Plasma Markers for Therapy Response Monitoring in Patients with Neuroendocrine Tumors Undergoing Peptide Receptor Radionuclide Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. [177Lu]Lu-DOTATOC-PRRT and Response Assessment
2.3. Evaluation
- Chromogranin A (CgA): <102 ng/mL;
- AST: <35 U/L (female), <50 U/L (male);
- ALT: ≤31 U/L (female), ≤41 U/L (male);
- ALP: 35–105 U/L (female), 40–130 U/L (male).
2.4. Statistical Analysis
3. Results
3.1. Patients
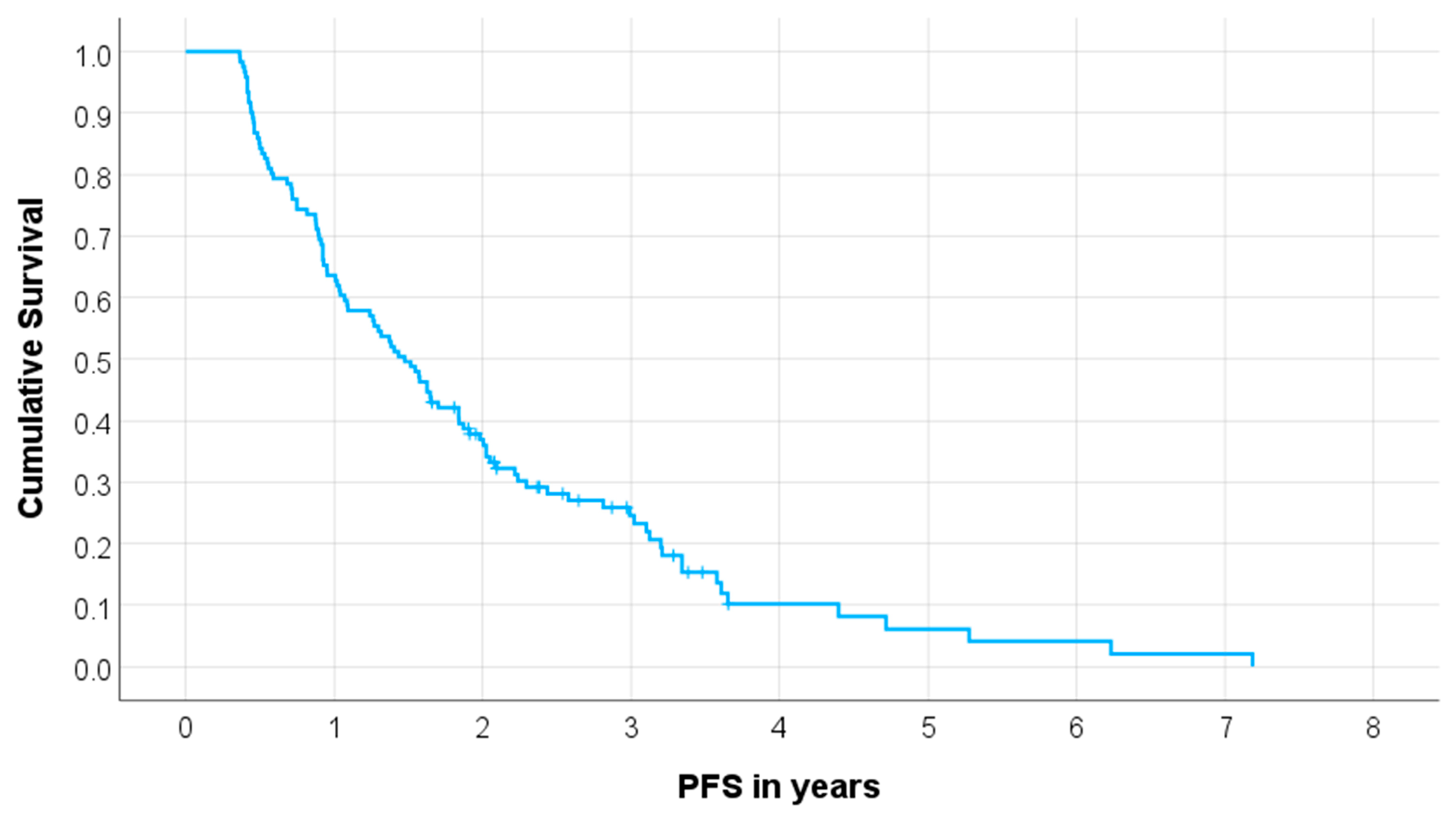
3.2. Progression-Free Survival
3.3. Changes in Laboratory Parameters
3.4. Alterations of Laboratory Parameters in Relation to the First Cycle PRRT
3.5. Prediction of PFS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knigge, U.; Capdevila, J.; Bartsch, D.K.; Baudin, E.; Falkerby, J.; Kianmanesh, R.; Kos-Kudla, B.; Niederle, B.; Nieveen van Dijkum, E.; O’Toole, D.; et al. ENETS Consensus Recommendations for the Standards of Care in Neuroendocrine Neoplasms: Follow-Up and Documentation. Neuroendocrinology 2017, 105, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Oberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Kidd, M.S.; Singh, A.; van der Zwan, W.A.; Severi, S.; Drozdov, I.A.; Malczewska, A.; Baum, R.P.; Kwekkeboom, D.J.; Paganelli, G.; et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: The NETest. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Huizing, D.M.V.; Aalbersberg, E.A.; Versleijen, M.W.J.; Tesselaar, M.E.T.; Walraven, I.; Lahaye, M.J.; de Wit-van der Veen, B.J.; Stokkel, M.P.M. Early response assessment and prediction of overall survival after peptide receptor radionuclide therapy. Cancer Imaging 2020, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, D.; Gronberg, M.; Landerholm, K.; Welin, S.; Ziolkowska, B.; Nordvall, D.; Janson, E.T. Assessment of hormonal levels as prognostic markers and of their optimal cut-offs in small intestinal neuroendocrine tumours grade 2. Endocrine 2021, 72, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Onesti, J.K.; Shirley, L.A.; Saunders, N.D.; Davidson, G.W.; Dillhoff, M.E.; Khabiri, H.; Guy, G.E.; Dowell, J.D.; Schmidt, C.R.; Shah, M.H.; et al. Elevated Alkaline Phosphatase Prior to Transarterial Chemoembolization for Neuroendocrine Tumors Predicts Worse Outcomes. J. Gastrointest. Surg. 2016, 20, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Andriantsoa, M.; Hoibian, S.; Autret, A.; Gilabert, M.; Sarran, A.; Niccoli, P.; Raoul, J.L. An elevated serum alkaline phosphatase level in hepatic metastases of grade 1 and 2 gastrointestinal neuroendocrine tumors is unusual and of prognostic value. PLoS ONE 2017, 12, e0177971. [Google Scholar] [CrossRef]
- Ruhwedel, T.; Rogasch, J.M.M.; Huang, K.; Jann, H.; Schatka, I.; Furth, C.; Amthauer, H.; Wetz, C. The Prognostic Value of the De Ritis Ratio for Progression-Free Survival in Patients with NET Undergoing [(177)Lu]Lu-DOTATOC-PRRT: A Retrospective Analysis. Cancers 2021, 13, 635. [Google Scholar] [CrossRef]
- Wetz, C.; Genseke, P.; Apostolova, I.; Furth, C.; Ghazzawi, S.; Rogasch, J.M.M.; Schatka, I.; Kreissl, M.C.; Hofheinz, F.; Grosser, O.S.; et al. The association of intra-therapeutic heterogeneity of somatostatin receptor expression with morphological treatment response in patients undergoing PRRT with [177Lu]-DOTATATE. PLoS ONE 2019, 14, e0216781. [Google Scholar] [CrossRef]
- Werner, R.A.; Lapa, C.; Ilhan, H.; Higuchi, T.; Buck, A.K.; Lehner, S.; Bartenstein, P.; Bengel, F.; Schatka, I.; Muegge, D.O.; et al. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget 2017, 8, 7039–7049. [Google Scholar] [CrossRef]
- Krenning, E.P.; Valkema, R.; Kooij, P.P.; Breeman, W.A.; Bakker, W.H.; deHerder, W.W.; vanEijck, C.H.; Kwekkeboom, D.J.; deJong, M.; Pauwels, S. Scintigraphy and radionuclide therapy with [indium-111-labelled-diethyl triamine penta-acetic acid-D-Phe1]-octreotide. Ital. J. Gastroenterol. Hepatol. 1999, 31 (Suppl. S2), S219–S223. [Google Scholar]
- Arnold, R.; Wilke, A.; Rinke, A.; Mayer, C.; Kann, P.H.; Klose, K.J.; Scherag, A.; Hahmann, M.; Müller, H.H.; Barth, P. Plasma chromogranin A as marker for survival in patients with metastatic endocrine gastroenteropancreatic tumors. Clin. Gastroenterol. Hepatol. 2008, 6, 820–827. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; de Herder, W.W.; Feelders, R.A.; Krenning, E.P.; Kwekkeboom, D.J. Pitfalls in the response evaluation after peptide receptor radionuclide therapy with [(177)Lu-DOTA(0),Tyr(3)]octreotate. Endocr. Relat. Cancer 2017, 24, 243–251. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.S.; Robertson, J.M.; Anscher, M.S.; Jirtle, R.L.; Ensminger, W.D.; Fajardo, L.F. Hepatic toxicity resulting from cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1237–1248. [Google Scholar] [CrossRef]
- Jiang, C.; Hu, F.; Xia, X.; Guo, X. Prognostic value of alkaline phosphatase and bone-specific alkaline phosphatase in breast cancer: A systematic review and meta-analysis. Int. J. Biol. Markers 2023, 38, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Janisch, F.; Parizi, M.K.; Mostafaei, H.; Lysenko, I.; Enikeev, D.V.; Kimura, S.; Egawa, S.; Shariat, S.F. Prognostic value of alkaline phosphatase in hormone-sensitive prostate cancer: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2020, 25, 247–257. [Google Scholar] [CrossRef]
- Ren, H.Y.; Sun, L.L.; Li, H.Y.; Ye, Z.M. Prognostic Significance of Serum Alkaline Phosphatase Level in Osteosarcoma: A Meta-Analysis of Published Data. BioMed Res. Int. 2015, 2015, 160835. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Kunz, P.L.; Hendifar, A.; Yao, J.; Bushnell, D.; Kulke, M.H.; Baum, R.P.; Caplin, M.; Ruszniewski, P.; Delpassand, E.; et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with (177)Lu-Dotatate: An analysis of the NETTER-1 study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2372–2382. [Google Scholar] [CrossRef]
- Bezan, A.; Mrsic, E.; Krieger, D.; Stojakovic, T.; Pummer, K.; Zigeuner, R.; Hutterer, G.C.; Pichler, M. The Preoperative AST/ALT (De Ritis) Ratio Represents a Poor Prognostic Factor in a Cohort of Patients with Nonmetastatic Renal Cell Carcinoma. J. Urol. 2015, 194, 30–35. [Google Scholar] [CrossRef]
- Hu, X.; Yang, W.X.; Wang, Y.; Shao, Y.X.; Xiong, S.C.; Li, X. The prognostic value of De Ritis (AST/ALT) ratio in patients after surgery for urothelial carcinoma: A systematic review and meta-analysis. Cancer Cell Int. 2020, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, S.; Wang, Y.; Hu, L. Pretreatment Aspartate Aminotransferase-to-Alanine Aminotransferase (De Ritis) Ratio Predicts the Prognosis of Nonmetastatic Nasopharyngeal Carcinoma. OncoTargets Ther. 2019, 12, 10077–10087. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.E.; Byun, S.S.; Kim, H.H.; Kwak, C.; Hong, S.K. De Ritis ratio (aspartate transaminase/alanine transaminase ratio) as a significant prognostic factor after surgical treatment in patients with clear-cell localized renal cell carcinoma: A propensity score-matched study. BJU Int. 2017, 119, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Ishihara, H.; Takagi, T.; Fukuda, H.; Yoshida, K.; Iizuka, J.; Kobayashi, H.; Okumi, M.; Ishida, H.; Kondo, T.; et al. The De Ritis (Aspartate Transaminase/Alanine Transaminase) Ratio as a Prognosticator in Patients With End-stage Renal Disease-associated Renal Cell Carcinoma. Clin. Genitourin. Cancer 2020, 18, 236–240 e231. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.D.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. De Ritis Ratio (Aspartate Transaminase/Alanine Transaminase) as a Significant Prognostic Factor in Patients Undergoing Radical Cystectomy with Bladder Urothelial Carcinoma: A Propensity Score-Matched Study. Dis. Markers 2019, 2019, 6702964. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, L.; Wang, Y.; Tan, W.; Huang, Z. Prognostic value of aspartate transaminase to alanine transaminase (De Ritis) ratio in solid tumors: A pooled analysis of 9,400 patients. OncoTargets Ther. 2019, 12, 5201–5213. [Google Scholar] [CrossRef]
- Mori, K.; Janisch, F.; Mostafaei, H.; Kimura, S.; Lysenko, I.; Karakiewicz, P.I.; Briganti, A.; Enikeev, D.V.; Roupret, M.; Margulis, V.; et al. Prognostic role of preoperative De Ritis ratio in upper tract urothelial carcinoma treated with nephroureterectomy. Urol. Oncol. 2020, 38, 601.e17–601.e24. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- Fantin, V.R.; St-Pierre, J.; Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 2006, 9, 425–434. [Google Scholar] [CrossRef]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Greenhouse, W.V.; Lehninger, A.L. Occurrence of the malate-aspartate shuttle in various tumor types. Cancer Res. 1976, 36, 1392–1396. [Google Scholar] [PubMed]
- Thornburg, J.M.; Nelson, K.K.; Clem, B.F.; Lane, A.N.; Arumugam, S.; Simmons, A.; Eaton, J.W.; Telang, S.; Chesney, J. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008, 10, R84. [Google Scholar] [CrossRef] [PubMed]
- Marotta, V.; Zatelli, M.C.; Sciammarella, C.; Ambrosio, M.R.; Bondanelli, M.; Colao, A.; Faggiano, A. Chromogranin A as circulating marker for diagnosis and management of neuroendocrine neoplasms: More flaws than fame. Endocr. Relat. Cancer 2018, 25, R11–R29. [Google Scholar] [CrossRef]
- Fossmark, R.; Jianu, C.S.; Martinsen, T.C.; Qvigstad, G.; Syversen, U.; Waldum, H.L. Serum gastrin and chromogranin A levels in patients with fundic gland polyps caused by long-term proton-pump inhibition. Scand. J. Gastroenterol. 2008, 43, 20–24. [Google Scholar] [CrossRef]
- Ceconi, C.; Ferrari, R.; Bachetti, T.; Opasich, C.; Volterrani, M.; Colombo, B.; Parrinello, G.; Corti, A. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur. Heart J. 2002, 23, 967–974. [Google Scholar] [CrossRef]
- Spadaro, A.; Ajello, A.; Morace, C.; Zirilli, A.; D’Arrigo, G.; Luigiano, C.; Martino, F.; Bene, A.; Migliorato, D.; Turiano, S.; et al. Serum chromogranin-A in hepatocellular carcinoma: Diagnostic utility and limits. World J. Gastroenterol. 2005, 11, 1987–1990. [Google Scholar] [CrossRef]
- Takiyyuddin, M.A.; Parmer, R.J.; Kailasam, M.T.; Cervenka, J.H.; Kennedy, B.; Ziegler, M.G.; Lin, M.C.; Li, J.; Grim, C.E.; Wright, F.A.; et al. Chromogranin A in human hypertension. Influence of heredity. Hypertension 1995, 26, 213–220. [Google Scholar] [CrossRef]


| Variable | n (%) or Median (Range) | p | ||
|---|---|---|---|---|
| Total Cohort | PFS Low | PFS High | ||
| Patient count | 141 | 67 | 54 | |
| Age in years | 64 (34–87) | 66 (42–87) | 63 (34–80) | 0.36 |
| Sex | 1.00 | |||
| Men | 89 (63%) | 44 (66%) | 36 (67%) | |
| Women | 52 (37%) | 23 (34%) | 18 (33%) | |
| Primary location | 0.009 | |||
| Small intestine | 67 (48%) | 29 (43%) | 26 (48%) | |
| Pancreas | 38 (27%) | 25 (37%) | 11 (20%) | |
| Colon/Rectum | 12 (9%) | 2 (3%) | 8 (15%) | |
| Lungs | 10 (7%) | 7 (10%) | 1 (2%) | |
| Stomach | 1 (1%) | 0 (0%) | 1 (2%) | |
| CUP | 13 (9%) | 4 (6%) | 7 (13%) | |
| Metastatic disease | 138 (98%) | 66 (99%) | 53 (98%) | 1.00 |
| Metastatic spread | ||||
| Hepatic | 135 (96%) | 64 (96%) | 52 (96%) | 1.00 |
| Lymphonodal | 118 (84%) | 59 (88%) | 43 (80%) | 0.22 |
| Osseous | 54 (38%) | 29 (43%) | 18 (33%) | 0.35 |
| Peritoneal | 24 (17%) | 11 (16%) | 9 (17%) | 1.00 |
| Pulmonal | 6 (4%) | 3 (5%) | 3 (6%) | 1.00 |
| Functional tumor | 46 (33%) | 19 (28%) | 19 (35%) | 0.44 |
| Hedinger syndrome | 6 (4%) | 3 (5%) | 1 (2%) | 0.63 |
| Grading | 0.98 | |||
| G1 | 30 (21%) | 12 (18%) | 11 (20%) | |
| G2 | 100 (71%) | 50 (75%) | 39 (72%) | |
| G3 | 5 (4%) | 3 (5%) | 2 (4%) | |
| Unknown | 6 (4%) | 2 (3%) | 2 (4%) | |
| Ki-67 index | 5 (1–40) | 10 (1–40) | 5 (1–25) | 0.10 |
| Number of PRRT cycles | 3 (2–6) | 3 (2–6) | 4 (2–6) | 0.001 |
| Previous treatment | ||||
| Operative resection | 93 (66%) | 44 (66%) | 36 (67%) | 1.00 |
| Somatostatin analogues | 108 (77%) | 51 (76%) | 41 (76%) | 1.00 |
| mTOR inhibitor | 17 (12%) | 11 (16%) | 6 (11%) | 0.44 |
| Tyrosine kinase inhibitor | 4 (3%) | 3 (5%) | 1 (2%) | 0.63 |
| Chemotherapy | 41 (29%) | 25 (37%) | 14 (26%) | 0.24 |
| Local ablative therapy | 12 (9%) | 6 (9%) | 5 (9%) | 1.00 |
| Radiation therapy | 5 (4%) | 3 (5%) | 2 (4%) | 1.00 |
| Transcatheter arterial chemoembolization | 7 (5%) | 4 (6%) | 1 (2%) | 0.38 |
| Laboratory Parameter | Median (Range) | |||
|---|---|---|---|---|
| 1st Cycle | 2nd Cycle | 3rd Cycle | 4th Cycle | |
| CgA (in µg/L) | 452 (14–601,700) | 427 (16–560,300) | 319 (27–419,300) | 324 (26–259,100) |
| AST (in U/L) | 29 (13–123) | 28 (14–104) | 27 (15–84) | 27 (15–75) |
| ALT (in U/L) | 27 (10–122) | 25 (7–215) | 24 (9–95) | 25 (14–97) |
| De Ritis ratio | 1.11 (0.52–2.87) | 1.09 (0.28–2.86) | 1.15 (0.38–3.38) | 1.05 (0.45–3.06) |
| ALP (in U/L) | 84 (36–470) | 81 (36–385) | 74 (34–263) | 77 (42–272) |
| Laboratory Parameter | Median (Range) | p | ||
|---|---|---|---|---|
| Total Cohort | PFS Low | PFS High | ||
| CgA | ||||
| 2nd Cycle/1st Cycle | 1.06 (0.19–3.73) | 1.05 (0.24–2.88) | 1.00 (0.19–3.73) | 0.46 |
| 3rd Cycle/1st Cycle | 1.07 (0.08–3.76) | 1.12 (0.24–3.76) | 0.95 (0.08–3.22) | 0.27 |
| 4th Cycle/1st Cycle | 1.15 (0.12–3.82) | 1.37 (0.24–3.69) | 1.10 (0.12–3.82) | 0.23 |
| AST | ||||
| 2nd Cycle/1st Cycle | 0.96 (0.26–2.31) | 0.96 (0.26–2.31) | 0.94 (0.48–2.02) | 0.75 |
| 3rd Cycle/1st Cycle | 0.98 (0.45–2.12) | 0.99 (0.45–2.05) | 1.00 (0.57–2.12) | 0.84 |
| 4th Cycle/1st Cycle | 1.00 (0.47–2.21) | 0.96 (0.65–1.46) | 0.98 (0.47–2.21) | 0.73 |
| ALT | ||||
| 2nd Cycle/1st Cycle | 0.84 (0.16–4.78) | 0.82 (0.16–2.43) | 0.87 (0.35–4.78) | 0.42 |
| 3rd Cycle/1st Cycle | 0.84 (0.26–2.65) | 0.94 (0.26–2.00) | 0.83 (0.46–2.65) | 0.74 |
| 4th Cycle/1st Cycle | 0.92 (0.39–2.88) | 0.89 (0.46–1.82) | 0.94 (0.39–2.88) | 0.47 |
| De Ritis ratio | ||||
| 2nd Cycle/1st Cycle | 0.99 (0.39–1.88) | 1.00 (0.48–1.88) | 1.03 (0.53–1.84) | 0.27 |
| 3rd Cycle/1st Cycle | 1.04 (0.40–2.16) | 1.07 (0.40–1.86) | 1.04 (0.64–2.16) | 0.47 |
| 4th Cycle/1st Cycle | 1.03 (0.61–1.67) | 0.93 (0.65–1.61) | 1.09 (0.67–1.40) | 0.76 |
| ALP | ||||
| 2nd Cycle/1st Cycle | 0.99 (0.51–2.95) | 1.00 (0.61–2.95) | 0.94 (0.51–2.46) | 0.07 |
| 3rd Cycle/1st Cycle | 1.00 (0.41–1.45) | 1.04 (0.57–1.45) | 0.95 (0.41–1.40) | 0.014 |
| 4th Cycle/1st Cycle | 1.03 (0.44–1.77) | 1.14 (0.73–1.77) | 0.97 (0.44–1.53) | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wetz, C.; Ruhwedel, T.; Schatka, I.; Grabowski, J.; Jann, H.; Metzger, G.; Galler, M.; Amthauer, H.; Rogasch, J.M.M. Plasma Markers for Therapy Response Monitoring in Patients with Neuroendocrine Tumors Undergoing Peptide Receptor Radionuclide Therapy. Cancers 2023, 15, 5717. https://doi.org/10.3390/cancers15245717
Wetz C, Ruhwedel T, Schatka I, Grabowski J, Jann H, Metzger G, Galler M, Amthauer H, Rogasch JMM. Plasma Markers for Therapy Response Monitoring in Patients with Neuroendocrine Tumors Undergoing Peptide Receptor Radionuclide Therapy. Cancers. 2023; 15(24):5717. https://doi.org/10.3390/cancers15245717
Chicago/Turabian StyleWetz, Christoph, Tristan Ruhwedel, Imke Schatka, Jane Grabowski, Henning Jann, Giulia Metzger, Markus Galler, Holger Amthauer, and Julian M. M. Rogasch. 2023. "Plasma Markers for Therapy Response Monitoring in Patients with Neuroendocrine Tumors Undergoing Peptide Receptor Radionuclide Therapy" Cancers 15, no. 24: 5717. https://doi.org/10.3390/cancers15245717







