.3. siRNA Transfection
2.4. Western Blot
2.5. PCR
2.6. Immunocytochemistry
2.7. Single Cell Assay
2.8. Limited Dilution Assay
2.9. Colony Formation Assay and Irradiation
2.10. Wound Healing Assay
2.11. Invasion and Migration Assays
2.12. Cytokine Array
2.13. Antibodies
2.14. Kaplan–Meier Survival Analysis
2.15. Statistical Analysis
3. Results
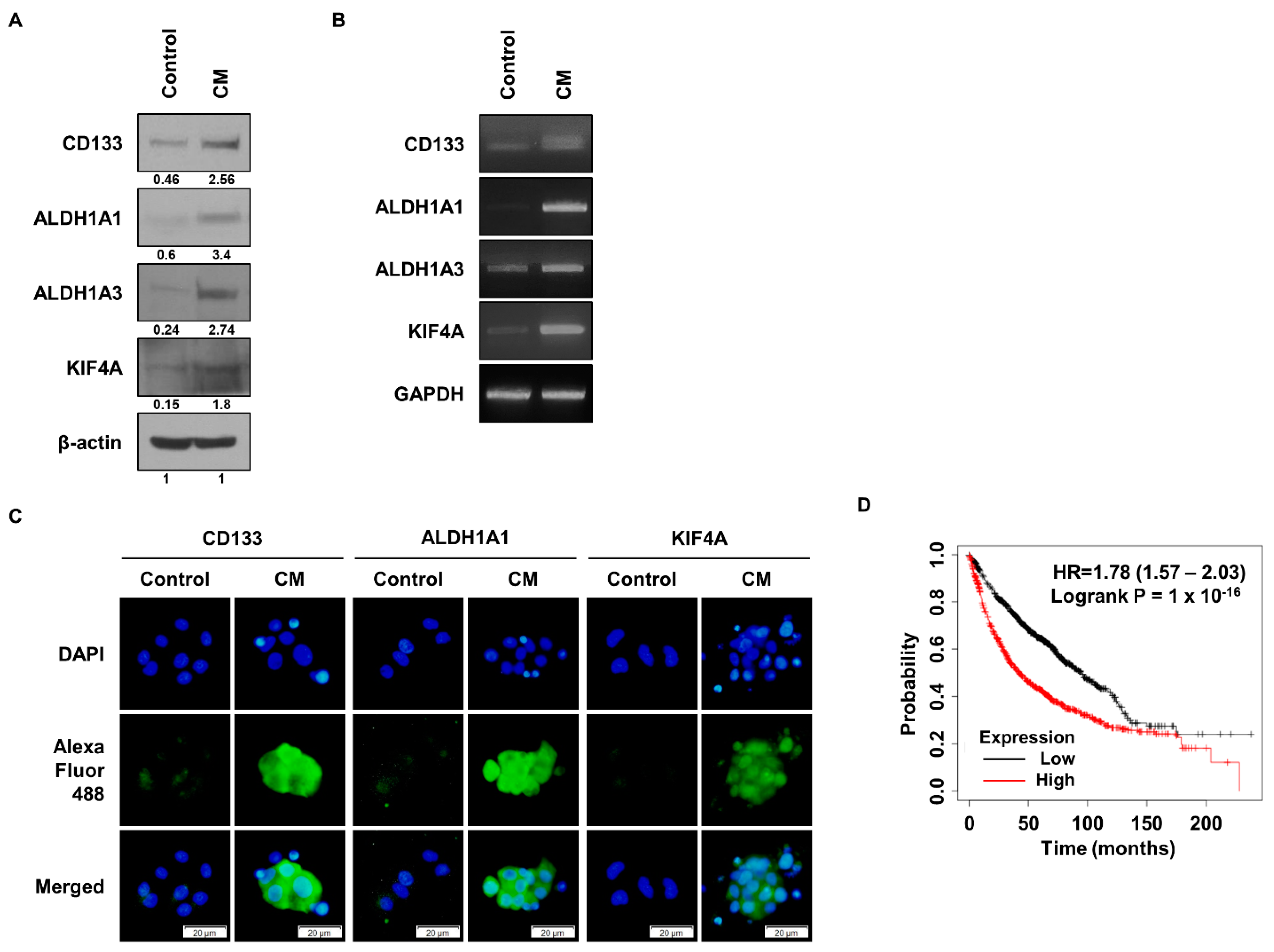
3.1. KIF4A Is Highly Expressed in Malignant Lung Cancer Cells
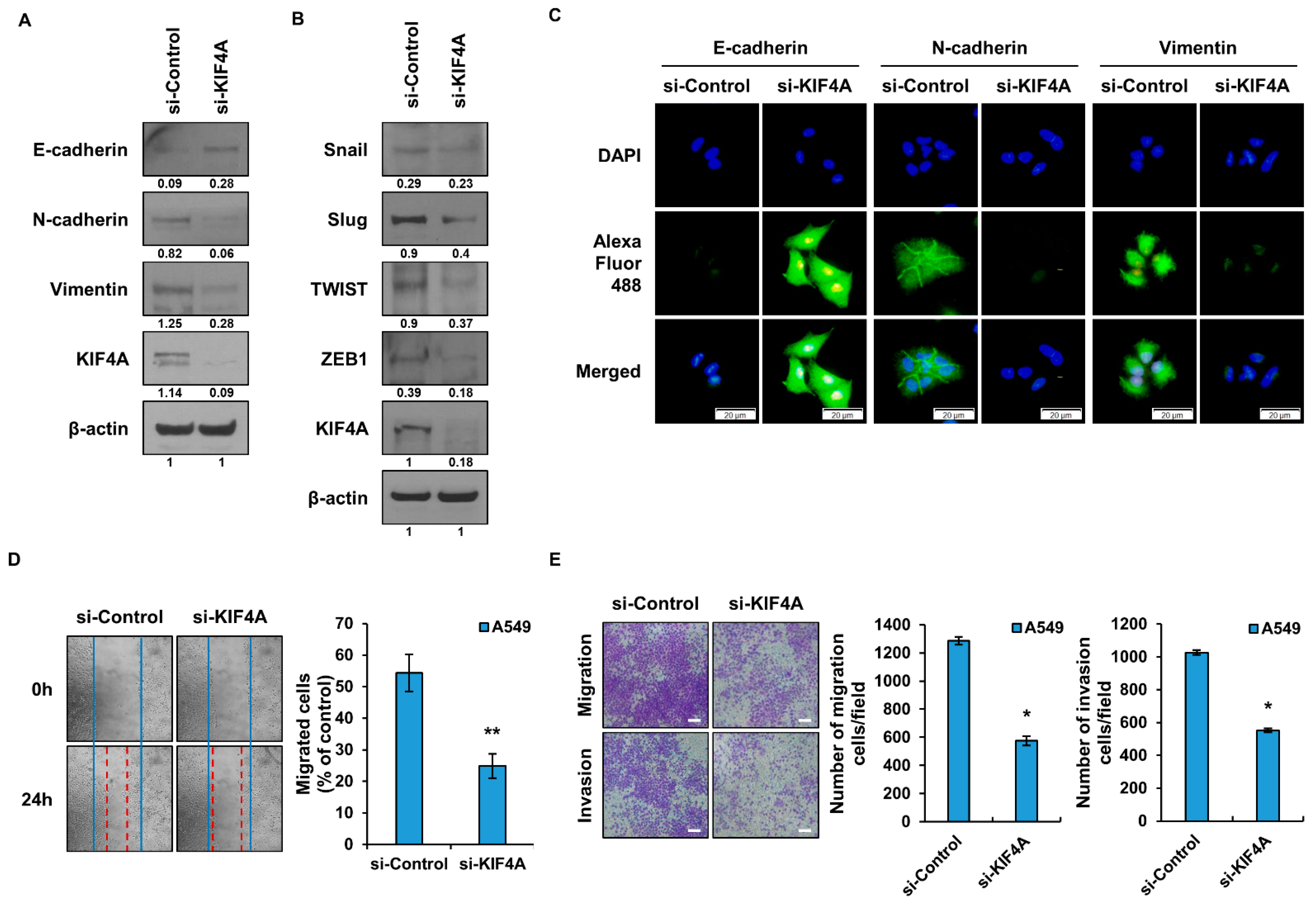
3.2. KIF4A Regulates the Properties of Lung Cancer Stem Cells
3.3. The Epithelial Mesenchymal Transition Is Regulated by KIF4A
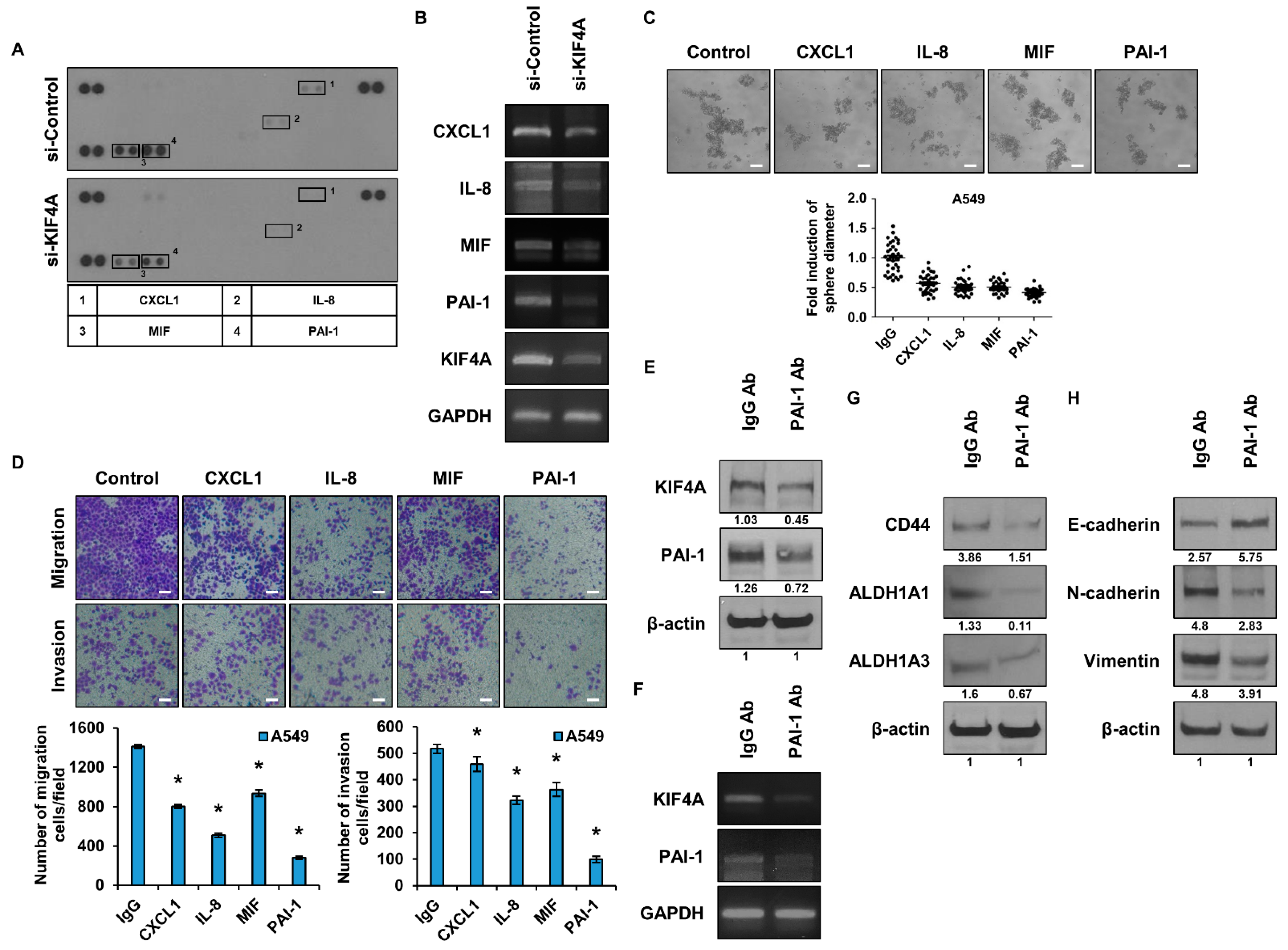
3.4. Identification and Functional Analysis of Cytokines Regulated by KIF4A
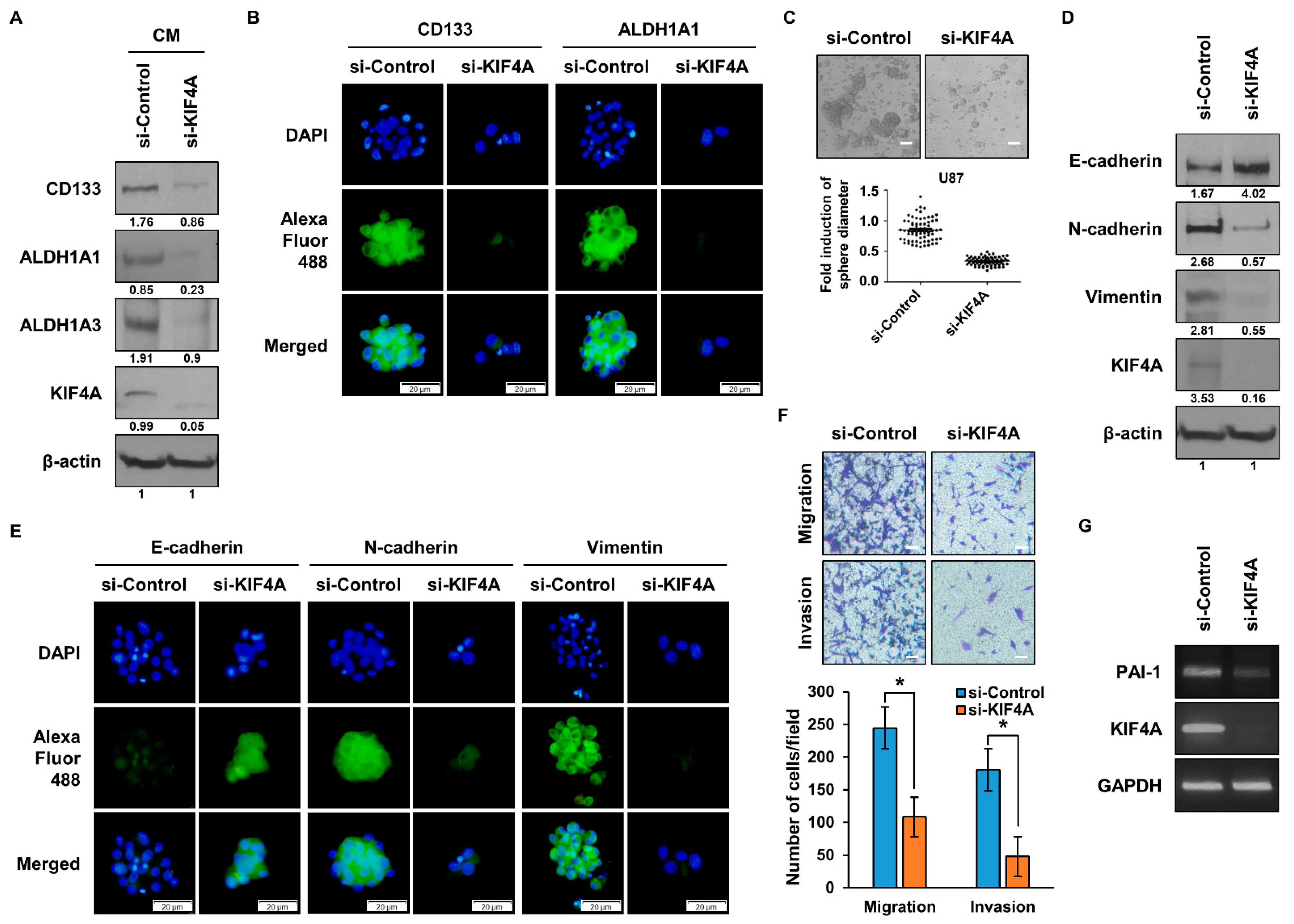
3.5. Functional Analysis of KIF4A in Glioma Stem Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasser, K.A.; Geoffrey, J.M.; Dingcheng, G.; Jeffrey, L.P.; Ashish, S.; Brendon, S.; Timothy, M.G.; Vivek, M. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Krex, D.; Klink, B.; Hartmann, C.; von Deimling, A.; Pietsch, T.; Simon, M.; Sabel, M.; Steinbach, J.P.; Heese, O.; Reifenberger, G.; et al. Long-term survival with glioblastoma multiforme. Brain 2007, 130, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, H.; Liang, Q.; Chen, L.; Ren, J. Radiotherapy for glioblastoma: Clinical issues and nanotechnology strategies. Biomater. Sci. 2022, 10, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Lee, J.H.; Kim, R.K.; Jung, U.; Kahm, Y.J.; Cho, E.W.; Kim, I.G. HSPA1L Enhances Cancer Stem Cell-Like Properties by Activating IGF1Rβ and Regulating β-Catenin Transcription. Int. J. Mol. Sci. 2020, 21, 6957. [Google Scholar] [CrossRef]
- Yan, X.; Ma, L.; Yi, D.; Yoon, J.G.; Diercks, A.; Foltz, G.; Price, N.D.; Hood, L.E.; Tian, Q. A CD133-related gene expression signature identifies an aggressive glioblastoma subtype with excessive mutations. Proc. Natl. Acad. Sci. USA 2011, 108, 1591–1596. [Google Scholar] [CrossRef]
- Geiman, T.M.; Sankpal, U.T.; Robertson, A.K.; Chen, Y.; Mazumdar, M.; Heale, J.T.; Schmiesing, J.A.; Kim, W.; Yokomori, K.; Zhao, Y.; et al. Isolation and characterization of a novel DNA methyltransferase complex linking DNMT3B with components of the mitotic chromosome condensation machinery. Nucleic Acids Res. 2004, 32, 2716–2729. [Google Scholar] [CrossRef]
- Takata, H.; Madung, M.; Katoh, K.; Fukui, K. Cdk1-dependent phosphorylation of KIF4A at S1186 triggers lateral chromosome compaction during early mitosis. PLoS ONE 2018, 13, e0209614. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Q.; Lou, J.; Zou, L.; Wang, Y.; Zhang, P.; Yang, G.; Zhang, J.; Yu, L.; Yan, D.; et al. A novel PHD-finger protein 14/KIF4A complex overexpressed in lung cancer is involved in cell mitosis regulation and tumorigenesis. Oncotarget 2017, 8, 19684–19698. [Google Scholar] [CrossRef] [PubMed]
- Vukušić, K.; Ponjavić, I.; Buđa, R.; Risteski, P.; Tolić, I.M. Microtubule-sliding modules based on kinesins EG5 and PRC1-dependent KIF4A drive human spindle elongation. Dev. Cell 2021, 56, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Setou, M.; Kaneshiro, K.; Hirokawa, N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc. Natl. Acad. Sci. USA 2001, 98, 7004–7011. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tang, W.; Li, H. Identification of KIF4A and its effect on the progression of lung adenocarcinoma based on the bioinformatics analysis. Biosci. Rep. 2021, 41, BSR20203973. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, S.; Chu, K.; Chu, S.; Fan, Y.C.; Bai, J.; Yu, Z.Q. KIF4A drives gliomas growth by transcriptional repression of Rac1/Cdc42 to induce cytoskeletal remodeling in glioma cells. J. Cancer 2022, 13, 3640–3651. [Google Scholar] [CrossRef]
- Hou, P.F.; Jiang, T.; Chen, F.; Shi, P.C.; Li, H.Q.; Bai, J.; Song, J. KIF4A facilitates cell proliferation via induction of p21-mediated cell cycle progression and promotes metastasis in colorectal cancer. Cell Death Dis. 2018, 9, 477. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Qu, D.; Wang, K.; Zhang, L.; Jing, X.; Li, C.; Wei, F.; Qu, X. Kif4A mediate the accumulation and reeducation of THP-1 derived macrophages via regulation of CCL2-CCR2 expression in crosstalking with OSCC. Sci. Rep. 2017, 7, 2226. [Google Scholar] [CrossRef]
- Rouam, S.; Moreau, T.; Broët, P. Identifying common prognostic factors in genomic cancer studies: A novel index for censored outcomes. BMC Bioinform. 2010, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Lee, H.J.; Chang, J.E. Inflammatory Cytokine: An Attractive Target for Cancer Treatment. Biomedicines 2022, 10, 2116. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Bolon, I.; Gazzeri, S.; Veyrenc, S.; Brambilla, C.; Brambilla, E. Expression of plasminogen activator inhibitors 1 and 2 in lung cancer and their role in tumor progression. Clin. Cancer Res. 1999, 5, 2094–2102. [Google Scholar]
- Xi, X.; Liu, N.; Wang, Q.; Chu, Y.; Yin, Z.; Ding, Y.; Lu, Y. ACT001, a novel PAI-1 inhibitor, exerts synergistic effects in combination with cisplatin by inhibiting PI3K/AKT pathway in glioma. Cell Death Dis. 2019, 10, 757. [Google Scholar] [CrossRef]
- Lemaire, R.; Burwell, T.; Sun, H.; Delaney, T.; Bakken, J.; Cheng, L.; Rebelatto, M.C.; Czapiga, M.; de-Mendez, I.; Coyle, A.J.; et al. Resolution of Skin Fibrosis by Neutralization of the Antifibrinolytic Function of Plasminogen Activator Inhibitor 1. Arthritis Rheumatol. 2016, 68, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Meng, L.; Qu, L.; Zhao, C.; Wang, L.; Liu, C.; Shou, C. Anti-PAI-1 Monoclonal Antibody Inhibits the Metastasis and Growth of Esophageal Squamous Cell Carcinoma. J. Cancer 2023, 14, 114–128. [Google Scholar] [CrossRef]
- Wang, D.; Yang, L.Y.; Liu, Z.; Yu, J.; Zhang, M.J.; Zhang, Y.; Cai, Y.; Xu, X.; Hao, J.J.; Wang, M.R. PAI-1 overexpression promotes invasion and migration of esophageal squamous carcinoma cells. Yi Chuan 2020, 42, 287–295. [Google Scholar]
- Isogai, C.; Laug, W.E.; Shimada, H.; Declerck, P.J.; Stins, M.F.; Durden, D.L.; Erdreich-Epstein, A.; DeClerck, Y.A. Plasminogen activator inhibitor-1 promotes angiogenesis by stimulating endothelial cell migration toward fibronectin. Cancer Res. 2001, 61, 5587–5594. [Google Scholar]
- Wei, X.; Li, S.; He, J.; Du, H.; Liu, Y.; Yu, W.; Hu, H.; Han, L.; Wang, C.; Li, H.; et al. Tumor-secreted PAI-1 promotes breast cancer metastasis via the induction of adipocyte-derived collagen remodeling. Cell Commun. Signal. 2019, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Mota de Oliveira, M.; Peterle, G.T.; Monteiro da Silva Couto, C.V.; de Lima Maia, L.; Kühl, A.; Gasparini Dos Santos, J.; Moysés, R.A.; Trivilin, L.O.; Borçoi, A.R.; Archanjo, A.B.; et al. PAI-1 expression in intratumoral inflammatory infiltrate contributes to lymph node metastasis in oral cancer: A cross-sectional study. Ann. Med. Surg. 2021, 65, 102303. [Google Scholar] [CrossRef]
- Tzekaki, E.E.; Geromichalos, G.; Lavrentiadou, S.N.; Tsantarliotou, M.P.; Pantazaki, A.A.; Papaspyropoulos, A. Oleuropein is a natural inhibitor of PAI-1-mediated proliferation in human ER-/PR- breast cancer cells. Breast Cancer Res. Treat. 2021, 186, 305–316. [Google Scholar] [CrossRef]
- Masuda, T.; Nakashima, T.; Namba, M.; Yamaguchi, K.; Sakamoto, S.; Horimasu, Y.; Miyamoto, S.; Iwamoto, H.; Fujitaka, K.; Miyata, Y.; et al. Inhibition of PAI-1 limits chemotherapy resistance in lung cancer through suppressing myofibroblast characteristics of cancer-associated fibroblasts. J. Cell. Mol. Med. 2019, 23, 2984–2994. [Google Scholar] [CrossRef] [PubMed]
- Shioya, S.; Masuda, T.; Senoo, T.; Horimasu, Y.; Miyamoto, S.; Nakashima, T.; Iwamoto, H.; Fujitaka, K.; Hamada, H.; Hattori, N. Plasminogen activator inhibitor-1 serves an important role in radiation-induced pulmonary fibrosis. Exp. Ther. Med. 2018, 16, 3070–3076. [Google Scholar] [CrossRef] [PubMed]
- Kahm, Y.-J.; Jung, U.; Kim, R.-K. Regulation of Cancer Stem Cells and Epithelial-Mesenchymal Transition by CTNNAL1 in Lung Cancer and Glioblastoma. Biomedicines 2023, 11, 1462. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Primer Sequence (5′ to 3′) | °C | Cycle |
|---|---|---|---|
| ALDH1A1 | F: TTAGCAGGCTGCATCAAAAC | 56 | 34 |
| R: GCACTGGTCCAAAAATCTCC | |||
| ALDH1A3 | F: ACCTGGAGGGCTGTATTAGA | 57.5 | 34 |
| R: GGTTGAAGAACACTCCCTGA | |||
| CD133 | F: CATGGCCCATCGCACT | 55 | 34 |
| R: TCTCAAAGTATCTGG | |||
| CXCL1 | F: ATGGCCCGCGCTGCTCTCTC | 56 | 34 |
| R: TCAGTTGGATTTGTCACTGTTC | |||
| GAPDH | F: AGTCAACGGATTTGGTCGTA | 56 | 34 |
| R: GTCATGAGTCCTTCCACGAT | |||
| IL-8 | F: ATGGCTGCTCAAGGCTGGTC | 56 | 34 |
| R: AGGCTTTTCATGCTCAACACTAT | |||
| KIF4A | F: GAATAAAGCGCTTGCACTGA | 55.5 | 34 |
| R: ACCACGCACTTCAGTAAGG | |||
| MIF | F: GCGCGTGCGTCTGTGCC | 56.5 | 34 |
| R: GACCACGTGCACCGCGATGTA | |||
| PAI-1 | F: CAGGCGGACTTCTCCAGTT | 56.5 | 34 |
| R: CATTCGGGCTGAGACTACAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahm, Y.-J.; Kim, I.-G.; Jung, U.; Lee, J.H.; Kim, R.-K. Impact of KIF4A on Cancer Stem Cells and EMT in Lung Cancer and Glioma. Cancers 2023, 15, 5523. https://doi.org/10.3390/cancers15235523
Kahm Y-J, Kim I-G, Jung U, Lee JH, Kim R-K. Impact of KIF4A on Cancer Stem Cells and EMT in Lung Cancer and Glioma. Cancers. 2023; 15(23):5523. https://doi.org/10.3390/cancers15235523
Chicago/Turabian StyleKahm, Yeon-Jee, In-Gyu Kim, Uhee Jung, Jei Ha Lee, and Rae-Kwon Kim. 2023. "Impact of KIF4A on Cancer Stem Cells and EMT in Lung Cancer and Glioma" Cancers 15, no. 23: 5523. https://doi.org/10.3390/cancers15235523
APA StyleKahm, Y.-J., Kim, I.-G., Jung, U., Lee, J. H., & Kim, R.-K. (2023). Impact of KIF4A on Cancer Stem Cells and EMT in Lung Cancer and Glioma. Cancers, 15(23), 5523. https://doi.org/10.3390/cancers15235523







