Chordoma: A Comprehensive Systematic Review of Clinical Trials
Abstract
:Simple Summary
Abstract
1. Introduction
2. Objective
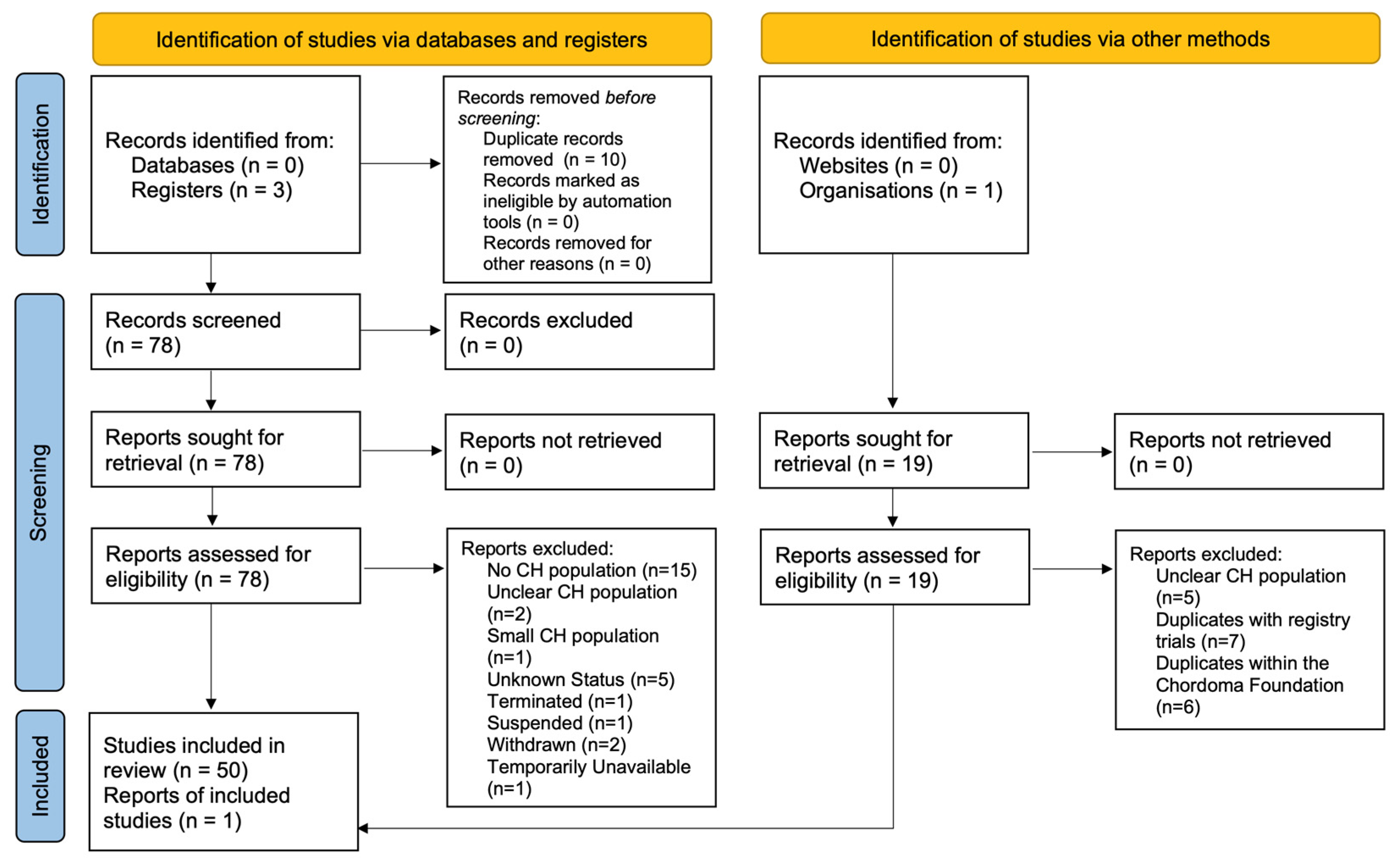
3. Materials & Methods
4. Results
4.1. Clinical Trials Data
4.2. Therapeutic Clinical Trials (Table 1a)
4.2.1. Biologics & Drugs
Monoclonal Antibody (mAb)-Based Immunotherapy
Enhancer of Zeste Homolog 2 (EZH2) Inhibitors
Tyrosine Kinase Inhibitors (TKIs)
Cyclin-Dependent Kinases (CDK) Inhibitors
Brachyury-Targeting Interventions (Figure 5)
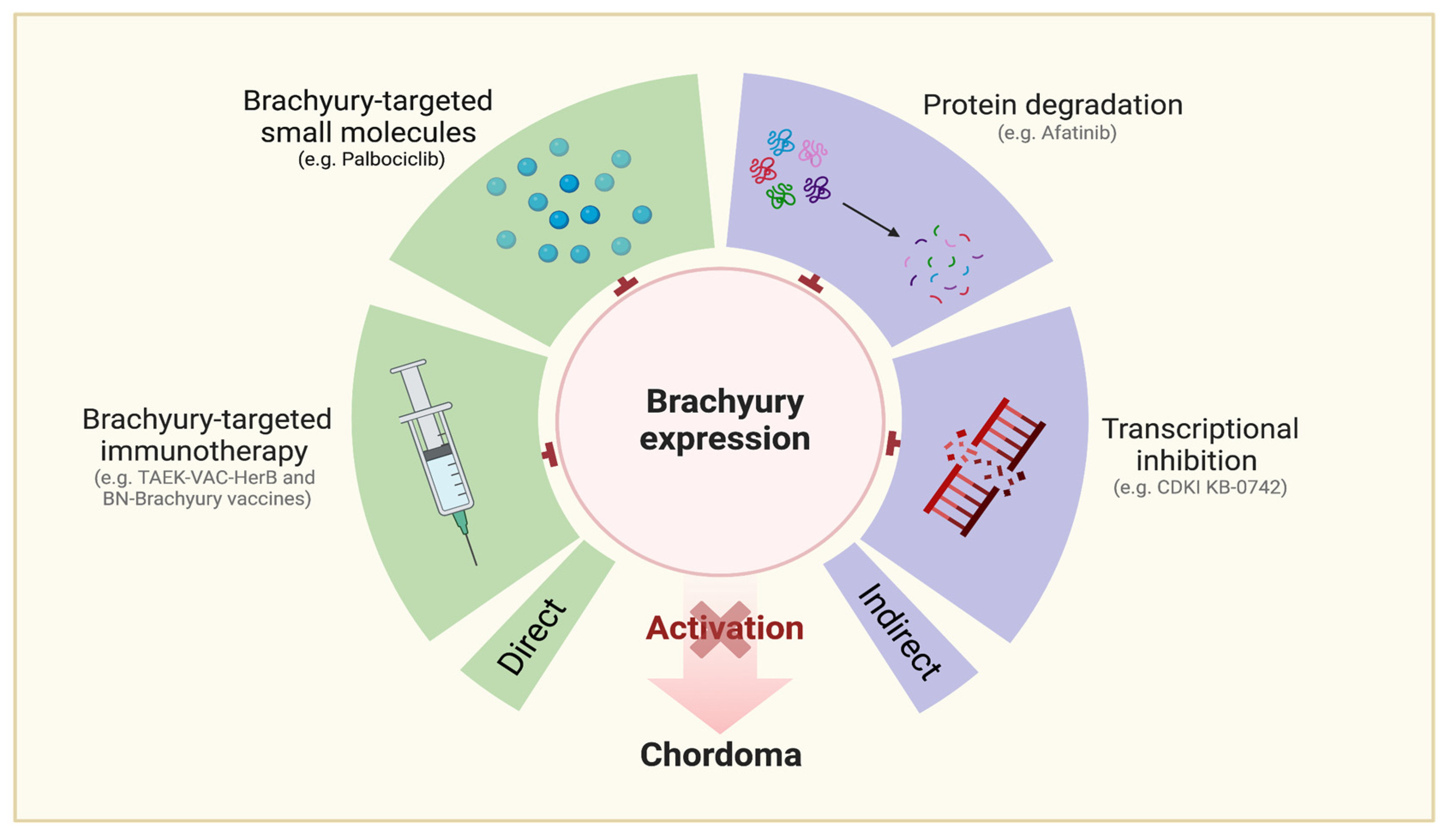
Ancillary Agents
4.2.2. Radiation (Table 1a)
4.3. Observational (Table 1b)
4.3.1. Imaging
4.3.2. Genomic/Molecular
4.3.3. Quality of Life (QoL)/Natural History
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hung, G.Y.; Horng, J.L.; Yen, H.J.; Yen, C.C.; Chen, W.M.; Chen, P.C.H.; Wu, H.T.H.; Chiou, H.J. Incidence Patterns of Primary Bone Cancer in Taiwan (2003–2010): A Population-Based Study. Ann. Surg. Oncol. 2014, 21, 2490–2498. [Google Scholar] [CrossRef]
- McMaster, M.L.; Goldstein, A.M.; Bromley, C.M.; Ishibe, N.; Parry, D.M. Chordoma: Incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control 2001, 12, 1–11. [Google Scholar] [CrossRef]
- Smoll, N.R.; Gautschi, O.P.; Radovanovic, I.; Schaller, K.; Weber, D.C. Incidence and relative survival of chordomas: The standardized mortality ratio and the impact of chordomas on a population. Cancer 2013, 119, 2029–2037. [Google Scholar] [CrossRef]
- Whelan, J.; McTiernan, A.; Cooper, N.; Wong, Y.K.; Francis, M.; Vernon, S.; Strauss, S.J. Incidence and survival of malignant bone sarcomas in England 1979–2007. Int. J. Cancer 2012, 131, E508–E517. [Google Scholar] [CrossRef]
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef]
- Sun, X.; Hornicek, F.; Schwab, J.H. Chordoma: An update on the pathophysiology and molecular mechanisms. Curr. Rev. Musculoskelet. Med. 2015, 8, 344–352. [Google Scholar] [CrossRef]
- Kremenevski, N.; Schlaffer, S.M.; Coras, R.; Kinfe, T.M.; Graillon, T.; Buchfelder, M. Skull Base Chordomas and Chondrosarcomas. Neuroendocrinology 2020, 110, 836–847. [Google Scholar] [CrossRef]
- Pillai, S.; Govender, S. Sacral chordoma: A review of literature. J. Orthop. 2018, 15, 679–684. [Google Scholar] [CrossRef]
- Sciubba, D.M.; Cheng, J.J.; Petteys, R.J.; Weber, K.L.; Frassica, D.A.; Gokaslan, Z.L. Chordoma of the sacrum and vertebral bodies. J. Am. Acad. Orthop. Surg. 2009, 17, 708–717. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef]
- Barber, S.M.; Sadrameli, S.S.; Lee, J.J.; Fridley, J.S.; Teh, B.S.; Oyelese, A.A.; Telfeian, A.E.; Gokaslan, Z.L. Chordoma-Current Understanding and Modern Treatment Paradigms. J. Clin. Med. 2021, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.J.; Bloch, O.G.; Yang, I.; Han, S.J.; Aranda, D.; Tihan, T.; Parsa, A.T. Adjuvant radiation therapy and chondroid chordoma subtype are associated with a lower tumor recurrence rate of cranial chordoma. J. Neuro-Oncol. 2010, 98, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Pallini, R.; Maira, G.; Pierconti, F.; Falchetti, M.L.; Alvino, E.; Cimino-Reale, G.; Fernandez, E.; D’Ambrosio, E.; Larocca, L.M. Chordoma of the skull base: Predictors of tumor recurrence. J. Neurosurg. 2003, 98, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Gronchi, A.; Fossati, P.; Akiyama, T.; Alapetite, C.; Baumann, M.; Blay, J.Y.; Bolle, S.; Boriani, S.; Bruzzi, P.; et al. Best practices for the management of local-regional recurrent chordoma: A position paper by the Chordoma Global Consensus Group. Ann. Oncol. 2017, 28, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.J.; Neale, N.; Sun, J.; Yang, M.; Bai, H.X.; Tang, L.; Zhan, Z.S.; Landi, A.; Wang, Y.Y.; Huang, R.Y.; et al. Prognostic Factors in Clival Chordomas: An Integrated Analysis of 347 Patients. World Neurosurg. 2018, 118, E375–E387. [Google Scholar] [CrossRef] [PubMed]
- Freed, D. Chordoma Foundation Labs—A resource for accelerating the development of new systemic therapies. In Proceedings of the International Chordoma Research Workshop, Boston, MA, USA, 13–14 July 2023. [Google Scholar]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Novartis Pharmaceuticals. A Study of FAZ053 Single Agent and in Combination with PDR001 in Patients with Advanced Malignancies. 2016. Available online: https://classic.clinicaltrials.gov/show/NCT02936102 (accessed on 30 January 2023).
- National Cancer Institute (NCI). Tiragolumab and Atezolizumab for the Treatment of Relapsed or Refractory SMARCB1 or SMARCA4 Deficient Tumors. 2022. Available online: https://classic.clinicaltrials.gov/show/NCT05286801 (accessed on 30 January 2023).
- Sarcoma Oncology Research Center, LLC; Aadi Bioscience, Inc. Nivolumab (Opdivo®) Plus ABI-009 (Nab-Rapamycin) for Advanced Sarcoma and Certain Cancers. 2017. Available online: https://classic.clinicaltrials.gov/show/NCT03190174 (accessed on 30 January 2023).
- Dana-Farber Cancer Institute; Gateway for Cancer Research. Study of Nivolumab and Ipilimumab in Children and Young Adults with INI1-Negative Cancers. 2020. Available online: https://classic.clinicaltrials.gov/show/NCT04416568 (accessed on 30 January 2023).
- Centre Léon Bérard. RAR-Immune: A Randomised, Comparative, Prospective, Multicentre Study of the Efficacy of Nivolumab + Ipilimumab versus Pazopanib Alone in Patients with Metastatic or Unresectable Advanced Sarcoma of Rare Subtype; Centre Léon Bérard: Lyon, France, 2020. [Google Scholar]
- Jonsson Comprehensive Cancer Center; Bristol-Myers Squibb. Nivolumab and Relatlimab in Treating Participants with Advanced Chordoma. 2019. Available online: https://classic.clinicaltrials.gov/show/NCT03623854 (accessed on 30 January 2023).
- Sarcoma Oncology Research Center, LLC. Talimogene Laherparepvec, Nivolumab and Trabectedin for Sarcoma. 2019. Available online: https://classic.clinicaltrials.gov/show/NCT03886311 (accessed on 30 January 2023).
- Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; National Cancer Institute (NCI); Bristol-Myers Squibb; Chordoma Foundation. Nivolumab with or Without Stereotactic Radiosurgery in Treating Patients with Recurrent, Advanced, or Metastatic Chordoma. 2017. Available online: https://classic.clinicaltrials.gov/show/NCT02989636 (accessed on 30 January 2023).
- UNICANCER. Secured Access to Pembrolizumab for Adult Patients with Selected Rare Cancer; UNICANCER: Paris, France, 2017. [Google Scholar]
- M.D. Anderson Cancer Center. Cetuximab for the Treatment of Advanced Unresectable or Metastatic Chordoma. 2022. Available online: https://classic.clinicaltrials.gov/show/NCT05041127 (accessed on 30 January 2023).
- Epizyme, Inc. A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects with Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma. 2016. Available online: https://classic.clinicaltrials.gov/show/NCT02601937 (accessed on 30 January 2023).
- Epizyme, Inc.; Ipsen. A Study of Tazemetostat in Adult Participants with Soft Tissue Sarcoma. 2015. Available online: https://classic.clinicaltrials.gov/show/NCT02601950 (accessed on 30 January 2023).
- Susan Chi MD; Bristol-Myers Squibb; Epizyme, Inc.; Dana-Farber Cancer Institute. Tazemetostat+Nivo/Ipi in INI1-Neg/SMARCA4-Def Tumors. 2023. Available online: https://classic.clinicaltrials.gov/show/NCT05407441 (accessed on 30 January 2023).
- Massachusetts General Hospital. Nilotinib with Radiation for High Risk Chordoma. 2011. Available online: https://classic.clinicaltrials.gov/show/NCT01407198 (accessed on 30 January 2023).
- Isitituto Nazionale Per La Cura Tumori. Phase II Study of Lapatinib in egfr/her2neu Positive Advanced Chordoma. 2009. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2009-014456-29/IT (accessed on 30 January 2023).
- Leiden University Medical Center; Chordoma Foundation; Boehringer Ingelheim. Afatinib in Locally Advanced and Metastatic Chordoma. 2018. Available online: https://classic.clinicaltrials.gov/show/NCT03083678 (accessed on 30 January 2023).
- Huashan Hospital; Boehringer Ingelheim. Study to Evaluate the Efficacy of Afatinib in Skull Base Chordoma. 2022. Available online: https://classic.clinicaltrials.gov/show/NCT05519917 (accessed on 30 January 2023).
- Novartis Pharmaceuticals; Novartis Pharmaceuticals. Efficacy and Safety of Imatinib in Chordoma. 2004. Available online: https://classic.clinicaltrials.gov/show/NCT00150072 (accessed on 30 January 2023).
- Isitituto Nazionale Per La Cura Tumori. Phase II Study on Imatinib in Combination with RAD001 in Advanced Chordoma. 2010. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-021755-34/IT (accessed on 30 January 2023).
- American Society of Clinical Oncology; AstraZeneca; Bayer; Bristol-Myers Squibb; Eli Lilly and Company; Genentech, Inc.; Merck Sharp & Dohme LLC; Pfizer; Boehringer Ingelheim; Seagen Inc.; et al. TAPUR: Testing the Use of Food and Drug Administration (FDA) Approved Drugs That Target a Specific Abnormality in a Tumor Gene in People with Advanced Stage Cancer. 2016. Available online: https://classic.clinicaltrials.gov/show/NCT02693535 (accessed on 30 January 2023).
- Bio, K. A Dose Escalation and Cohort Expansion Study of KB-0742 in Participants with Relapsed or Refractory Solid Tumors or Non-Hodgkin Lymphoma. 2021. Available online: https://classic.clinicaltrials.gov/show/NCT04718675 (accessed on 30 January 2023).
- University Hospital Heidelberg. CDK4/6 Inhibition in Locally Advanced/Metastatic Chordoma. 2017. Available online: https://classic.clinicaltrials.gov/show/NCT03110744 (accessed on 30 January 2023).
- Grupo Espanol de Investigacion en Sarcomas. Trial of Palbociclib in Second Line of Advanced Sarcomas with CDK4 Overexpression. 2017. Available online: https://classic.clinicaltrials.gov/show/NCT03242382 (accessed on 30 January 2023).
- Bavarian Nordic. TAEK-VAC-HerBy Vaccine for Brachyury and HER2 Expressing Cancer. 2020. Available online: https://classic.clinicaltrials.gov/show/NCT04246671 (accessed on 30 January 2023).
- Bavarian Nordic. BN Brachyury and Radiation in Chordoma. 2018. Available online: https://classic.clinicaltrials.gov/show/NCT03595228 (accessed on 30 January 2023).
- Intensity Therapeutics, Inc.; Merck Sharp & Dohme LLC; Bristol-Myers Squibb. A Phase 1/2 Safety Study of Intratumorally Dosed INT230-6. 2017. Available online: https://classic.clinicaltrials.gov/show/NCT03058289 (accessed on 30 January 2023).
- Saint John’s Cancer Institute; Eli Lilly and Company; Chordoma Foundation. Pemetrexed for the Treatment of Chordoma. 2019. Available online: https://classic.clinicaltrials.gov/show/NCT03955042 (accessed on 30 January 2023).
- Erasca, Inc. A Dose Escalation/Expansion Study of ERAS-601 in Patients with Advanced or Metastatic Solid Tumors. 2020. Available online: https://classic.clinicaltrials.gov/show/NCT04670679 (accessed on 30 January 2023).
- M.D. Anderson Cancer Center. Proton Beam Therapy for Chordoma Patients. 2006. Available online: https://classic.clinicaltrials.gov/show/NCT00496119 (accessed on 30 January 2023).
- Mercy Research. Stereotactic Body Radiotherapy for Head and Neck Tumors. 2008. Available online: https://classic.clinicaltrials.gov/show/NCT01344356 (accessed on 30 January 2023).
- Massachusetts General Hospital; M.D. Anderson Cancer Center; National Cancer Institute (NCI). High Dose Intensity Modulated Proton Radiation Treatment +/− Surgical Resection of Sarcomas of the Spine, Sacrum and Base of Skull. 2012. Available online: https://classic.clinicaltrials.gov/show/NCT01346124 (accessed on 30 January 2023).
- University College, London; Cancer Research UK; NCRI Radiotherapy Trials QA (RTTQA) Group. A Study of IMRT in Primary Bone and Soft Tissue Sarcoma. 2016. Available online: https://classic.clinicaltrials.gov/show/NCT02520128 (accessed on 30 January 2023).
- Hospices Civils de Lyon. Randomized Carbon Ions vs Standard Radiotherapy for Radioresistant Tumors. 2017. Available online: https://classic.clinicaltrials.gov/show/NCT02838602 (accessed on 30 January 2023).
- Abramson Cancer Center at Penn Medicine. Proton Radiation for Chordomas and Chondrosarcomas. 2010. Available online: https://classic.clinicaltrials.gov/show/NCT01449149 (accessed on 30 January 2023).
- Massachusetts General Hospital. Charged Particle RT for Chordomas and Chondrosarcomas of the Base of Skull or Cervical Spine. 1999. Available online: https://classic.clinicaltrials.gov/show/NCT00592748 (accessed on 30 January 2023).
- Massachusetts General Hospital; National Cancer Institute (NCI). Hypoxia-positron Emission Tomography (PET) and Intensity Modulated Proton Therapy (IMPT) Dose Painting in Patients with Chordomas. 2008. Available online: https://classic.clinicaltrials.gov/show/NCT00713037 (accessed on 30 January 2023).
- Institut Curie. Improvement of Local Control in Skull Base, Spine and Sacral Chordomas Treated by Surgery and Protontherapy Targeting Hypoxic Cells Revealed by 18F FAZA) PET/CT Tracers. 2016. Available online: https://classic.clinicaltrials.gov/show/NCT02802969 (accessed on 30 January 2023).
- Leiden University Medical Center; HollandPTC; Varian Medical Systems. Image Assisted Optimization of Proton Radiation Therapy in Chordomas and Chondrosarcomas. 2021. Available online: https://classic.clinicaltrials.gov/show/NCT04832620 (accessed on 30 January 2023).
- National Cancer Institute (NCI); National Institutes of Health Clinical Center (CC). Genetic Aspects of Chordoma: A Collaboration with SEER Registries to Identify Chordoma Families. 1999. Available online: https://classic.clinicaltrials.gov/show/NCT00341627 (accessed on 30 January 2023).
- National Cancer Institute (NCI); National Institutes of Health Clinical Center (CC). Chordoma Family Study. 2003. Available online: https://classic.clinicaltrials.gov/show/NCT00410670 (accessed on 30 January 2023).
- National Cancer Institute (NCI); National Institutes of Health Clinical Center (CC). Genetic Clues to Chordoma Etiology: A Protocol to Identify Sporadic Chordoma Patients for Studies of Cancer-Susceptibility Genes. 2011. Available online: https://classic.clinicaltrials.gov/show/NCT01200680 (accessed on 30 January 2023).
- Children’s Oncology Group; National Cancer Institute (NCI). Studying Genes in Tissue Samples From Younger and Adolescent Patients with Soft Tissue Sarcomas. 2016. Available online: https://classic.clinicaltrials.gov/show/NCT01567046 (accessed on 30 January 2023).
- Centre Hospitalier Universitaire de Saint Etienne. Immunohistochemical Study of Chordomas to Improve Their Diagnosis and Prognosis Care. 2020. Available online: https://classic.clinicaltrials.gov/show/NCT04486820 (accessed on 30 January 2023).
- Children’s Oncology Group; National Cancer Institute (NCI). Collecting and Storing Tissue, Blood, and Bone Marrow Samples From Patients with Rhabdomyosarcoma or Other Soft Tissue Sarcoma. 1999. Available online: https://classic.clinicaltrials.gov/show/NCT00919269 (accessed on 30 January 2023).
- Ohio State University. Long-Term Longitudinal QoL in Patients Undergoing EEA. 2019. Available online: https://classic.clinicaltrials.gov/show/NCT04087902 (accessed on 30 January 2023).
- Mayo Clinic. Comparing Carbon Ion Therapy, Surgery, and Proton Therapy for the Management of Pelvic Sarcomas Involving the Bone, the PROSPER Study. 2022. Available online: https://classic.clinicaltrials.gov/show/NCT05033288 (accessed on 30 January 2023).
- University of Florida. Proton Therapy for Chordomas and/or Chondrosarcomas. 2007. Available online: https://classic.clinicaltrials.gov/show/NCT00797602 (accessed on 30 January 2023).
- Italian Sarcoma Group. Sacral Chordoma: Surgery Versus Definitive Radiation Therapy in Primary Localized Disease. 2017. Available online: https://classic.clinicaltrials.gov/show/NCT02986516 (accessed on 30 January 2023).
- National Cancer Institute (NCI); National Institutes of Health Clinical Center (CC). Children and Adults with Chordoma. 2019. Available online: https://classic.clinicaltrials.gov/show/NCT03910465 (accessed on 30 January 2023).
- National Cancer Institute (NCI); National Institutes of Health Clinical Center (CC). Natural History and Biospecimen Acquisition for Children and Adults with Rare Solid Tumors. 2019. Available online: https://classic.clinicaltrials.gov/show/NCT03739827 (accessed on 30 January 2023).
- The South Australian Health and Medical Research Institute (SAHMRI). Australian Particle Therapy Clinical Quality Registry (ASPIRE) Is a Prospective, Observational, Longitudinal Study of Paediatric, Adolescent, Young Adult and Rare Adult Tumour Patients from a Select Group of Tumour Streams Treated with Radiation Therapy; The South Australian Health and Medical Research Institute (SAHMRI): Adelaide, Australia, 2021. [Google Scholar]
- Traylor, J.I.; Pernik, M.N.; Plitt, A.R.; Lim, M.; Garzon-Muvdi, T. Immunotherapy for Chordoma and Chondrosarcoma: Current Evidence. Cancers 2021, 13, 2408. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Ptaszynski, K.; Szumera-Cieckiewicz, A.; Owczarek, J.; Mrozkowiak, A.; Pekul, M.; Baranska, J.; Rutkowski, P. Epidermal Growth Factor Receptor (Egfr) Status In Chordoma. Pol. J. Pathol. 2009, 60, 81–87. [Google Scholar]
- Scheipl, S.; Barnard, M.; Cottone, L.; Jorgensen, M.; Drewry, D.H.; Zuercher, W.J.; Turlais, F.; Ye, H.; Leite, A.P.; Smith, J.A.; et al. EGFR inhibitors identified as a potential treatment for chordoma in a focused compound screen. J. Pathol. 2016, 239, 320–334. [Google Scholar] [CrossRef]
- Straining, R.; Eighmy, W. Tazemetostat: EZH2 Inhibitor. J. Adv. Pract. Oncol. 2022, 13, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Raso, A.; Mascelli, S.; Gessi, M.; Nozza, P.; Coli, A.; Gardiman, M.P.; Arcella, A.; Massimino, M.; Buttarelli, F.R.; et al. SMARCB1/INI1 Involvement in Pediatric Chordoma A Mutational and Immunohistochemical Analysis. Am. J. Surg. Pathol. 2017, 41, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.N.; Bourdeaut, F.; Casanova, M.; Kilburn, L.B.; Hargrave, D.R.; McCowage, G.B.; Pinto, N.R.; Yang, J.; Chadha, R.; Kahali, B.; et al. Update on phase 1 study of tazemetostat, an enhancer of zeste homolog 2 inhibitor, in pediatric patients with relapsed or refractory integrase interactor 1-negative tumors. J. Clin. Oncol. 2022, 40, 1. [Google Scholar] [CrossRef]
- Meng, T.; Jin, J.; Jiang, C.; Huang, R.Z.; Yin, H.B.; Song, D.W.; Cheng, L.M. Molecular Targeted Therapy in the Treatment of Chordoma: A Systematic Review. Front. Oncol. 2019, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Akhavan-Sigari, R.; Gaab, M.R.; Rohde, V.; Abili, M.; Ostertag, H. Expression of PDGFR-alpha, EGFR and c-MET in Spinal Chordoma: A Series of 52 Patients. Anticancer Res. 2014, 34, 623–630. [Google Scholar]
- Zhai, Y.X.; Bai, J.W.; Wang, S.; Gao, H.; Li, M.X.; Li, C.Z.; Gui, S.B.; Zhang, Y.Z. Analysis of clinical factors and PDGFR-beta in predicting prognosis of patients with clival chordoma. J. Neurosurg. 2018, 129, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Rossari, F.; Minutolo, F.; Orciuolo, E. Past, present, and future of Bcr-Abl inhibitors: From chemical development to clinical efficacy. J. Hematol. Oncol. 2018, 11, 14. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Longhi, A.; Ferraresi, V.; Grignani, G.; Comandone, A.; Stupp, R.; Bertuzzi, A.; Tamborini, E.; Pilotti, S.; Messina, A.; et al. Phase II Study of Imatinib in Advanced Chordoma. J. Clin. Oncol. 2012, 30, 914–920. [Google Scholar] [CrossRef]
- Bompas, E.; Le Cesne, A.; Tresch-Bruneel, E.; Lebellec, L.; Laurence, V.; Collard, O.; Saada-Bouzid, E.; Isambert, N.; Blay, J.Y.; Amela, E.Y.; et al. Sorafenib in patients with locally advanced and metastatic chordomas: A phase II trial of the French Sarcoma Group (GSF/GETO). Ann. Oncol. 2015, 26, 2168–2173. [Google Scholar] [CrossRef]
- Le Cesne, A.; Chevreau, C.; Perrin, C.; Italiano, A.; Hervieu, A.; Blay, J.Y.; Piperno-Neumann, S.; Saada-Bouzid, E.; Bertucci, F.; Firmin, N.; et al. Regorafenib in patients with relapsed advanced or metastatic chordoma: Results of a non-comparative, randomised, double-blind, placebo-controlled, multicentre phase II study. ESMO Open 2023, 8, 101569. [Google Scholar] [CrossRef]
- Schettini, F.; De Santo, I.; Rea, C.G.; De Placido, P.; Formisano, L.; Giuliano, M.; Arpino, G.; De Laurentiis, M.; Puglisi, F.; De Placido, S.; et al. CDK 4/6 Inhibitors as Single Agent in Advanced Solid Tumors. Front. Oncol. 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Cottone, L.; Eden, N.; Usher, I.; Lombard, P.; Ye, H.T.; Ligammari, L.; Lindsay, D.; Brandner, S.; Pizem, J.; Pillay, N.; et al. Frequent alterations in p16/CDKN2A identified by immunohistochemistry and FISH in chordoma. J. Pathol. Clin. Res. 2020, 6, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shen, J.K.; Choy, E.; Zhang, Y.; Mankin, H.J.; Hornicek, F.J.; Duan, Z.F. CDK4 expression in chordoma: A potential therapeutic target. J. Orthop. Res. 2018, 36, 1581–1589. [Google Scholar] [CrossRef]
- Shen, S.; Deane, D.C.; Yu, Z.J.; Hornicek, F.; Kan, Q.C.; Duan, Z.F. Aberrant CDK9 expression within chordoma tissues and the therapeutic potential of a selective CDK9 inhibitor LDC000067. J. Cancer 2020, 11, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Ieni, A.; Branca, G.; Tuccari, G. Brachyury: A Diagnostic Marker for the Differential Diagnosis of Chordoma and Hemangioblastoma versus Neoplastic Histological Mimickers. Dis. Markers 2014, 2014, 7. [Google Scholar] [CrossRef]
- Sankhala, K.K.; Carrillo, J.; Wagle, N.; Sharma, A.; Truong, J.; Nguyen, M.; Heng, A.; Gill, J.; Nersesian, R.; Kesari, S. Preliminary Safety Results of A Pilot Study of High Dose Pemetrexed For Chordoma. In Proceedings of the Connective Tissue Oncology Society Annual Meeting, Virtual, 10–13 November 2021. [Google Scholar]
- Thomas, J.S.; El-Khoueiry, A.B.; Olszanski, A.J.; Azad, N.S.; Whalen, G.F.; Hanna, D.L.; Ingham, M.; Camacho, L.H.; Mahmood, S.; Bender, L.H.; et al. Effect of intratumoral INT230-6 on tumor necrosis and promotion of a systemic immune response: Results from a multicenter phase 1/2 study of solid tumors with and without pembrolizumab (PEM) [Intensity IT-01; Merck KEYNOTE-A10]. J. Clin. Oncol. 2022, 40, 2520. [Google Scholar] [CrossRef]
- Trikalinos, T.A.; Terasawa, T.; Ip, S.; Raman, G.; Lau, J. Particle Beam Radiation Therapies for Cancer; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2009. [Google Scholar]
- Taylor, A.; Powell, M.E.B. Intensity-modulated radiotherapy—What is it? Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2004, 4, 68–73. [Google Scholar] [CrossRef]
- Rockwell, S.; Dobrucki, I.T.; Kim, E.Y.; Marrison, S.T.; Vu, V.T. Hypoxia and Radiation Therapy: Past History, Ongoing Research, and Future Promise. Curr. Mol. Med. 2009, 9, 442–458. [Google Scholar] [CrossRef]
- Sorensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 11. [Google Scholar] [CrossRef]
- Simoes, R.; Miles, E.; Yang, H.; Le Grange, F.; Bhat, R.; Forsyth, S.; Seddon, B. IMRiS phase II study of IMRT in limb sarcomas: Results of the pre-trial QA facility questionnaire and workshop. Radiography 2020, 26, 71–75. [Google Scholar] [CrossRef]
- Scheipl, S.; Igrec, J.; Leithner, A.; Smolle, M.; Haybäck, J.; Liegl, B. Chordoma: Is there a molecular basis for diagnosis and treatment? Pathologe 2020, 41, 153–162. [Google Scholar] [CrossRef] [PubMed]
- John, L.; Smith, H.; Ilanchezhian, M.; Lockridge, R.; Reilly, K.M.; Raygada, M.; Dombi, E.; Sandler, A.; Thomas, B.J.; Glod, J.; et al. The NIH pediatric/young adult chordoma clinic and natural history study: Making advances in a very rare tumor. Pediatr. Blood Cancer 2023, 70, e30358. [Google Scholar] [CrossRef] [PubMed]
- Chordoma Foundation. Available online: https://www.chordomafoundation.org/ (accessed on 30 January 2023).
- Zenonos, G.A.; Fernandez-Miranda, J.C.; Mukherjee, D.; Chang, Y.-F.; Panayidou, K.; Snyderman, C.H.; Wang, E.W.; Seethala, R.R.; Gardner, P.A. Prospective validation of a molecular prognostication panel for clival chordoma. J. Neurosurg. JNS 2019, 130, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Baluszek, S.; Kober, P.; Rusetska, N.; Wągrodzki, M.; Mandat, T.; Kunicki, J.; Bujko, M. DNA methylation, combined with RNA sequencing, provide novel insight into molecular classification of chordomas and their microenvironment. Acta Neuropathol. Commun. 2023, 11, 113. [Google Scholar] [CrossRef]

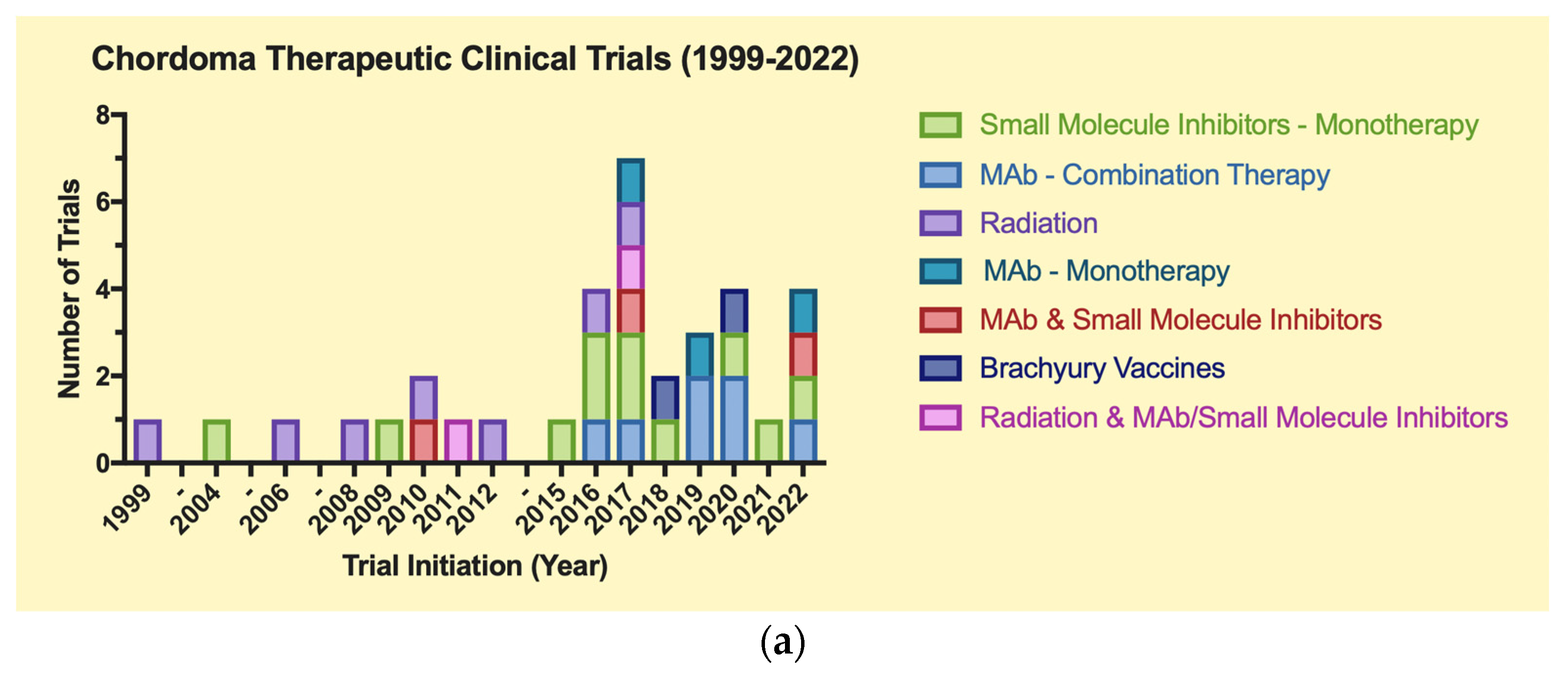
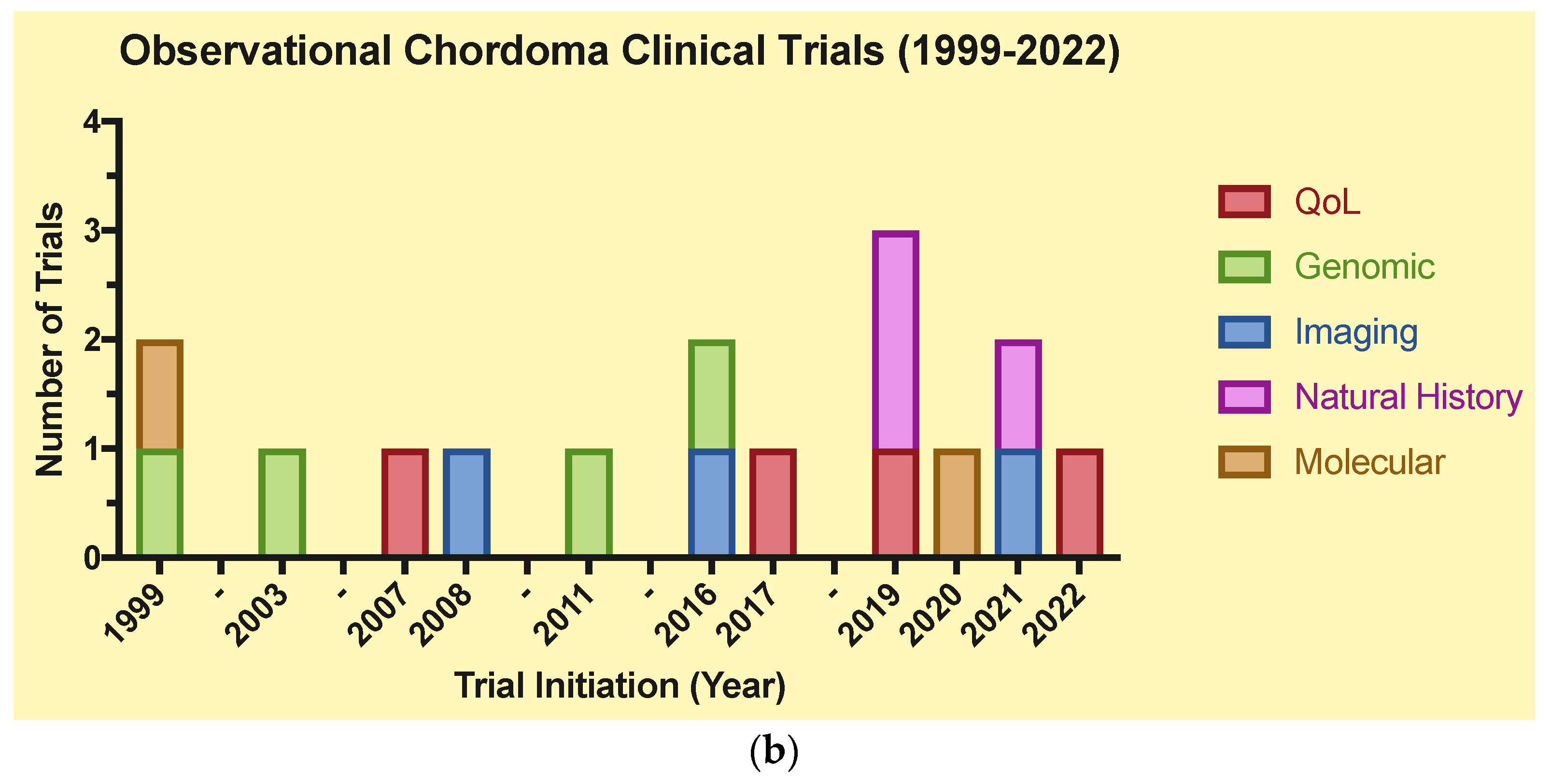
| (a) | |||
|---|---|---|---|
| Intervention | Phase | Subcategory | Primary Outcome Measures |
| Biologic + Drug | Phase I Phase I + II Phase II | α-PD-1 mAb: |
|
| Phase II | α-EGFR mAb: Cetuximab [27] | Assess efficacy by measuring ORR | |
| Phase I Phase II | EZH2 inhibitors: Tazemetostat [28,29,30] | Assess safety and tolerability with monotherapy vs. combination with Nivolumab & Ipilimumab Evaluate efficacy in EZH2 GOF mutation and INI1-negative tumors | |
| Phase I Phase II | TKIs: |
| |
| Phase I Phase II | CDK inhibitors:
|
| |
| Phase I Phase II | Brachyury vaccines: |
| |
| Phase I Phase I + II | Other: |
| |
| Radiation | Phase II | Photon Beam Radiation Therapy [46] | Assess safety, efficacy, and LC in combination with or without PBT |
| Phase IV N/A | Stereotactic Radiation Therapy [47] | Evaluate safety, efficacy, and LC | |
| N/A | Intensity Modulated Radiotherapy [48,49] | Assess efficacy of monotherapy vs. combination with surgery Assess ability in reducing late normal tissue toxicity and optimal radiation dose for CH | |
| Phase I + II Phase III N/A | Charged Particle Radiation Therapy [50,51,52] | Assess PFS of CIRT vs. proton RT or photon RT or proton & photon RT Assess the possibility of delivering an optimal dose within normal tissue tolerances Assess proton therapy’s feasibility & acute toxicity for CH patients | |
| (b) | |||
| Intervention | Phase | Subcategory | Primary Outcome Measures |
| Imaging | N/A Phase II | CT/MRI and FMISO-PET [53] CT/MRI and [18F]FAZA-PET [54] | Visualize hypoxic tumor areas via tracer to improve radiation dosing/planning |
| N/A | MRI post-PBT [55] | Determine if MRI parameters change within 6 months after the start of PBT and prior to volumetric changes | |
| Genomic/Molecular | N/A | Gene Mapping [56,57,58,59] | Study germline and somatic mutations associated with CH |
| N/A | Immunohistochemical [60] | Retrospectively explore protein expression, including brachyury, in CH | |
| N/A | Biomarker analysis [61] | Biobanking network of tissue specimens | |
| QoL/Natural History | N/A | Quality of Life [62,63,64,65] |
|
| N/A | Characterize history of skull base/spine CH [66,67] | Characterize clinical presentation and disease progression patterns | |
| N/A | Characterize patterns of care and long term toxicities [68] | Assess long-term effects of photon and PBT in CH patients (chart review) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Ulloa, R.; Soffer, J.; Alcazar-Felix, R.J.; Snyderman, C.H.; Gardner, P.A.; Patel, V.A.; Polster, S.P. Chordoma: A Comprehensive Systematic Review of Clinical Trials. Cancers 2023, 15, 5800. https://doi.org/10.3390/cancers15245800
Chen S, Ulloa R, Soffer J, Alcazar-Felix RJ, Snyderman CH, Gardner PA, Patel VA, Polster SP. Chordoma: A Comprehensive Systematic Review of Clinical Trials. Cancers. 2023; 15(24):5800. https://doi.org/10.3390/cancers15245800
Chicago/Turabian StyleChen, Sonja, Ruben Ulloa, Justin Soffer, Roberto J. Alcazar-Felix, Carl H. Snyderman, Paul A. Gardner, Vijay A. Patel, and Sean P. Polster. 2023. "Chordoma: A Comprehensive Systematic Review of Clinical Trials" Cancers 15, no. 24: 5800. https://doi.org/10.3390/cancers15245800
APA StyleChen, S., Ulloa, R., Soffer, J., Alcazar-Felix, R. J., Snyderman, C. H., Gardner, P. A., Patel, V. A., & Polster, S. P. (2023). Chordoma: A Comprehensive Systematic Review of Clinical Trials. Cancers, 15(24), 5800. https://doi.org/10.3390/cancers15245800






