Cardiac Arrhythmias in Patients Treated for Lung Cancer: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
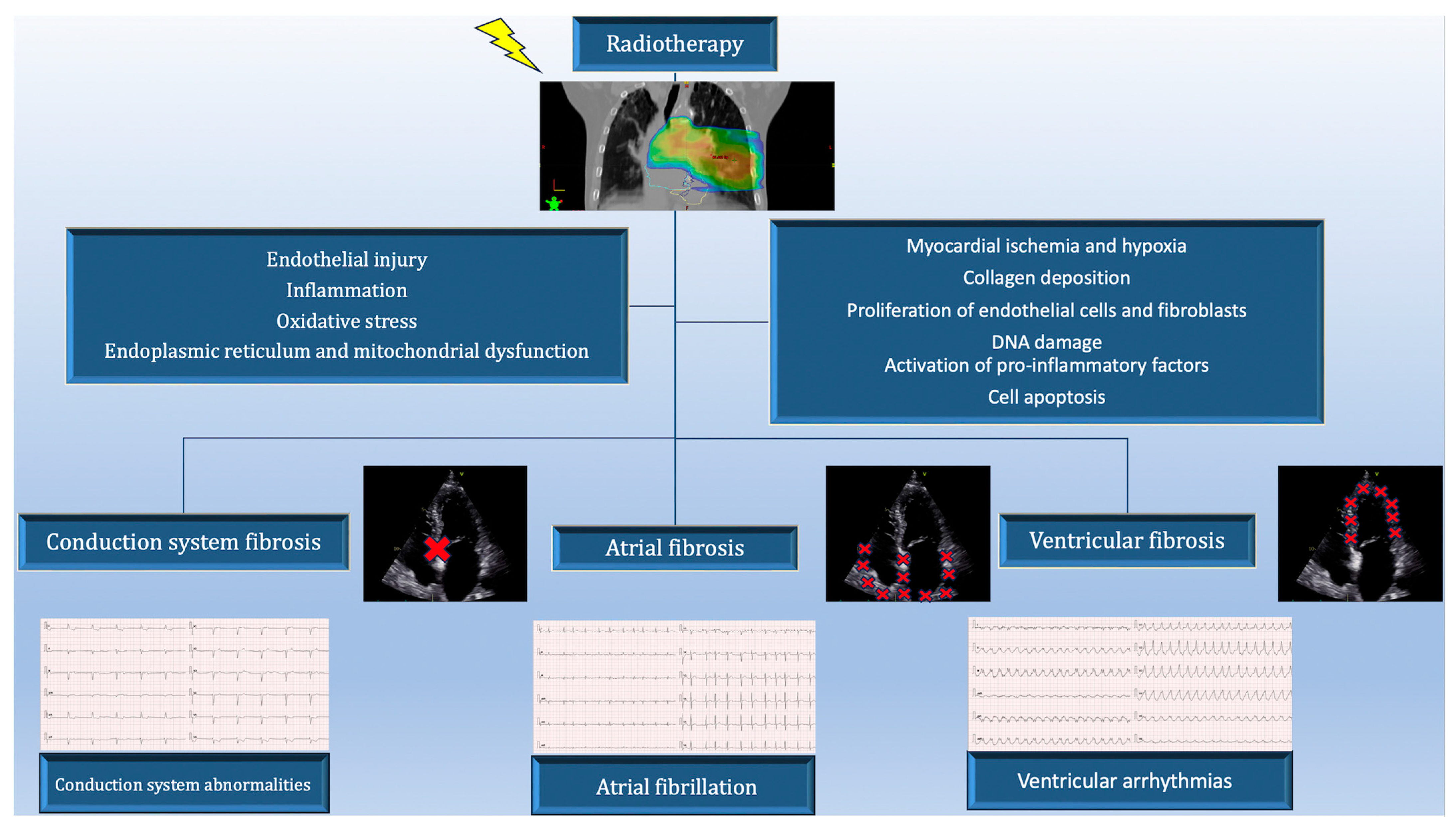
2. Pathogenesis of Cardiac Arrhythmias in Patients Treated for Lung Cancer
3. Diagnostics
4. Supraventricular Arrhythmias
4.1. Atrial Fibrillation
4.2. Atrial Flutter and Atrial Tachycardia
5. Management of Supraventricular Arrhythmias in Lung Cancer Patients
6. Ventricular Arrhythmias
7. Management of Ventricular Arrhythmias in Lung Cancer Patients
8. Implantable Cardioverter-Defibrillator (ICD)
9. Atrio-Ventricular Conduction Disturbances and Sinus Bradycardia
10. Lung Cancer Treatment
10.1. Surgery
10.2. Radiotherapy
10.3. Chemotherapy and Targeted Therapies
11. Directions
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Chen, V.W.; Ruiz, B.A.; Hsieh, M.; Wu, X.; Ries, L.A.G.; Lewis, D.R. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer 2014, 120, 3781–3792. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.M.; Zheng, M.M.; Pan, Y.; Liu, S.Y.; Li, Y.; Wu, Y.L. Emerging evidence and treatment paradigm of non-small cell lung cancer. J. Hematol. Oncol. 2023, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef]
- Han, X.; Zhou, Y.; Liu, W. Precision cardio-oncology: Understanding the cardiotoxicity of cancer therapy. NPJ Precis. Oncol. 2017, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Vinod, S.K.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25 (Suppl. S2), 61–71. [Google Scholar] [CrossRef]
- Fendler, W.; Tomasik, B.; Atkins, K.; Stawiski, K.; Chałubińska-Fendler, J.; Kozono, D. The clinician’s guide to radiotherapy complications. Pol. Arch. Intern. Med. 2022, 132, 16190. [Google Scholar] [CrossRef]
- Gomez, D.R.; Yusuf, S.W.; Munsell, M.F.; Welsh, J.W.; Liao, Z.; Lin, S.H.; Pan, H.Y.; Chang, J.Y.; Komaki, R.; Cox, J.D.; et al. Prospective exploratory analysis of cardiac biomarkers and electrocardiogram abnormalities in patients receiving thoracic radiation therapy with high-dose heart exposure. J. Thorac. Oncol. 2014, 9, 1554–1560. [Google Scholar] [CrossRef]
- Vivekanandan, S.; Landau, D.B.; Counsell, N.; Warren, D.R.; Khwanda, A.; Rosen, S.D.; Parsons, E.; Ngai, Y.; Farrelly, L.; Hughes, L.; et al. The Impact of Cardiac Radiation Dosimetry on Survival After Radiation Therapy for Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 51–60. [Google Scholar] [CrossRef]
- Hotca, A.; Thor, M.; Deasy, J.O.; Rimner, A. Dose to the cardio-pulmonary system and treatment-induced electrocardiogram abnormalities in locally advanced non-small cell lung cancer. Clin. Transl. Radiat. Oncol. 2019, 19, 96–102. [Google Scholar] [CrossRef]
- Lee, C.; Maan, A.; Singh, J.P.; Fradley, M.G. Arrhythmias and device therapies in patients with cancer therapy-induced cardiomyopathy. Heart Rhythm. 2021, 18, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Fradley, M.G.; Beckie, T.M.; Brown, S.A.; Cheng, R.K.; Dent, S.F.; Nohria, A.; Patton, K.K.; Singh, J.P.; Olshansky, B. Recognition, Prevention, and Management of Arrhythmias and Autonomic Disorders in Cardio-Oncology: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e41–e55. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Buza, V.; Rajagopalan, B.; Curtis, A.B. Cancer Treatment–Induced Arrhythmias. Circ. Arrhythm. Electrophysiol. 2017, 10, e005443. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Ghosh, R.K.; Wongsaengsak, S.; Bandyopadhyay, D.; Ghosh, G.C.; Aronow, W.S.; Fonarow, G.C.; Lenihan, D.J.; Bhatt, D.L. Cardiovascular Toxicities of Immune Checkpoint Inhibitors: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 1714–1727. [Google Scholar] [CrossRef]
- Kilickap, S.; Barista, I.; Akgul, E.; Aytemir, K.; Aksoy, S.; Tekuzman, G. Early and late arrhythmogenic effects of doxorubicin. South. Med. J. 2007, 100, 262–265. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, F.Z.; Dai, S.; Hu, H.Y.; Fu, S.Y.; Liu, J.W.; Luo, F. Clinical insights into cisplatin-induced arrhythmia in a patient with locally advanced non-small cell lung cancer: A case report. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6–10. [Google Scholar]
- Morcos, P.N.; Bogman, K.; Hubeaux, S.; Sturm-Pellanda, C.; Ruf, T.; Bordogna, W.; Golding, S.; Zeaiter, A.; Abt, M.; Balas, B. Effect of alectinib on cardiac electrophysiology: Results from intensive electrocardiogram monitoring from the pivotal phase II NP28761 and NP28673 studies. Cancer Chemother. Pharmacol. 2017, 79, 559–568. [Google Scholar] [CrossRef]
- Khozin, S.; Blumenthal, G.M.; Zhang, L.; Tang, S.; Brower, M.; Fox, E.; Helms, W.; Leong, R.; Song, P.; Pan, Y.; et al. FDA Approval: Ceritinib for the Treatment of Metastatic Anaplastic Lymphoma Kinase–Positive Non–Small Cell Lung Cancer. Clin. Cancer Res. 2015, 21, 2436–2439. [Google Scholar] [CrossRef]
- Ou, S.H.I.; Tang, Y.; Polli, A.; Wilner, K.D.; Schnell, P. Factors associated with sinus bradycardia during crizotinib treatment: A retrospective analysis of two large-scale multinational trials (PROFILE 1005 and 1007). Cancer Med. 2016, 5, 617–622. [Google Scholar] [CrossRef]
- Kaira, K.; Ogiwara, Y.; Naruse, I. Occurrence of Ventricular Fibrillation in a Patient With Lung Cancer Receiving Osimertinib. J. Thorac. Oncol. 2020, 15, e54–e55. [Google Scholar] [CrossRef]
- Guo, G.G.; Luo, X.; Zhu, K.; Li, L.L.; Ou, Y.F. Fatal ventricular arrhythmias after osimertinib treatment for lung adenocarcinoma: A case report. J. Geriatr. Cardiol. 2023, 20, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Ott, P.A.; Hodi, F.S.; Husain, A.N.; Tajmir-Riahi, A.; Tawbi, H.; Pauschinger, M.; Gajewski, T.F.; Lipson, E.J.; Luke, J.J. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J. Immunother. Cancer 2016, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Behling, J.; Kaes, J.; Münzel, T.; Grabbe, S.; Loquai, C. New-onset third-degree atrioventricular block because of autoimmune-induced myositis under treatment with anti-programmed cell death-1 (nivolumab) for metastatic melanoma. Melanoma Res. 2017, 27, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wei, Q.; Wu, X. Cardiac arrhythmias associated with immune checkpoint inhibitors: A comprehensive disproportionality analysis of the FDA adverse event reporting system. Front. Pharmacol. 2022, 13, 986357. [Google Scholar] [CrossRef] [PubMed]
- Bergom, C.; Bradley, J.A.; Ng, A.K.; Samson, P.; Robinson, C.; Lopez-Mattei, J.; Mitchell, J.D. Past, Present, and Future of Radiation-Induced Cardiotoxicity: Refinements in Targeting, Surveillance, and Risk Stratification. JACC CardioOncol. 2021, 3, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Koyanagi, T.; Kasegawa, H.; Miyazaki, M. Three-day magnesium administration prevents atrial fibrillation after coronary artery bypass grafting. Ann. Thorac. Surg. 2005, 79, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ghezel-Ahmadi, V.; Ghezel-Ahmadi, D.; Beck, G.; Bölükbas, S. Perioperative systemic magnesium sulfate minimizes the incidence of atrial fibrillation after thoracotomy for lung resection: A prospective observational study. J. Thorac. Dis. 2023, 15, 4648–4656. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, F.; Reisenbauer, D.; Herkner, H.; Oppenauer, J.; Schuetz, N.; Niederdoeckl, J.; Schnaubelt, S.; Gupta, S.; Lutnik, M.; Simon, A.; et al. Association of Intravenous Potassium and Magnesium Administration With Spontaneous Conversion of Atrial Fibrillation and Atrial Flutter in the Emergency Department. JAMA Netw. Open. 2022, 5, e2237234. [Google Scholar] [CrossRef]
- Elbey, M.A.; Young, D.; Kanuri, S.H.; Akella, K.; Murtaza, G.; Garg, J.; Atkins, D.; Bommana, S.; Sharma, S.; Turagam, M.; et al. Diagnostic Utility of Smartwatch Technology for Atrial Fibrillation Detection—A Systematic Analysis. J. Atr. Fibrillation 2021, 13, 20200446. [Google Scholar]
- Pay, L.; Yumurtaş, A.Ç.; Satti, D.I.; Hui, J.M.H.; Chan, J.S.K.; Mahalwar, G.; Lee, Y.H.A.; Tezen, O.; Birdal, O.; Inan, D.; et al. Arrhythmias Beyond Atrial Fibrillation Detection Using Smartwatches: A Systematic Review. Anatol. J. Cardiol. 2023, 27, 126–131. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Madnick, D.L.; Fradley, M.G. Atrial Fibrillation and Cancer Patients: Mechanisms and Management. Curr. Cardiol. Rep. 2022, 24, 1517–1527. [Google Scholar] [CrossRef]
- Font, J.; Milliez, P.; Ouazar, A.B.; Klok, F.A.; Alexandre, J. Atrial fibrillation, cancer and anticancer drugs. Arch. Cardiovasc. Dis. 2023, 116, 219–226. [Google Scholar] [CrossRef]
- Imperatori, A.; Mariscalco, G.; Riganti, G.; Rotolo, N.; Conti, V.; Dominioni, L. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J. Cardiothorac. Surg. 2012, 7, 4. [Google Scholar] [CrossRef]
- Cheng, C.W.; Yang, N.I.; Ng, K.K.; Cherng, W.J. Metastatic cardiac tumor presenting as atrial fibrillation in a previously healthy woman. Medicine 2017, 96, e7649. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Kong, L.; Jia, B.; Chi, Y.; Zhai, X.; Jin, H.; Wang, Z. Clinical Characteristics of Cardiac and Pericardial Metastasis in Small Cell Lung Cancer with Arrhythmia. J. Cardiol. Cardiovasc. Sci. 2021, 5, 7–15. [Google Scholar]
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. J. Am. Coll. Cardiol. 2014, 63, 945–953. [Google Scholar] [CrossRef]
- Shabtaie, S.A.; Tan, N.Y.; Ward, R.C.; Lewis, B.R.; Yang, E.H.; Holmes, D.R., Jr.; Herrmann, J. Left Atrial Appendage Occlusion in Patients With Atrial Fibrillation and Cancer. JACC CardioOncol. 2023, 5, 203–212. [Google Scholar] [CrossRef]
- Kumar, S.; Yoon, S.; Milioglou, I.; Tashtish, N.; Farmakis, I.; Dallan, L.A.P.; Mogalapalli, A.; Arruda, M.; Filby, S.J. Left Atrial Appendage Closure Outcomes in Patients With Cancer at a Single Tertiary Center. Am. J. Cardiol. 2023, 202, 176–181. [Google Scholar] [CrossRef]
- Enevoldsen, C.; Borregaard, B.; Riber, S.S.; Riber, L.P.S. Amiodarone for Postoperative Atrial Fibrillation in Lung Cancer Patients: A 6-Year Follow-Up Study. Open J. Thorac. Surg. 2020, 10, 6–18. [Google Scholar] [CrossRef]
- Phillips, J.D.; Porter, E.D.; Beaulieu-Jones, B.R.; Fay, K.A.; Hasson, R.M.; Millington, T.M.; Finley, D.J. Postoperative atrial fibrillation prophylaxis using a novel amiodarone order set. J. Thorac. Dis. 2020, 12, 3110–3124. [Google Scholar] [CrossRef]
- Riber, L.P.; Christensen, T.D.; Jensen, H.K.; Hoejsgaard, A.; Pilegaard, H.K. Amiodarone Significantly Decreases Atrial Fibrillation in Patients Undergoing Surgery for Lung Cancer. Ann. Thorac. Surg. 2012, 94, 339–346. [Google Scholar] [CrossRef]
- Amar, D.; Zhang, H.; Chung, M.K.; Tan, K.S.; Desiderio, D.; Park, B.J.; Pedoto, A.; Roistacher, N.; Isbell, J.M.; Molena, D.; et al. Amiodarone with or without N. -Acetylcysteine for the Prevention of Atrial Fibrillation after Thoracic Surgery: A Double-blind, Randomized Trial. Anesthesiology 2022, 136, 916–926. [Google Scholar] [CrossRef]
- Cardinale, D.; Sandri, M.T.; Colombo, A.; Salvatici, M.; Tedeschi, I.; Bacchiani, G.; Beggiato, M.; Meroni, C.A.; Civelli, M.; Lamantia, G.; et al. Prevention of Atrial Fibrillation in High-risk Patients Undergoing Lung Cancer Surgery. Ann. Surg. 2016, 264, 244–251. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Ren, Y.; Han, J.; Li, G.; Guo, X. Pharmacological interventions for preventing atrial fibrillation after lung surgery: Systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2022, 78, 1777–1790. [Google Scholar] [CrossRef]
- Asnani, A.; Manning, A.; Mansour, M.; Ruskin, J.; Hochberg, E.P.; Ptaszek, L.M. Management of atrial fibrillation in patients taking targeted cancer therapies. Cardiooncology 2017, 3, 2. [Google Scholar] [CrossRef]
- Kumar, M.; Lopetegui-Lia, N.; Malouf CAl Almnajam, M.; Coll, P.P.; Kim, A.S. Atrial fibrillation in older adults with cancer. J. Geriatr. Cardiol. 2022, 19, 1–8. [Google Scholar]
- Tamura, M.; Kaneko, Y.; Nakajima, T.; Irie, T.; Kato, T.; Iijima, T.; Kurabayashi, M. A case of atrial tachycardia originating from pulmonary vein invaded by lung cancer. J. Cardiol. Cases 2012, 5, e118–e121. [Google Scholar] [CrossRef]
- Giustozzi, M.; Ali, H.; Reboldi, G.; Balla, C.; Foresti, S.; de Ambroggi, G.; Lupo, P.P.; Agnelli, G.; Cappato, R. Safety of catheter ablation of atrial fibrillation in cancer survivors. J. Interv. Card. Electrophysiol. 2021, 60, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Abraham, S.; Kumar, A.; Parikh, R.; Patel, R.; Khadke, S.; Kumar, A.; Liu, V.; Diaz, A.N.R.; Neilan, T.G.; et al. Efficacy and safety of catheter ablation for atrial fibrillation in patients with history of cancer. Cardio-Oncology 2023, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Rhea, I.; Burgos, P.H.; Fradley, M.G. Arrhythmogenic Anticancer Drugs in Cardio-Oncology. Cardiol. Clin. 2019, 37, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Roomi, S.; Lashari, B.H.; Khan, M.A.A. Non-Sustained Ventricular Tachycardia as a Sign of Lung Cancer. Cureus 2019, 11, e6090. [Google Scholar] [CrossRef] [PubMed]
- Porta-Sánchez, A.; Gilbert, C.; Spears, D.; Amir, E.; Chan, J.; Nanthakumar, K.; Thavendiranathan, P. Incidence, Diagnosis, and Management of QT Prolongation Induced by Cancer Therapies: A Systematic Review. J. Am. Heart Assoc. 2017, 6, e007724. [Google Scholar] [CrossRef] [PubMed]
- Grehn, M.; Mandija, S.; Miszczyk, M.; Krug, D.; Tomasik, B.; Stickney, K.E.; Alcantara, P.; Alongi, F.; Anselmino, M.; Aranda, R.S.; et al. STereotactic Arrhythmia Radioablation (STAR): The Standardized Treatment and Outcome Platform for Stereotactic Therapy Of Re-entrant tachycardia by a Multidisciplinary consortium (STOPSTORM.eu) and review of current patterns of STAR practice in Europe. Europace 2023, 25, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Siedow, M.; Brownstein, J.; Prasad, R.N.; Loccoh, E.; Harfi, T.T.; Okabe, T.; Tong, M.S.; Afzal, M.R.; Williams, T. Cardiac radioablation in the treatment of ventricular tachycardia. Clin. Transl. Radiat. Oncol. 2021, 31, 71–79. [Google Scholar] [CrossRef]
- Cuculich, P.S.; Schill, M.R.; Kashani, R.; Mutic, S.; Lang, A.; Cooper, D.; Faddis, M.; Gleva, M.; Noheria, A.; Smith, T.W.; et al. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N. Engl. J. Med. 2017, 377, 2325–2336. [Google Scholar] [CrossRef]
- Zhang, D.M.; Szymanski, J.; Bergom, C.; Cuculich, P.S.; Robinson, C.G.; Schwarz, J.K.; Rentschler, S.L. Leveraging Radiobiology for Arrhythmia Management: A New Treatment Paradigm? Clin. Oncol. R. Coll. Radiol. 2021, 33, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Navara, R.; Yin, T.; Szymanski, J.; Goldsztejn, U.; Kenkel, C.; Lang, A.; Mpoy, C.; Lipovsky, C.E.; Qiao, Y.; et al. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat. Commun. 2021, 12, 5558. [Google Scholar] [CrossRef]
- Wang, S.; Luo, H.; Mao, T.; Xiang, C.; Hu, H.; Zhao, J.; Wang, X.; Wang, J.; Liu, H.; Yu, L.; et al. Stereotactic arrhythmia radioablation: A novel therapy for cardiac arrhythmia. Heart Rhythm. 2023, 20, 1327–1336. [Google Scholar] [CrossRef]
- Viani, G.A.; Gouveia, A.G.; Pavoni, J.F.; Louie, A.V.; Detsky, J.; Spratt, D.E.; Moraes, F.Y. A Meta-analysis of the Efficacy and Safety of Stereotactic Arrhythmia Radioablation (STAR) in Patients with Refractory Ventricular Tachycardia. Clin. Oncol. 2023, 35, 611–620. [Google Scholar] [CrossRef]
- Krug, D.; Zaman, A.; Eidinger, L.; Grehn, M.; Boda-Heggemann, J.; Rudic, B.; Mehrhof, F.; Boldt, L.H.; Hohmann, S.; Merten, R.; et al. Radiosurgery for ventricular tachycardia (RAVENTA): Interim analysis of a multicenter multiplatform feasibility trial. Strahlenther. Onkol. 2023, 199, 621–630. [Google Scholar] [CrossRef]
- van der Ree, M.H.; Dieleman, E.M.T.; Visser, J.; Planken, R.N.; Boekholdt, S.M.; de Bruin-Bon, R.H.A.; Rasch, C.R.N.; Hoeksema, W.F.; de Jong, R.M.A.J.; Kemme, M.J.B.; et al. Non-invasive stereotactic arrhythmia radiotherapy for ventricular tachycardia: Results of the prospective STARNL-1 trial. Europace 2023, 25, 1015–1024. [Google Scholar] [CrossRef]
- Gianni, C.; Rivera, D.; Burkhardt, J.D.; Pollard, B.; Gardner, E.; Maguire, P.; Al-Ahmad, A. Stereotactic arrhythmia radioablation for refractory scar-related ventricular tachycardia. Heart Rhythm. 2020, 17, 1241–1248. [Google Scholar] [CrossRef]
- Robinson, C.G.; Samson, P.P.; Moore, K.M.S.; Hugo, G.D.; Knutson, N.; Mutic, S.; Goddu, S.M.; Lang, A.; Cooper, D.H.; Faddis, M.; et al. Phase I/II Trial of Electrophysiology-Guided Noninvasive Cardiac Radioablation for Ventricular Tachycardia. Circulation 2019, 139, 313–321. [Google Scholar] [CrossRef]
- Miszczyk, M.; Sajdok, M.; Bednarek, J.; Latusek, T.; Wojakowski, W.; Tomasik, B.; Wita, K.; Jadczyk, T.; Kurzelowski, R.; Drzewiecka, A.; et al. Stereotactic management of arrhythmia—Radiosurgery in treatment of ventricular tachycardia (SMART-VT). Results of a prospective safety trial. Radiother. Oncol. 2023, 188, 109857. [Google Scholar] [CrossRef]
- Shangguan, W.; Xu, G.; Wang, X.; Zhang, N.; Liu, X.; Li, G.; Tse, G.; Liu, T. Stereotactic Radiotherapy: An Alternative Option for Refractory Ventricular Tachycardia to Drug and Ablation Therapy. J. Clin. Med. 2022, 11, 3549. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.; Biagi, J.; Redfearn, D.; Boles, U.; Kamel, D.; Ali, F.S.; Hopman, W.M.; Michael, K.A.; Simpson, C.; Abdollah, H.; et al. Increased Incidence of Ventricular Arrhythmias in Patients With Advanced Cancer and Implantable Cardioverter-Defibrillators. JACC Clin. Electrophysiol. 2017, 3, 50–56. [Google Scholar] [CrossRef]
- Itzhaki Ben Zadok, O.; Nardi Agmon, I.; Neiman, V.; Eisen, A.; Golovchiner, G.; Bental, T.; Schamroth-Pravda, N.; Kadmon, E.; Goldenberg, G.R.; Erez, A.; et al. Implantable Cardioverter Defibrillator for the Primary Prevention of Sudden Cardiac Death among Patients With Cancer. Am. J. Cardiol. 2023, 191, 32–38. [Google Scholar] [CrossRef]
- Christensen, A.M.; Bjerre, J.; Schou, M.; Jons, C.; Vinther, M.; Gislason, G.H.; Johansen, J.B.; Nielsen, J.C.; Petersen, H.H.; Riahi, S.; et al. Clinical outcome in patients with implantable cardioverter-defibrillator and cancer: A nationwide study. Europace 2019, 21, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Seol, K.H. Inevitable high-dose irradiation to lead of implantable cardioverter defibrillator in small cell lung cancer: A case report. J. Med. Case Rep. 2019, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Ilangovan, B.; Venkatraman, M.; Balasundaram, S.; Janarthinakani, M. Radiotherapy for patients with cardiovascular implantable electronic device-a single institutional experience. IHJ Cardiovasc. Case Rep. (CVCR) 2022, 6, 16–20. [Google Scholar] [CrossRef]
- Sławiński, G.; Wrona, A.; Dąbrowska-Kugacka, A.; Raczak, G.; Lewicka, E. Immune Checkpoint Inhibitors and Cardiac Toxicity in Patients Treated for Non-Small Lung Cancer: A Review. Int. J. Mol. Sci. 2020, 21, 7195. [Google Scholar] [CrossRef] [PubMed]
- Bukamur, H.S.; Mezughi, H.; Karem, E.; Shahoub, I.; Shweihat, Y. Nivolumab-induced Third Degree Atrioventricular Block in a Patient with Stage IV Squamous Cell Lung Carcinoma. Cureus 2019, 11, e4869. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhao, L.; Zhou, C.; Wang, H.; Jiang, S.; Li, Y.; Peng, Y.; Deng, C.; Ma, F.; Pan, Y.; et al. Pacemakers and methylprednisolone pulse therapy in immune-related myocarditis concomitant with complete heart block. Open Med. 2022, 17, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Pallangyo, P.; Kweka, G.; Lyimo, F.; Mayala, H.; Swai, H.J.; Mkojera, Z.; Misidai, N.; Komba, M.; Millinga, J.; Bhalia, S.; et al. Complete heart block ensuing from a metastatic small cell carcinoma: A case report. J. Med. Case Rep. 2022, 16, 77. [Google Scholar] [CrossRef]
- Mocini, D.; Longo, R.; Colivicchi, F.; Morabito, A.; Gasparini, G.; Santini, M. A complete atrioventricular block secondary to myocardial metastases of lung cancer. Case Rep. Ital. Heart J. 2005, 6, 931–932. [Google Scholar]
- Vasic, N.; Stevic, R.; Pesut, D.; Jovanovic, D. Acute left bundle branch block as a complication of brachytherapy for lung cancer. Respir. Med. 2011, 105, S78–S80. [Google Scholar] [CrossRef] [PubMed]
- Pruis, M.A.; Veerman, G.D.M.; Hassing, H.C.; Lanser, D.A.C.; Paats, M.S.; van Schaik, R.H.N.; Mathijssen, R.H.J.; Manintveld, O.; Dingemans, A.C. Cardiac Toxicity of Alectinib in Patients With ALK+ Lung Cancer. JACC CardioOncol. 2023, 5, 102–113. [Google Scholar] [CrossRef]
- Harδardottir, H.; Jonsson, S.; Gunnarsson, O.; Hilmarsdottir, B.; Asmundsson, J.; Gudmundsdottir, I.; Saevarsdottir, V.Y.; Hansdottir, S.; Hannesson, P.; Gudbjartsson, T. Advances in lung cancer diagnosis and treatment—A review. Laeknabladid 2022, 108, 17–29. [Google Scholar]
- Stefanidis, K.; Konstantellou, E.; Yusuf, G.; Moser, J.; Tan, C.; Vlahos, I. The Evolving Landscape of Lung Cancer Surgical Resection: An Update for Radiologists With Focus on Key Chest CT Findings. AJR Am. J. Roentgenol. 2022, 218, 52–65. [Google Scholar] [CrossRef]
- Kasprzyk, M.; Sławiński, G.; Musik, M.; Marciniak, Ł.; Dyszkiewicz, W.; Piwkowski, C.; Gałęcki, B. THORACIC SURGERY Completion pneumonectomy and chemoradiotherapy as treatment options in local recurrence of non-small-cell lung cancer. Pol. J. Cardio-Thorac. Surg. 2015, 1, 18–25. [Google Scholar] [CrossRef]
- Rozencwajg, S.; Desthieux, C.; Szymkiewicz, O.; Ynineb, Y.; Fulgencio, J.P.; Bonnet, F. The risk of atrial fibrillation after pneumonectomy is not impaired by preoperative administration of dexamethasone. Cohort Study. Anaesth. Crit. Care Pain. Med. 2017, 36, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Vaporciyan, A.A.; Correa, A.M.; Rice, D.C.; Roth, J.A.; Smythe, W.R.; Swisher, S.G.; Walsh, G.L.; Putnam, J.B., Jr. Risk factors associated with atrial fibrillation after noncardiac thoracic surgery: Analysis of 2588 patients. J. Thorac. Cardiovasc. Surg. 2004, 127, 779–786. [Google Scholar] [CrossRef] [PubMed]
- No, H.J.; Guo, F.B.; Park, N.J.I.; Kastelowitz, N.; Rhee, J.W.; Clark, D.E.; Chin, A.L.C.; Vitzthum, L.K.; Horst, K.C.; Moding, E.J.; et al. Predicting Adverse Cardiac Events After Radiotherapy for Locally Advanced Non–Small Cell Lung Cancer. JACC CardioOncol. 2023; in press. [Google Scholar] [CrossRef]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Bosch, W.; Galvin, J.M.; et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Banfill, K.; Giuliani, M.; Aznar, M.; Franks, K.; McWilliam, A.; Schmitt, M.; Sun, F.; Vozenin, M.C.; Faivre Finn, C. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J. Thorac. Oncol. 2021, 16, 216–227. [Google Scholar] [CrossRef]
- Mo, Y.; Tian, B.; Wu, M.; Chen, M.; Chen, D.; Yu, J. Dual effect of radiotherapy related concomitant cardiovascular diseases in non-small cell lung cancer. Cancer Med. 2023, 12, 1025–1034. [Google Scholar] [CrossRef]
- Mery, B.; Guichard, J.B.; Guy, J.B.; Vallard, A.; Barthelemy, J.C.; Da Costa, A.; Magné, N.; Bertoletti, L. Atrial fibrillation in cancer patients: Hindsight, insight and foresight. Int. J. Cardiol. 2017, 240, 196–202. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Q.; Ma, S. Left atrial fibrosis in atrial fibrillation: Mechanisms, clinical evaluation and management. J. Cell Mol. Med. 2021, 25, 2764–2775. [Google Scholar] [CrossRef]
- Huang, Y.J.; Harrison, A.; Sarkar, V.; Rassiah-Szegedi, P.; Zhao, H.; Szegedi, M.; Huang, L.; Wilson, B.; Gaffney, D.K.; Salter, B.J. Detection of late radiation damage on left atrial fibrosis using cardiac late gadolinium enhancement magnetic resonance imaging. Adv. Radiat. Oncol. 2016, 1, 106–114. [Google Scholar] [CrossRef]
- Bedi, R.; Ahmad, A.; Horbal, P.; Mar, P.L. Radiation-associated Arrhythmias: Putative Pathophysiological Mechanisms, Prevalence, Screening and Management Strategies. Arrhythm. Electrophysiol. Rev. 2023, 12, e24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.W.; Snir, J.; Boldt, R.G.; Rodrigues, G.B.; Louie, A.V.; Gaede, S.; McGarry, R.C.; Urbanic, J.J.; Daly, M.E.; Palma, D.A. Is the Importance of Heart Dose Overstated in the Treatment of Non-Small Cell Lung Cancer? A Systematic Review of the Literature. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 582–589. [Google Scholar] [CrossRef]
- Kim, K.H.; Oh, J.; Yang, G.; Lee, J.; Kim, J.; Gwak, S.Y.; Cho, I.; Lee, S.H.; Byun, H.K.; Choi, H.K.; et al. Association of Sinoatrial Node Radiation Dose With Atrial Fibrillation and Mortality in Patients With Lung Cancer. JAMA Oncol. 2022, 8, 1624–1634. [Google Scholar] [CrossRef]
- Borkenhagen, J.F.; Bergom, C.; Rapp, C.T.; Klawikowski, S.J.; Rein, L.E.; Gore, E.M. Dosimetric Predictors of Cardiotoxicity in Thoracic Radiotherapy for Lung Cancer. Clin. Lung Cancer 2019, 20, 435–441. [Google Scholar] [CrossRef]
- Atkins, K.M.; Nikolova, A.; Guthier, C.V.; Bitterman, D.S.; Kozono, D.E.; Nohria, A.; Mak, R.H. Association of Cardiac Sub-Structure Radiation Dose with Bradyarrhythmias and Tachyarrhythmias after Lung Cancer Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, S58–S59. [Google Scholar] [CrossRef]
- Walls, G.M.; McCann, C.; Ball, P.; Atkins, K.M.; Mak, R.H.; Bedair, A.; O’Hare, J.; McAleese, J.; Harrison, C.; Tumelty, K.A.; et al. A pulmonary vein atlas for radiotherapy planning. Radiother. Oncol. 2023, 184, 109680. [Google Scholar] [CrossRef]
- Niska, J.R.; Thorpe, C.S.; Allen, S.M.; Daniels, T.B.; Rule, W.G.; Schild, S.E.; Vargas, C.E.; Mookadam, F. Radiation and the heart: Systematic review of dosimetry and cardiac endpoints. Expert. Rev. Cardiovasc. Ther. 2018, 16, 931–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Pearlstein, K.A.; Patchett, N.D.; Deal, A.M.; Mavroidis, P.; Jensen, B.C.; Lipner, M.B.; Zagar, T.M.; Wang, Y.; Lee, C.B.; et al. Heart dosimetric analysis of three types of cardiac toxicity in patients treated on dose-escalation trials for Stage III non-small-cell lung cancer. Radiother Oncol. 2017, 125, 293–300. [Google Scholar] [CrossRef]
- Chang, W.T.; Lin, H.W.; Chang, T.C.; Lin, S.H.; Li, Y.H. The association between tyrosine kinase inhibitors and fatal arrhythmia in patients with non-small cell lung cancer in Taiwan. Front. Oncol. 2023, 13, 1172036. [Google Scholar] [CrossRef] [PubMed]
| Drug | Drug Class | Associated Arrhythmias |
|---|---|---|
| Doxorubicin | Anthracyclines | Sinus bradycardia, sinus tachycardia, supraventricular and ventricular arrhythmias (rarely reported life-threatening arrhythmias), AV block, other conduction disturbances [17] |
| Methotrexate | Antimetabolites | Sinus bradycardia, supraventricular tachycardia, VT, VF [15] |
| Vincristine | Vinca alkaloids | AF, PVCs (very rarely) [15] |
| Cyclophosphamide | Alkylating agents | Sinus bradycardia, PACs, PVCs, supraventricular tachycardia, AF, ventricular arrhythmias, AV block [15] |
| Cisplatin | Platinum compounds | Sinus bradycardia, supraventricular tachycardia, AF (>10%), PACs, PVCs, VT (1–10%) [15,18] |
| Paclitaxel | Taxanes | Sinus bradycardia, sinus tachycardia (>10%), AF (<1%), AV block, VT (<1%) [15] |
| Alectinib | Multitargeted TKIs | Sinus bradycardia (5.1–20%), mean QTc change of 5.3 ms, QTc > 500 ms (0.45%) [15,19] |
| Ceritinib | Multi-targeted TKIs | Sinus bradycardia (3%), PACs, QTc > 500 ms (0.33%) [15,20] |
| Crizotinib | Multi-targeted TKIs | Sinus bradycardia (5–69%), mean QTc change of 9–13.3 ms, QTc > 500 ms (1.3%) [15,21] |
| Osimertinib | Multi-targeted TKIs | VT [22,23] |
| Pembrolizumab | ICIs | Rare cases of sinus tachycardia, ventricular bigeminy, AF, SCD [15,24] |
| Nivolumab | ICIs | VT (<1%), AV block [15,25] |
| Atezolizumab | ICIs | Complete AV block, AF [26] |
| Ipilimumab | ICIs | Limited reports on AF and malignant ventricular arrhythmias [15] |
| (A) CHA2DS2-VASc Score for Atrial Fibrillation | Score | (B) HAS-BLED Bleeding Risk Score | Score |
|---|---|---|---|
| For risk stratification of ischemic stroke and thromboembolism in patients with AF | |||
| Congestive heart failure | 1 | Hypertension | 1 |
| Signs/symptoms of heart failure confirmed with objective evidence of cardiac dysfunction | |||
| Hypertension | 1 | Abnormal liver function | 1 |
| Resting BP > 140/90 mmHg on at least 2 occasions or current antihypertensive pharmacologic treatment | Liver disease, bilirubin > 2× ULN with ASAT/ALAT/ALP > 3× ULN | ||
| Age 75 years or older | 2 | Abnormal liver function | 1 |
| Dialysis, kidney transplantation, creatinine ≥ 200 µmol/L (≥2.26 mg/dL) | |||
| Diabetes mellitus | 1 | Stroke | 1 |
| Fasting glucose > 125 mg/dL or treatment with oral hypoglycemic agent and/or insulin | |||
| Stroke, TIA, or SE | 2 | Drugs | 1 |
| Includes any history of cerebral ischemia | Concomitant use of antiplatelet/NSAIDs | ||
| Vascular disease | 1 | Labile INR | 1 |
| Prior myocardial infarction (MI), peripheral arterial disease (PAD), or aortic plaque | <60% time in therapeutic INR range | ||
| Age 65 to 74 years | 1 | Bleeding | 1 |
| Previous major bleeding or predisposition to bleeding | |||
| Sex category (female) | 1 | Alcohol | 1 |
| Female gender confers higher risk | ≥8 units/week | ||
| Elderly: age greater than 65 years | 1 | ||
| Maximum 9 | Maximum 9 | ||
| Score: 0 low risk 1–2 moderate/intermediate risk ≥3 high risk Score ≥ 2–recommend anticoagulation | Score: 0 Low risk for major bleeding 1–2 Moderate risk for major bleeding ≥3 High risk of major bleeding >5 Very high risk of bleeding If a score is ≥3 a caution is needed and regular patient review is recommended Consider LAA closure in high risk patients (score > 4) with a life expectancy > 12 months | ||
| Study (Ref) | Findings |
|---|---|
| Atkins KM et al. (2022) [97] | V5Gy ≥ 60 cm3 for the left atrium associated with increased risk of AF |
| Atkins KM et al. (2022) [97] | V60Gy ≥ 0.03 cm3 for the right atrium associated with increased the risk of SVT |
| Atkins KM et al. (2022) [97] | V5Gy ≥ 1 cm3 for the left main coronary artery was a risk factor of VT occurrence |
| Atkins KM et al. (2022) [97] | V10Gy ≥ 1 cm3 for the left main coronary artery increased the risk of bradyarrhythmias |
| Kim K [95] et al. (2022) | The maximum dose delivered to the sinoatrial node increased the risk of atrial fibrillation |
| Wang K et al. (2017) [100] | Risk factors for arrhythmic events: heart V5, right atrium V60, heart V30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawryszko, M.; Sławiński, G.; Tomasik, B.; Lewicka, E. Cardiac Arrhythmias in Patients Treated for Lung Cancer: A Review. Cancers 2023, 15, 5723. https://doi.org/10.3390/cancers15245723
Hawryszko M, Sławiński G, Tomasik B, Lewicka E. Cardiac Arrhythmias in Patients Treated for Lung Cancer: A Review. Cancers. 2023; 15(24):5723. https://doi.org/10.3390/cancers15245723
Chicago/Turabian StyleHawryszko, Maja, Grzegorz Sławiński, Bartłomiej Tomasik, and Ewa Lewicka. 2023. "Cardiac Arrhythmias in Patients Treated for Lung Cancer: A Review" Cancers 15, no. 24: 5723. https://doi.org/10.3390/cancers15245723





