Multisite Is Superior to Single-Site Intratumoral Chemotherapy to Retard the Outcomes of Pancreatic Ductal Adenocarcinoma in a Murine Model
Abstract
:Simple Summary
Abstract
1. Introduction
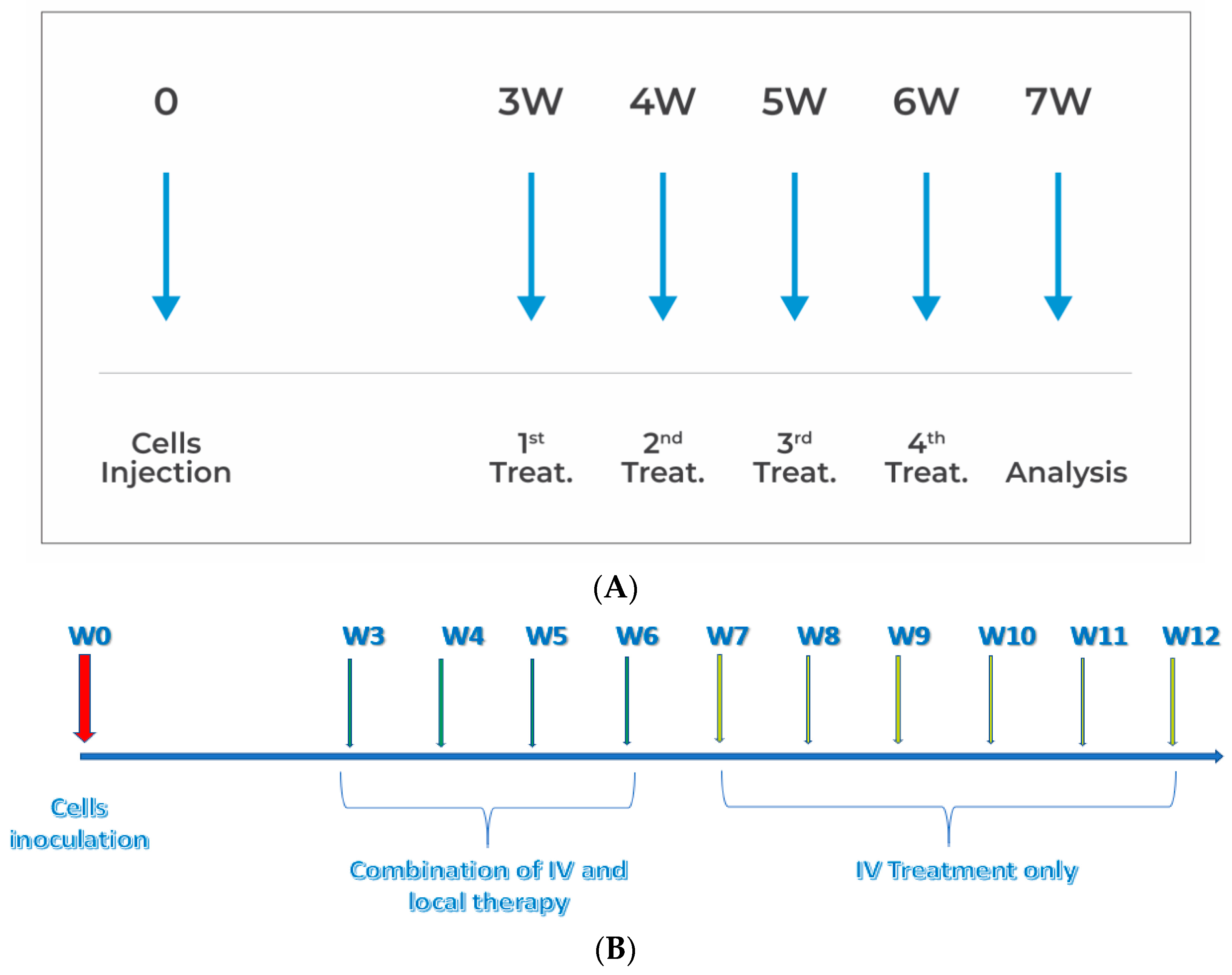
2. Materials and Methods
2.1. PANC1 Orthotopic Pancreatic Tumor Mouse Model
2.2. Drugs and Drugs Administration
2.3. In Vivo Experiments
2.4. Efficacy Measurements and Analysis
2.5. Statistical Methods
3. Results
3.1. Study S-21-27
3.2. Study S-21-371
3.3. Study S-22-357
3.4. Survival Study S-22-357
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bengtsson, A.; Andersson, R.; Ansari, D. The Actual 5-Year Survivors of Pancreatic Ductal Adenocarcinoma Based on Real-World Data. Sci. Rep. 2020, 10, 16425. [Google Scholar] [CrossRef]
- Survival Rates for Pancreatic Cancer. Available online: https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 29 January 2023).
- Jain, R.K. Barriers to Drug Delivery in Solid Tumors. Sci. Am. 1994, 271, 58–65. [Google Scholar] [CrossRef]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The Pancreas Cancer Microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef]
- Ramos, A.; Sadeghi, S.; Tabatabaeian, H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int. J. Mol. Sci. 2021, 22, 9451. [Google Scholar] [CrossRef]
- Cho, S.H.; Oh, D.; Seo, D.-W. Therapeutic EUS. Mini-Invasive Surg. 2021, 5, 20. [Google Scholar] [CrossRef]
- Hwang, J.S.; Joo, H.D.; Song, T.J. Endoscopic Ultrasound-Guided Local Therapy for Pancreatic Neoplasms. Clin. Endosc. 2020, 53, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Su, G.H. Development of Orthotopic Pancreatic Tumor Mouse Models. Methods Mol. Biol. 2013, 980, 215–223. [Google Scholar] [CrossRef]
- Pancreatic Cancer Treatment Protocols: Treatment Protocols. Available online: https://emedicine.medscape.com/article/2007067-overview (accessed on 29 January 2023).
- Gemzar, Infugem (Gemcitabine) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/infugem-gemzar-gemcitabine-342218 (accessed on 29 January 2023).
- Abraxane (Paclitaxel Protein Bound) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/abraxane-paclitaxel-protein-bound-999775 (accessed on 29 January 2023).
- Eloxatin (Oxaliplatin) Dosing, Indications, Interactions, Adverse Effects, and More. Available online: https://reference.medscape.com/drug/eloxatin-oxaliplatin-342106 (accessed on 29 January 2023).
- Argyriou, A.A.; Bruna, J.; Marmiroli, P.; Cavaletti, G. Chemotherapy-Induced Peripheral Neurotoxicity (CIPN): An Update. Crit. Rev. Oncol. Hematol. 2012, 82, 51–77. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W.; Secomb, T.W. Transport of Drugs from Blood Vessels to Tumour Tissue. Nat. Rev. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Lin, F.; Zhou, Q.; Huang, L.; Jin, K. Strategies to Improve Drug Distribution in Solid Tumor. Int. J. Clin. Exp. Med. 2016, 9, 658–667. [Google Scholar]
- Brekken, C.; Bruland, Ø.S.; de Lange Davies, C. Interstitial Fluid Pressure in Human Osteosarcoma Xenografts: Significance of Implantation Site and the Response to Intratumoral Injection of Hyaluronidase. Anticancer Res. 2000, 20, 3503–3512. [Google Scholar]
- Netti, P.A.; Berk, D.A.; Swartz, M.A.; Grodzinsky, A.J.; Jain, R.K. Role of Extracellular Matrix Assembly in Interstitial Transport in Solid Tumors. Cancer Res. 2000, 60, 2497–2503. [Google Scholar] [PubMed]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug Resistance and the Solid Tumor Microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Truong, V.G.; Choi, J.; Jeong, H.J.; Oh, S.-J.; Park, J.-S.; Kang, H.W. Endoscopic Ultrasound-Guided Laser Ablation Using a Diffusing Applicator for Locally Advanced Pancreatic Cancer Treatment. Cancers 2022, 14, 2274. [Google Scholar] [CrossRef]
- de Nucci, G.; Imperatore, N.; Mandelli, E.D.; di Nuovo, F.; d’Urbano, C.; Manes, G. Endoscopic Ultrasound-Guided Radiofrequency Ablation of Pancreatic Neuroendocrine Tumors: A Case Series. Endosc. Int. Open 2020, 8, E1754–E1758. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Jaruvongvanich, V.; Chandrasekhara, V. Endoscopic Ultrasound-Guided Injectable Therapy for Pancreatic Cancer: A Systematic Review. World J. Gastroenterol. 2022, 28, 2383–2395. [Google Scholar] [CrossRef]
- Levy, M.J.; Alberts, S.R.; Bamlet, W.R.; Burch, P.A.; Farnell, M.B.; Gleeson, F.C.; Haddock, M.G.; Kendrick, M.L.; Oberg, A.L.; Petersen, G.M.; et al. EUS-Guided Fine-Needle Injection of Gemcitabine for Locally Advanced and Metastatic Pancreatic Cancer. Gastrointest. Endosc. 2017, 86, 161–169. [Google Scholar] [CrossRef]
- Takahashi, K.; Ehata, S.; Koinuma, D.; Morishita, Y.; Soda, M.; Mano, H.; Miyazono, K. Pancreatic Tumor Microenvironment Confers Highly Malignant Properties on Pancreatic Cancer Cells. Oncogene 2018, 37, 2757–2772. [Google Scholar] [CrossRef]
- Maia, A.; Wiemann, S. Cancer-Associated Fibroblasts: Implications for Cancer Therapy. Cancers 2021, 13, 3526. [Google Scholar] [CrossRef]
- von Ahrens, D.; Bhagat, T.D.; Nagrath, D.; Maitra, A.; Verma, A. The Role of Stromal Cancer-Associated Fibroblasts in Pancreatic Cancer. J. Hematol. Oncol. 2017, 10, 76. [Google Scholar] [CrossRef]
- Li, M.; Li, M.; Yin, T.; Shi, H.; Wen, Y.; Zhang, B.; Chen, M.; Xu, G.; Ren, K.; Wei, Y. Targeting of Cancer-associated Fibroblasts Enhances the Efficacy of Cancer Chemotherapy by Regulating the Tumor Microenvironment. Mol. Med. Rep. 2016, 13, 2476–2484. [Google Scholar] [CrossRef]
- Jungwirth, U.; van Weverwijk, A.; Evans, R.J.; Jenkins, L.; Vicente, D.; Alexander, J.; Gao, Q.; Haider, S.; Iravani, M.; Isacke, C.M. Impairment of a Distinct Cancer-Associated Fibroblast Population Limits Tumour Growth and Metastasis. Nat. Commun. 2021, 12, 3516. [Google Scholar] [CrossRef] [PubMed]
- Napoletano, C.; Taurino, F.; Biffoni, M.; De Majo, A.; Coscarella, G.; Bellati, F.; Rahimi, H.; Pauselli, S.; Pellicciotta, I.; Burchell, J.M.; et al. RFA Strongly Modulates the Immune System and Anti-Tumor Immune Responses in Metastatic Liver Patients. Int. J. Oncol. 2008, 32, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Burz, C.; Berindan-Neagoe, I.B.-N.; Balacescu, O.; Tanaselia, C.; Ursu, M.; Gog, A.; Vlase, L.; Chintoanu, M.; Balacescu, L.; Leucuta, S.E.; et al. Clinical and Pharmacokinetics Study of Oxaliplatin in Colon Cancer Patients. J. Gastrointestin. Liver Dis. 2009, 18, 39–43. [Google Scholar]
- Vilalta-Lacarra, A.; Aldaz, A.; Sala-Elarre, P.; Urrizola, A.; Chopitea, A.; Arbea, L.; Rotellar, F.; Pardo, F.; Martí-Cruchaga, P.; Zozaya, G.; et al. Therapeutic Drug Monitoring of Neoadjuvant mFOLFIRINOX in Resected Pancreatic Ductal Adenocarcinoma. Pancreatology 2023, 23, 411–419. [Google Scholar] [CrossRef] [PubMed]





| Parameter (Field) | Magnification (X) | Score-0 | Score-1 | Score-2 | Score-3 | Score-4 | Score-5 |
|---|---|---|---|---|---|---|---|
| Inflammatory cells (H&E) | 10 | Rare or absent | <tumor cells | =tumor cells | Predominant | ||
| Lymphocytes infiltration (H&E) | 10 | Absent | <5 | 5–20 | >20 | ||
| Necrosis (H&E) | 10 | No sign | Small foci | <50% | 50–75% | 75–90% | >90% |
| Apoptotic cells (TUNEL) | 20 | No positive | <5 | 5–15 | 15–25 | 25–50 | >50 |
| Fibroblasts(SMA) | 20 | No positive | <5 | 5–15 | 15–25 | 25–50 | >50 |
| Parameter | Treatment/Number of Animal in Each Score | |||||
|---|---|---|---|---|---|---|
| Inflammatory Infiltration | Control | Gemcitabine | Abraxane | Oxaliplatin | Gemcitabine + Abraxane | Gemcitabine + Oxaliplatin |
| Score-1 | 7 | 3 | 1 | 0 | 2 | 0 |
| Score-2 | 1 | 2 | 4 | 3 | 5 | 6 |
| Score-3 | 0 | 3 | 3 | 5 | 1 | 2 |
| Mean | 1.13 | 2.00 | 2.25 | 2.63 | 1.88 | 2.25 |
| p-value | All groups = 0.003 | 0.021 | 0.003 | <0.0001 | 0.05 | 0.003 |
| Lymphocytes infiltration | ||||||
| Score-1 | 8 | 5 | 3 | 1 | 3 | 3 |
| Score-2 | 0 | 3 | 5 | 7 | 5 | 5 |
| Score-3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mean | 1.00 | 1.38 | 1.63 | 1.88 | 1.63 | 1.63 |
| p-value | All groups = 0.015 | 0.137 | 0.013 | 0.001 | 0.013 | 0.013 |
| Tumor necrosis | ||||||
| Score-2 | 2 | 1 | 1 | 0 | 2 | 1 |
| Score-3 | 6 | 4 | 2 | 3 | 2 | 4 |
| Score-4 | 0 | 3 | 5 | 3 | 4 | 3 |
| Score-5 | 0 | 0 | 0 | 2 | 0 | 0 |
| Mean | 2.75 | 3.25 | 3.50 | 3.88 | 3.25 | 3.25 |
| p-value | All groups = 0.120 | 0.163 | 0.032 | 0.005 | 0.133 | 0.163 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarovits, J.; Epelbaum, R.; Lachter, J.; Amikam, Y.; Ben Arie, J. Multisite Is Superior to Single-Site Intratumoral Chemotherapy to Retard the Outcomes of Pancreatic Ductal Adenocarcinoma in a Murine Model. Cancers 2023, 15, 5801. https://doi.org/10.3390/cancers15245801
Lazarovits J, Epelbaum R, Lachter J, Amikam Y, Ben Arie J. Multisite Is Superior to Single-Site Intratumoral Chemotherapy to Retard the Outcomes of Pancreatic Ductal Adenocarcinoma in a Murine Model. Cancers. 2023; 15(24):5801. https://doi.org/10.3390/cancers15245801
Chicago/Turabian StyleLazarovits, Janette, Ron Epelbaum, Jesse Lachter, Yaron Amikam, and Jacob Ben Arie. 2023. "Multisite Is Superior to Single-Site Intratumoral Chemotherapy to Retard the Outcomes of Pancreatic Ductal Adenocarcinoma in a Murine Model" Cancers 15, no. 24: 5801. https://doi.org/10.3390/cancers15245801





