Adaptation Time as a Determinant of the Dosimetric Effectiveness of Online Adaptive Radiotherapy for Bladder Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Online Adaptive Radiotherapy
2.2. Contouring
2.3. Modelling of the Bladder and CTV Volume at Different Time Points
2.4. Equivalent Uniform Dose
2.5. Dose Accumulation
2.6. Dependence of the EUD on Margin around CTV
2.7. Statistics
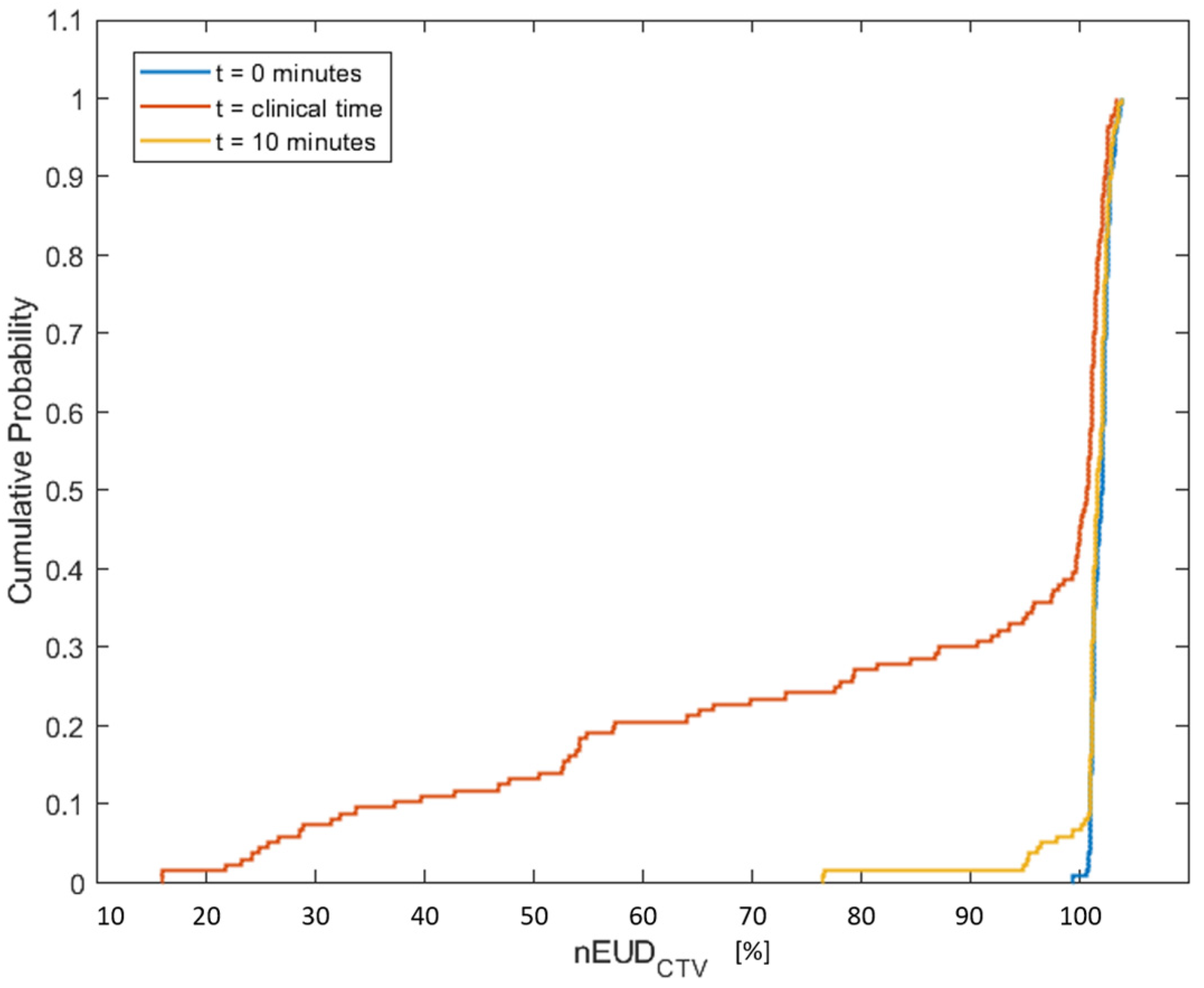
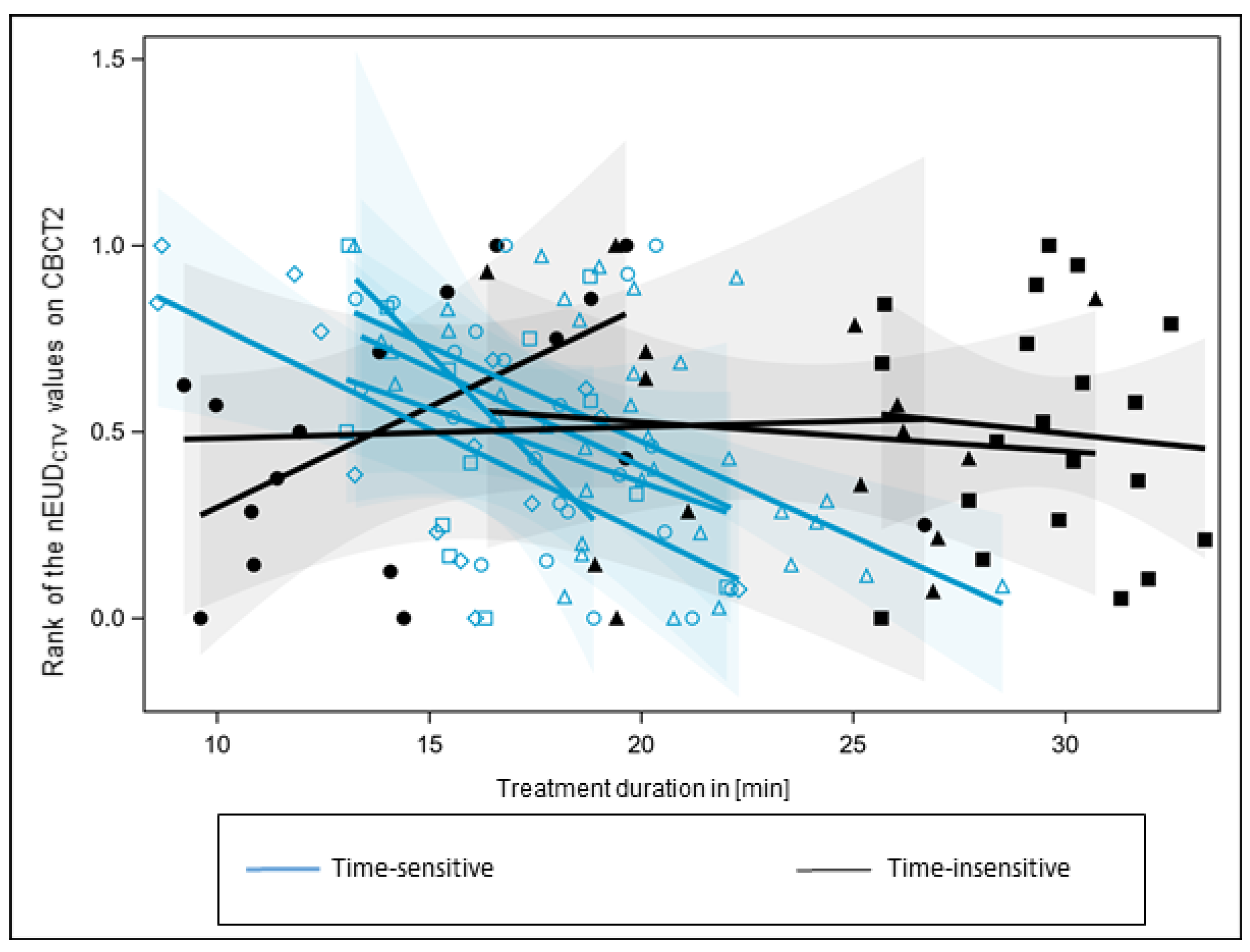
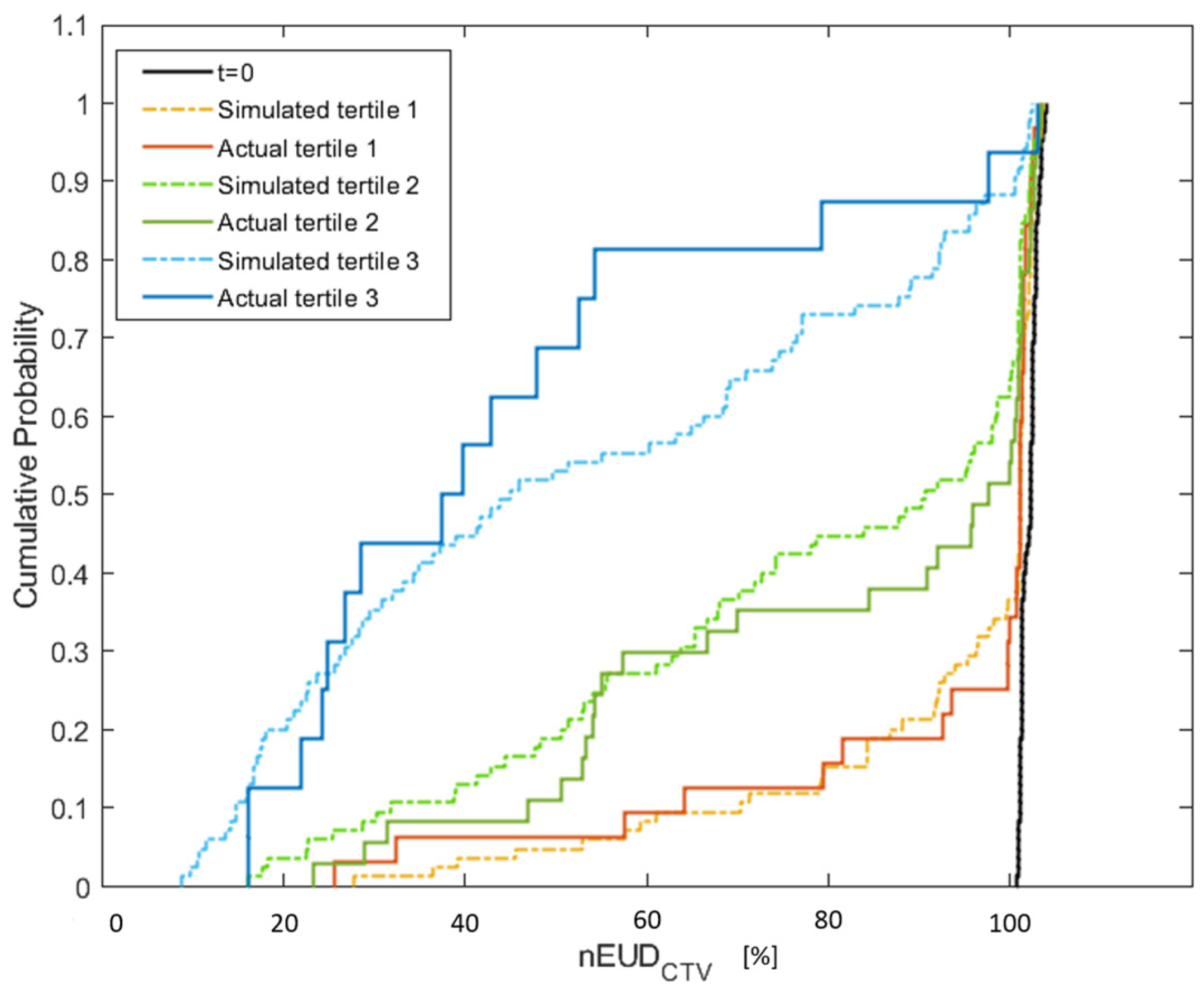
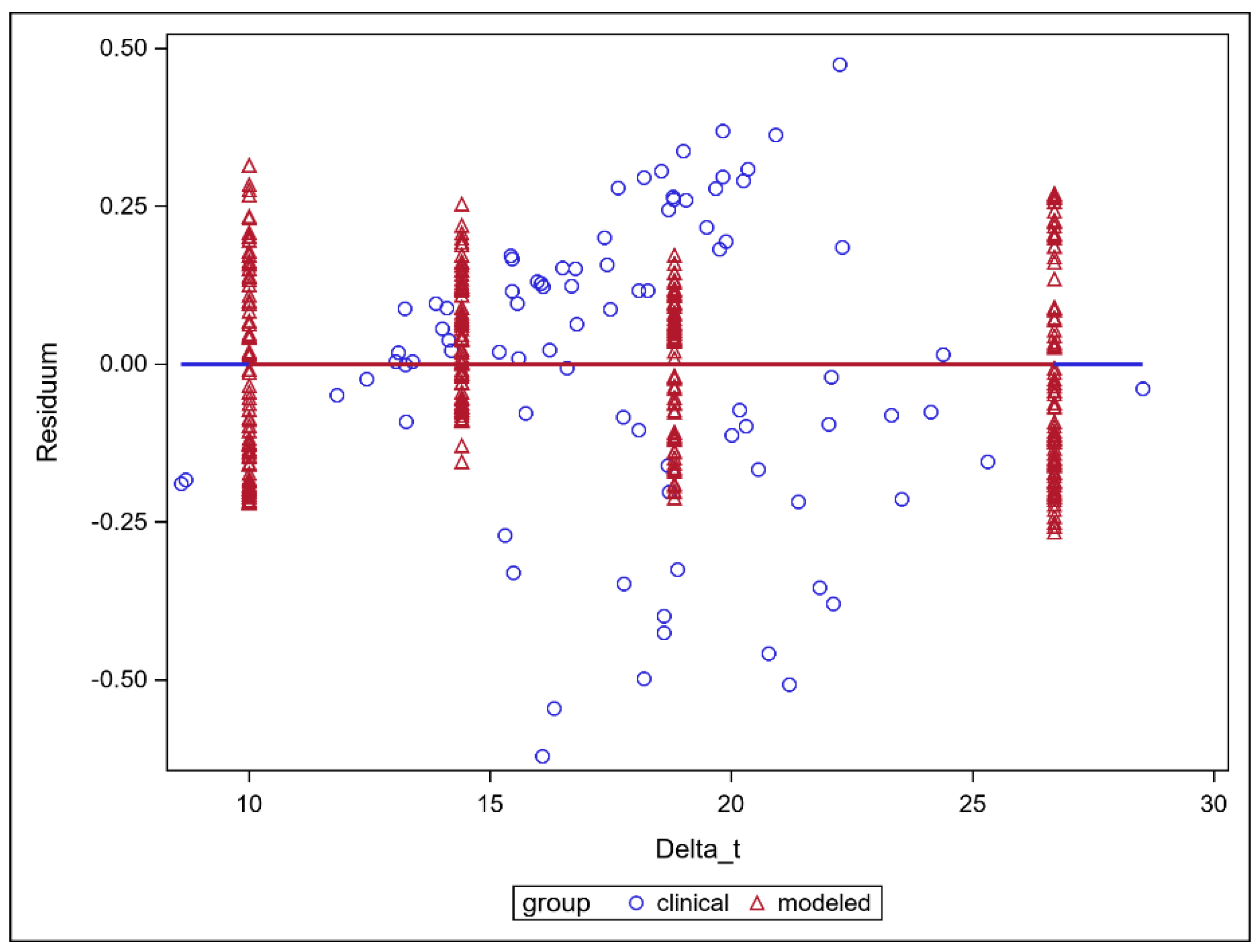
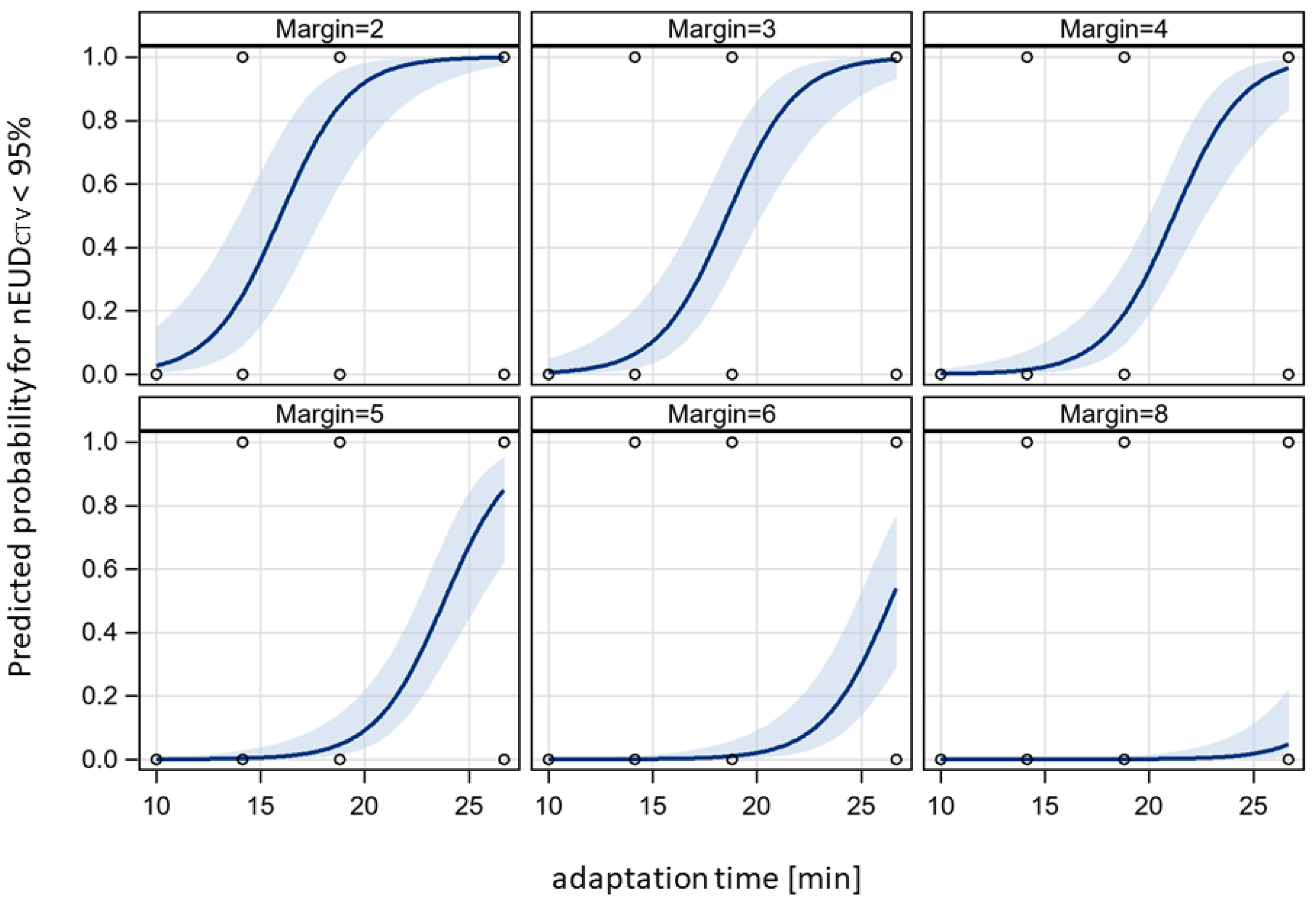
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foroudi, F.; Wong, J.; Haworth, A.; Baille, A.; McAlpine, J.; Rolfo, A.; Kron, T.; Roxby, P.; Paneghel, A.; Williams, S.; et al. Offline adaptive radiotherapy for bladder cancer using cone beam computed tomography. J. Med. Imaging Radiat. Oncol. 2009, 53, 226–233. [Google Scholar] [CrossRef]
- Pos, F.; Remeijer, P. Adaptive Management of Bladder Cancer Radiotherapy. Semin. Radiat. Oncol. 2010, 20, 116–120. [Google Scholar] [CrossRef]
- El-Bared, N.; Portelance, L.; Spieler, B.O.; Kwon, D.; Padgett, K.R.; Brown, K.M.; Mellon, E.A. Dosimetric Benefits and Practical Pitfalls of Daily Online Adaptive MRI-Guided Stereotactic Radiation Therapy for Pancreatic Cancer. Pract. Radiat. Oncol. 2019, 9, e46–e54. [Google Scholar] [CrossRef]
- Li, X.A.; Liu, F.; Tai, A.; Ahunbay, E.; Chen, G.; Kelly, T.; Lawton, C.; Erickson, B. Development of an online adaptive solution to account for inter- and intra-fractional variations. Radiother. Oncol. 2011, 100, 370–374. [Google Scholar] [CrossRef]
- Liu, F.; Erickson, B.; Peng, C.; Li, X.A. Characterization and Management of Interfractional Anatomic Changes for Pancreatic Cancer Radiotherapy. Int. J. Radiat. Oncol. 2012, 83, e423–e429. [Google Scholar] [CrossRef]
- Henke, L.; Kashani, R.; Robinson, C.; Curcuru, A.; DeWees, T.; Bradley, J.; Green, O.; Michalski, J.; Mutic, S.; Parikh, P.; et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother. Oncol. 2017, 126, 519–526. [Google Scholar] [CrossRef]
- Guberina, M.; Garcia, A.S.; Khouya, A.; Pöttgen, C.; Holubyev, K.; Ringbaek, T.P.; Lachmuth, M.; Alberti, Y.; Hoffmann, C.; Hlouschek, J.; et al. Comparison of Online-Onboard Adaptive Intensity-Modulated Radiation Therapy or Volumetric-Modulated Arc Radiotherapy With Image-Guided Radiotherapy for Patients With Gynecologic Tumors in Dependence on Fractionation and the Planning Target Volume Margin. JAMA Netw. Open 2023, 6, e234066. [Google Scholar] [CrossRef]
- Winkel, D.; Bol, G.H.; Kroon, P.S.; van Asselen, B.; Hackett, S.S.; Werensteijn-Honingh, A.M.; Intven, M.P.; Eppinga, W.S.; Tijssen, R.H.; Kerkmeijer, L.G.; et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin. Transl. Radiat. Oncol. 2019, 18, 54–59. [Google Scholar] [CrossRef]
- Tetar, S.U.; Bruynzeel, A.M.; Lagerwaard, F.J.; Slotman, B.J.; Bohoudi, O.; Palacios, M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys. Imaging Radiat. Oncol. 2019, 9, 69–76. [Google Scholar] [CrossRef]
- Bertelsen, A.S.; Schytte, T.; Møller, P.K.; Mahmood, F.; Riis, H.L.; Gottlieb, K.L.; Agergaard, S.N.; Dysager, L.; Hansen, O.; Gornitzka, J.; et al. First clinical experiences with a high field 1.5 T MR linac. Acta Oncol. 2019, 58, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- de Leon, J.; Crawford, D.; Moutrie, Z.; Alvares, S.; Hogan, L.; Pagulayan, C.; Jelen, U.; Loo, C.; Aylward, J.D.; Condon, K.; et al. Early experience with MR-guided adaptive radiotherapy using a 1.5 T MR-Linac: First 6 months of operation using adapt to shape workflow. J. Med. Imaging Radiat. Oncol. 2022, 66, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.; Archibald-Heeren, B.; Hu, Y.; Teh, A.; Beserminji, R.; Cai, E.; Liu, G.; Yates, A.; Rijken, J.; Collett, N.; et al. Varian ethos online adaptive radiotherapy for prostate cancer: Early results of contouring accuracy, treatment plan quality, and treatment time. J. Appl. Clin. Med. Phys. 2022, 23, e13479. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.W.; Lin, H.; Alonso-Basanta, M.; Anderson, N.; Apinorasethkul, O.; Cooper, K.; Dong, L.; Kempsey, B.; Marcel, J.; Metz, J.; et al. Initial Evaluation of a Novel Cone-Beam CT-Based Semi-Automated Online Adaptive Radiotherapy System for Head and Neck Cancer Treatment—A Timing and Automation Quality Study. Cureus 2020, 12, e9660. [Google Scholar] [CrossRef]
- Mangar, S.A.; Scurr, E.; Huddart, R.A.; Sohaib, S.A.; Horwich, A.; Dearnaley, D.P.; Khoo, V.S. Assessing intra-fractional bladder motion using cine-MRI as initial methodology for Predictive Organ Localization (POLO) in radiotherapy for bladder cancer. Radiother. Oncol. 2007, 85, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Cui, Z.; Li, Y.; Liu, P.; Wang, Y.; Zhu, J.; Zhu, J.; Yin, Y.; Li, Z. Online MR evaluation of inter- and intra-fraction uterus motions and bladder volume changes during cervical cancer external beam radiotherapy. Radiat. Oncol. 2021, 16, 179. [Google Scholar] [CrossRef]
- Kong, V.; Hansen, V.; Hafeez, S. Image-guided Adaptive Radiotherapy for Bladder Cancer. Clin. Oncol. 2021, 33, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Meijer, G.J.; Rasch, C.; Remeijer, P.; Lebesque, J. Three-dimensional analysis of delineation errors, setup errors, and organ motion during radiotherapy of bladder cancer. Int. J. Radiat. Oncol. 2003, 55, 1277–1287. [Google Scholar] [CrossRef]
- Muren, L.P.; Smaaland, R.; Dahl, O. Organ motion, set-up variation and treatment margins in radical radiotherapy of urinary bladder cancer. Radiother. Oncol. 2003, 69, 291–304. [Google Scholar] [CrossRef]
- Muren, L.P.; Redpath, A.T.; McLaren, D.B. Treatment margins and treatment fractionation in conformal radiotherapy of muscle-invading urinary bladder cancer. Radiother. Oncol. 2004, 71, 65–71. [Google Scholar] [CrossRef]
- Khalifa, J.; Supiot, S.; Pignot, G.; Hennequin, C.; Blanchard, P.; Pasquier, D.; Magné, N.; de Crevoisier, R.; Graff-Cailleaud, P.; Riou, O.; et al. Recommendations for planning and delivery of radical radiotherapy for localized urothelial carcinoma of the bladder. Radiother. Oncol. 2021, 161, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Pos, F.J.; Koedooder, K.; Hulshof, M.C.; van Tienhoven, G.; González, D.G. Influence of bladder and rectal volume on spatial variability of a bladder tumor during radical radiotherapy. Int. J. Radiat. Oncol. 2003, 55, 835–841. [Google Scholar] [CrossRef]
- Murthy, V.; Masodkar, R.; Kalyani, N.; Mahantshetty, U.; Bakshi, G.; Prakash, G.; Joshi, A.; Prabhash, K.; Ghonge, S.; Shrivastava, S. Clinical Outcomes With Dose-Escalated Adaptive Radiation Therapy for Urinary Bladder Cancer: A Prospective Study. Int. J. Radiat. Oncol. 2016, 94, 60–66. [Google Scholar] [CrossRef]
- Ström, L.M.; Behrens, C.P.; Calmels, L.; Sjöström, D.; Geertsen, P.; Mouritsen, L.S.; Serup-Hansen, E.; Lindberg, H.; Sibolt, P. Online adaptive radiotherapy of urinary bladder cancer with full re-optimization to the anatomy of the day: Initial experience and dosimetric benefits. Radiother. Oncol. 2022, 171, 37–42. [Google Scholar] [CrossRef]
- Mitchell, A.; Ingle, M.; Smith, G.; Chick, J.; Diamantopoulos, S.; Goodwin, E.; Herbert, T.; Huddart, R.; McNair, H.; Oelfke, U.; et al. Feasibility of tumour-focused adaptive radiotherapy for bladder cancer on the MR-linac. Clin. Transl. Radiat. Oncol. 2022, 35, 27–32. [Google Scholar] [CrossRef]
- Pöttgen, C.; Hoffmann, C.; Gauler, T.; Guberina, M.; Guberina, N.; Ringbaek, T.; Garcia, A.S.; Krafft, U.; Hadaschik, B.; Khouya, A.; et al. Fractionation versus Adaptation for Compensation of Target Volume Changes during Online Adaptive Radiotherapy for Bladder Cancer: Answers from a Prospective Registry. Cancers 2023, 15, 4933. [Google Scholar] [CrossRef]
- Azzarouali, S.; Goudschaal, K.; Visser, J.; Hulshof, M.; Admiraal, M.; van Wieringen, N.; Nieuwenhuijzen, J.; Wiersma, J.; Daniëls, L.; Boer, D.D.; et al. Online adaptive radiotherapy for bladder cancer using a simultaneous integrated boost and fiducial markers. Radiat. Oncol. 2023, 18, 165. [Google Scholar] [CrossRef]
- Niemierko, A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef]
- Deasy, J.O.; Niemierko, A.; Herbert, D.; Yan, D.; Jackson, A.; Haken, R.K.T.; Langer, M.; Sapareto, S. Methodological issues in radiation dose-volume outcome analyses: Summary of a joint AAPM/NIH workshop. Med. Phys. 2002, 29, 2109–2127. [Google Scholar] [CrossRef]
- Chapet, O.; Thomas, E.; Kessler, M.L.; Fraass, B.A.; Haken, R.K.T. Esophagus sparing with IMRT in lung tumor irradiation: An EUD-based optimization technique. Int. J. Radiat. Oncol. 2005, 63, 179–187. [Google Scholar] [CrossRef]
- Berger, T.; Godart, J.; Jagt, T.; Vittrup, A.S.; Fokdal, L.U.; Lindegaard, J.C.; Jensen, N.B.K.; Zolnay, A.; Reijtenbagh, D.; Trnkova, P.; et al. Dosimetric Impact of Intrafraction Motion in Online-Adaptive Intensity Modulated Proton Therapy for Cervical Cancer. Int. J. Radiat. Oncol. 2020, 109, 1580–1587. [Google Scholar] [CrossRef]
- Brennan, V.S.; Burleson, S.; Kostrzewa, C.; Scripes, P.G.; Subashi, E.; Zhang, Z.; Tyagi, N.; Zelefsky, M.J. SBRT focal dose intensification using an MR-Linac adaptive planning for intermediate-risk prostate cancer: An analysis of the dosimetric impact of intra-fractional organ changes. Radiother. Oncol. 2023, 179, 109441. [Google Scholar] [CrossRef]
- Mannerberg, A.; Persson, E.; Jonsson, J.; Gustafsson, C.J.; Gunnlaugsson, A.; Olsson, L.E.; Ceberg, S. Dosimetric effects of adaptive prostate cancer radiotherapy in an MR-linac workflow. Radiat. Oncol. 2020, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, R.; Rigo, M.; Naccarato, S.; Gurrera, D.; Figlia, V.; Mazzola, R.; Ricchetti, F.; Nicosia, L.; Giaj-Levra, N.; Cuccia, F.; et al. Adaptive SBRT by 1.5 T MR-linac for prostate cancer: On the accuracy of dose delivery in view of the prolonged session time. Phys. Medica 2020, 80, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, K.; Shimizu, S.; Shinohara, N.; Ito, Y.M.; Abe, T.; Maruyama, S.; Katoh, N.; Kinoshita, R.; Hashimoto, T.; Miyamoto, N.; et al. Analysis of inter- and intra fractional partial bladder wall movement using implanted fiducial markers. Radiat. Oncol. 2017, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Eijkelenkamp, H.; Boekhoff, M.R.; Verweij, M.E.; Peters, F.P.; Meijer, G.J.; Intven, M.P. Planning target volume margin assessment for online adaptive MR-guided dose-escalation in rectal cancer on a 1.5 T MR-Linac. Radiother. Oncol. 2021, 162, 150–155. [Google Scholar] [CrossRef]
- Fry, C.H.; McCloskey, K.D. Spontaneous Activity and the Urinary Bladder. Adv. Exp. Med. Biol. 2019, 1124, 121–147. [Google Scholar]
- Junginger, B.; Baessler, K.; Sapsford, R.; Hodges, P.W. Effect of abdominal and pelvic floor tasks on muscle activity, abdominal pressure and bladder neck. Int. Urogynecol. J. 2010, 21, 69–77. [Google Scholar] [CrossRef]
- Langen, K.M.; Willoughby, T.R.; Meeks, S.L.; Santhanam, A.; Cunningham, A.; Levine, L.; Kupelian, P.A. Observations on Real-Time Prostate Gland Motion Using Electromagnetic Tracking. Int. J. Radiat. Oncol. 2008, 71, 1084–1090. [Google Scholar] [CrossRef]
- Elakshar, S.; Tsui, J.M.G.; Kucharczyk, M.J.; Tomic, N.; Fawaz, Z.S.; Bahoric, B.; Papayanatos, J.; Chaddad, A.; Niazi, T. Does Interfraction Cone Beam Computed Tomography Improve Target Localization in Prostate Bed Radiotherapy? Technol. Cancer Res. Treat 2019, 18, 1533033819831962. [Google Scholar] [CrossRef]
- Kishan, A.U.; Ma, T.M.; Lamb, J.M.; Casado, M.; Wilhalme, H.; Low, D.A.; Sheng, K.; Sharma, S.; Nickols, N.G.; Pham, J.; et al. Magnetic Resonance Imaging-Guided vs Computed Tomography-Guided Stereotactic Body Radiotherapy for Prostate Cancer: The MIRAGE Randomized Clinical Trial. JAMA Oncol. 2023, 9, 365–373. [Google Scholar] [CrossRef]
- Hafeez, S.; Warren-Oseni, K.; McNair, H.A.; Hansen, V.N.; Jones, K.; Tan, M.; Khan, A.; Harris, V.; McDonald, F.; Lalondrelle, S.; et al. Prospective Study Delivering Simultaneous Integrated High-dose Tumor Boost (≤70 Gy) With Image Guided Adaptive Radiation Therapy for Radical Treatment of Localized Muscle-Invasive Bladder Cancer. Int. J. Radiat. Oncol. 2016, 94, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Schaal, D.; Curry, H.; Clark, R.; Magliari, A.; Kupelian, P.; Khuntia, D.; Beriwal, S. Review of cone beam computed tomography based online adaptive radiotherapy: Current trend and future direction. Radiat. Oncol. 2023, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Jin, J.; Yi, J.; Han, C.; Zhou, Y.; Ai, Y.; Xie, C.; Jin, X. RefineNet-based 2D and 3D automatic segmentations for clinical target volume and organs at risks for patients with cervical cancer in postoperative radiotherapy. J. Appl. Clin. Med. Phys. 2022, 23, e13631. [Google Scholar] [CrossRef] [PubMed]
- Smolders, A.; Lomax, A.; Weber, D.; Albertini, F. Patient-specific neural networks for contour propagation in online adaptive radiotherapy. Phys. Med. Biol. 2023, 68, 095010. [Google Scholar] [CrossRef]
- Fransson, S.; Tilly, D.; Strand, R. Patient specific deep learning based segmentation for magnetic resonance guided prostate radiotherapy. Phys. Imaging Radiat. Oncol. 2022, 23, 38–42. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khouya, A.; Pöttgen, C.; Hoffmann, C.; Ringbaek, T.P.; Lübcke, W.; Indenkämpen, F.; Guberina, M.; Guberina, N.; Gauler, T.; Stuschke, M.; et al. Adaptation Time as a Determinant of the Dosimetric Effectiveness of Online Adaptive Radiotherapy for Bladder Cancer. Cancers 2023, 15, 5629. https://doi.org/10.3390/cancers15235629
Khouya A, Pöttgen C, Hoffmann C, Ringbaek TP, Lübcke W, Indenkämpen F, Guberina M, Guberina N, Gauler T, Stuschke M, et al. Adaptation Time as a Determinant of the Dosimetric Effectiveness of Online Adaptive Radiotherapy for Bladder Cancer. Cancers. 2023; 15(23):5629. https://doi.org/10.3390/cancers15235629
Chicago/Turabian StyleKhouya, Aymane, Christoph Pöttgen, Christian Hoffmann, Toke Printz Ringbaek, Wolfgang Lübcke, Frank Indenkämpen, Maja Guberina, Nika Guberina, Thomas Gauler, Martin Stuschke, and et al. 2023. "Adaptation Time as a Determinant of the Dosimetric Effectiveness of Online Adaptive Radiotherapy for Bladder Cancer" Cancers 15, no. 23: 5629. https://doi.org/10.3390/cancers15235629
APA StyleKhouya, A., Pöttgen, C., Hoffmann, C., Ringbaek, T. P., Lübcke, W., Indenkämpen, F., Guberina, M., Guberina, N., Gauler, T., Stuschke, M., & Santiago Garcia, A. (2023). Adaptation Time as a Determinant of the Dosimetric Effectiveness of Online Adaptive Radiotherapy for Bladder Cancer. Cancers, 15(23), 5629. https://doi.org/10.3390/cancers15235629






