Unlocking Glioblastoma Secrets: Natural Killer Cell Therapy against Cancer Stem Cells
Abstract
:Simple Summary
Abstract
1. Glioblastoma and Glioblastoma Stem Cells
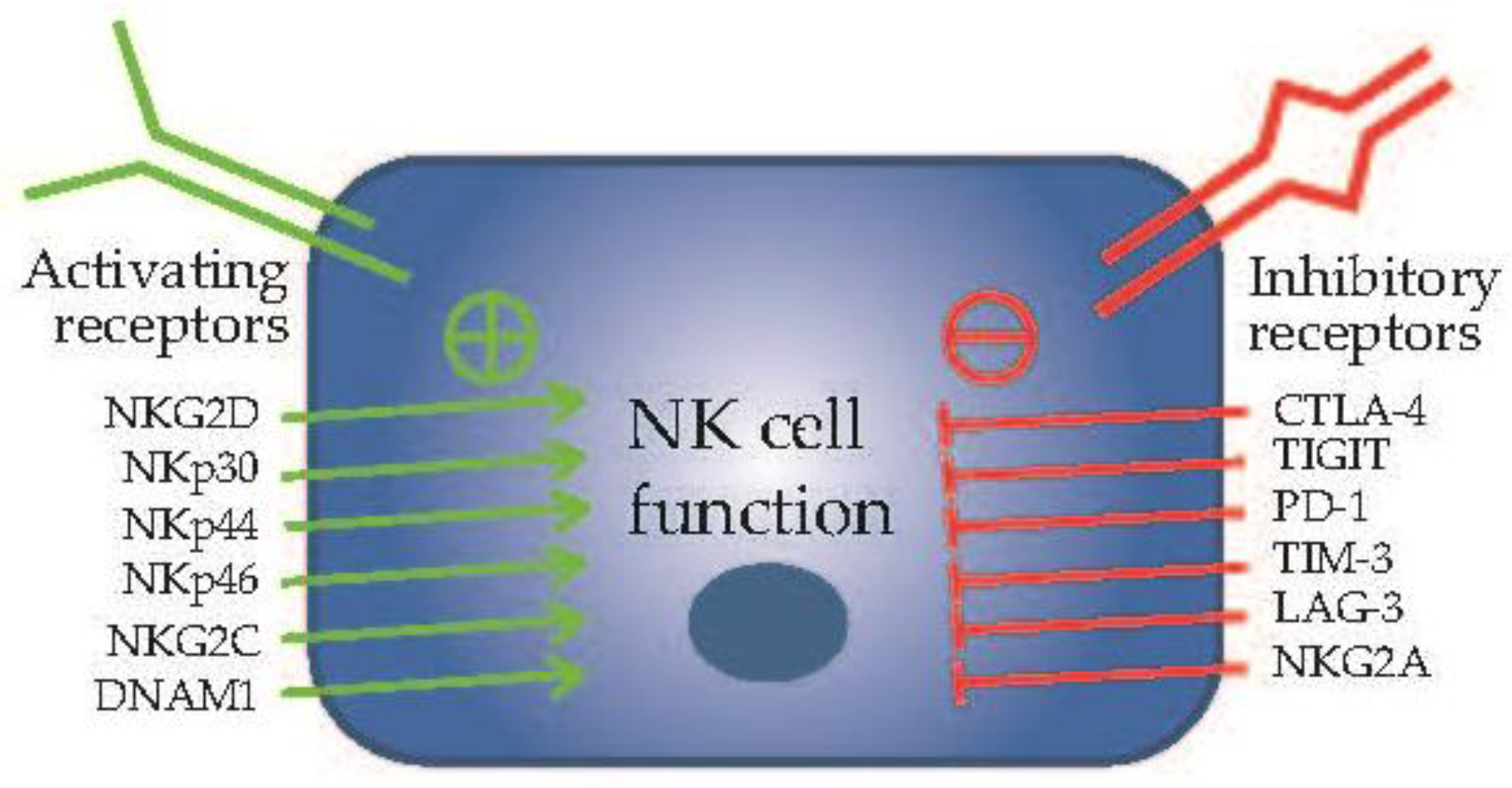
2. Natural Killer Cells in Cancer Immunotherapy
3. Natural Killer Cells Targeting of Glioblastoma Stem Cells
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and other primary brain malignancies in adults: A review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Yi, Y.; Hsieh, I.Y.; Huang, X.; Li, J.; Zhao, W. Glioblastoma stem-like cells: Characteristics, microenvironment, and therapy. Front. Pharmacol. 2016, 7, 477. [Google Scholar] [CrossRef]
- Helweg, L.P.; Storm, J.; Witte, K.E.; Schulten, W.; Wrachtrup, L.; Janotte, T.; Kitke, A.; Greiner, J.F.W.; Knabbe, C.; Kaltschmidt, B.; et al. Targeting key signaling pathways in glioblastoma stem cells for the development of efficient chemo- and immunotherapy. Int. J. Mol. Sci. 2022, 23, 12919. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.K.; Shen, D.; Chen, Y.S.; Yang, Q.Y.; Guo, C.C.; Feng, B.H.; Chen, Z.P. Enhanced mgmt expression contributes to temozolomide resistance in glioma stem-like cells. Chin. J. Cancer 2014, 33, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Dinesan, M.; Ajayakumar, T. Survival and quality of life analysis in glioblastoma multiforme with adjuvant chemoradiotherapy: A retrospective study. Rep. Pract. Oncol. Radiother. 2022, 27, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Galli, R.; Binda, E.; Orfanelli, U.; Cipelletti, B.; Gritti, A.; De Vitis, S.; Fiocco, R.; Foroni, C.; Dimeco, F.; Vescovi, A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004, 64, 7011–7021. [Google Scholar] [CrossRef] [PubMed]
- Uribe, D.; Niechi, I.; Rackov, G.; Erices, J.I.; San Martin, R.; Quezada, C. Adapt to persist: Glioblastoma microenvironment and epigenetic regulation on cell plasticity. Biology 2022, 11, 313. [Google Scholar] [CrossRef]
- Torres, A.; Vargas, Y.; Uribe, D.; Jaramillo, C.; Gleisner, A.; Salazar-Onfray, F.; Lopez, M.N.; Melo, R.; Oyarzun, C.; San Martin, R.; et al. Adenosine a3 receptor elicits chemoresistance mediated by multiple resistance-associated protein-1 in human glioblastoma stem-like cells. Oncotarget 2016, 7, 67373–67386. [Google Scholar] [CrossRef]
- Prager, B.C.; Bhargava, S.; Mahadev, V.; Hubert, C.G.; Rich, J.N. Glioblastoma stem cells: Driving resilience through chaos. Trends Cancer 2020, 6, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Safa, A.R.; Saadatzadeh, M.R.; Cohen-Gadol, A.A.; Pollok, K.E.; Bijangi-Vishehsaraei, K. Glioblastoma stem cells (gscs) epigenetic plasticity and interconversion between differentiated non-gscs and gscs. Genes Dis. 2015, 2, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Anido, J.; Saez-Borderias, A.; Gonzalez-Junca, A.; Rodon, L.; Folch, G.; Carmona, M.A.; Prieto-Sanchez, R.M.; Barba, I.; Martinez-Saez, E.; Prudkin, L.; et al. Tgf-beta receptor inhibitors target the cd44(high)/id1(high) glioma-initiating cell population in human glioblastoma. Cancer Cell 2010, 18, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Gallagher, J.; Heddleston, J.M.; Wang, J.; Eyler, C.E.; Macswords, J.; Wu, Q.; Vasanji, A.; McLendon, R.E.; Hjelmeland, A.B.; et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 2010, 6, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Son, M.J.; Woolard, K.; Nam, D.H.; Lee, J.; Fine, H.A. Ssea-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell 2009, 4, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Inoue, A.; Ohnishi, T.; Kohno, S.; Ohue, S.; Matsumoto, S.; Suehiro, S.; Yamashita, D.; Ozaki, S.; Watanabe, H.; et al. Significance of glioma stem-like cells in the tumor periphery that express high levels of cd44 in tumor invasion, early progression, and poor prognosis in glioblastoma. Stem Cells Int. 2018, 2018, 5387041. [Google Scholar] [CrossRef] [PubMed]
- Steponaitis, G.; Tamasauskas, A. Mesenchymal and proneural subtypes of glioblastoma disclose branching based on gsc associated signature. Int. J. Mol. Sci. 2021, 22, 4964. [Google Scholar] [CrossRef]
- Spinelli, C.; Montermini, L.; Meehan, B.; Brisson, A.R.; Tan, S.; Choi, D.; Nakano, I.; Rak, J. Molecular subtypes and differentiation programmes of glioma stem cells as determinants of extracellular vesicle profiles and endothelial cell-stimulating activities. J. Extracell. Vesicles 2018, 7, 1490144. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Xu, S.; Liu, Z.; Cheng, Q. The adaptive transition of glioblastoma stem cells and its implications on treatments. Signal Transduct. Target. Ther. 2021, 6, 124. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, J.; Ping, Y.; Yan, M.; Liao, G.; Yuan, H.; Zhou, Y.; Xiang, F.; Pang, B.; Xu, J.; et al. The heterogeneous cellular states of glioblastoma stem cells revealed by single cell analysis. Stem Cells 2023, 41, 111–125. [Google Scholar] [CrossRef]
- Suva, M.L.; Tirosh, I. The glioma stem cell model in the era of single-cell genomics. Cancer Cell 2020, 37, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Aulestia, F.J.; Neant, I.; Dong, J.; Haiech, J.; Kilhoffer, M.C.; Moreau, M.; Leclerc, C. Quiescence status of glioblastoma stem-like cells involves remodelling of Ca2+ signalling and mitochondrial shape. Sci. Rep. 2018, 8, 9731. [Google Scholar] [CrossRef]
- Osuka, S.; Van Meir, E.G. Overcoming therapeutic resistance in glioblastoma: The way forward. J. Clin. Investig. 2017, 127, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.; Rich, J.N. Cancer stem cells in glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Van Meir, E.G. Tumor initiating cells in malignant gliomas: Biology and implications for therapy. J. Mol. Med. 2009, 87, 363–374. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of gene expression and chemoresistance of cd133+ cancer stem cells in glioblastoma. Mol. Cancer 2006, 5, 67. [Google Scholar] [CrossRef]
- Chumakova, A.; Lathia, J.D. Outlining involvement of stem cell program in regulation of o6-methylguanine DNA methyltransferase and development of temozolomide resistance in glioblastoma: An editorial highlight for ‘transcriptional control of o(6)-methylguanine DNA methyltransferase expression and temozolomide resistance in glioblastoma’ on page 780. J. Neurochem. 2018, 144, 688–690. [Google Scholar] [CrossRef]
- Happold, C.; Stojcheva, N.; Silginer, M.; Weiss, T.; Roth, P.; Reifenberger, G.; Weller, M. Transcriptional control of o(6)-methylguanine DNA methyltransferase expression and temozolomide resistance in glioblastoma. J. Neurochem. 2018, 144, 780–790. [Google Scholar] [CrossRef]
- Pegg, A.E.; Byers, T.L. Repair of DNA containing o6-alkylguanine. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1992, 6, 2302–2310. [Google Scholar] [CrossRef]
- Tomar, M.S.; Kumar, A.; Srivastava, C.; Shrivastava, A. Elucidating the mechanisms of temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188616. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, A.J.; Xin, Y.; Li, L.T.; Cheng, Q.; Zheng, J.N. Progression of o(6)-methylguanine-DNA methyltransferase and temozolomide resistance in cancer research. Mol. Biol. Rep. 2014, 41, 6659–6665. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. Mgmt gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Lavon, I.; Fuchs, D.; Zrihan, D.; Efroni, G.; Zelikovitch, B.; Fellig, Y.; Siegal, T. Novel mechanism whereby nuclear factor kappab mediates DNA damage repair through regulation of o(6)-methylguanine-DNA-methyltransferase. Cancer Res. 2007, 67, 8952–8959. [Google Scholar] [CrossRef]
- Wang, X.; Jia, L.; Jin, X.; Liu, Q.; Cao, W.; Gao, X.; Yang, M.; Sun, B. Nf-kappab inhibitor reverses temozolomide resistance in human glioma tr/u251 cells. Oncol. Lett. 2015, 9, 2586–2590. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I.M. Nf-kappab, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Friedman, H.S.; McLendon, R.E.; Kerby, T.; Dugan, M.; Bigner, S.H.; Henry, A.J.; Ashley, D.M.; Krischer, J.; Lovell, S.; Rasheed, K.; et al. DNA mismatch repair and o6-alkylguanine-DNA alkyltransferase analysis and response to temodal in newly diagnosed malignant glioma. J. Clin. Oncol. 1998, 16, 3851–3857. [Google Scholar] [CrossRef]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes. Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.; Xu, J.; Zhang, H.; Zhou, J.; Li, H.; Zhang, G.; Xu, K.; Jing, Z. Glioblastoma-associated microglia-derived exosomal circkif18a promotes angiogenesis by targeting foxc2. Oncogene 2022, 41, 3461–3473. [Google Scholar] [CrossRef]
- Gujar, A.D.; Le, S.; Mao, D.D.; Dadey, D.Y.; Turski, A.; Sasaki, Y.; Aum, D.; Luo, J.; Dahiya, S.; Yuan, L.; et al. An nad+-dependent transcriptional program governs self-renewal and radiation resistance in glioblastoma. Proc. Natl. Acad. Sci. USA 2016, 113, E8247–E8256. [Google Scholar] [CrossRef]
- Panizza, E.; Regalado, B.D.; Wang, F.; Nakano, I.; Vacanti, N.M.; Cerione, R.A.; Antonyak, M.A. Proteomic analysis reveals microvesicles containing nampt as mediators of radioresistance in glioma. Life Sci. Alliance 2023, 6, e202201680. [Google Scholar] [CrossRef]
- Garnier, D.; Renoult, O.; Alves-Guerra, M.C.; Paris, F.; Pecqueur, C. Glioblastoma stem-like cells, metabolic strategy to kill a challenging target. Front. Oncol. 2019, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Harland, A.; Liu, X.; Ghirardello, M.; Galan, M.C.; Perks, C.M.; Kurian, K.M. Glioma stem-like cells and metabolism: Potential for novel therapeutic strategies. Front. Oncol. 2021, 11, 743814. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. The microenvironmental landscape of brain tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef]
- Xuan, W.; Hsu, W.H.; Khan, F.; Dunterman, M.; Pang, L.; Wainwright, D.A.; Ahmed, A.U.; Heimberger, A.B.; Lesniak, M.S.; Chen, P. Circadian regulator clock drives immunosuppression in glioblastoma. Cancer Immunol. Res. 2022, 10, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Bayik, D.; Bartels, C.F.; Lovrenert, K.; Watson, D.C.; Zhang, D.; Kay, K.; Lee, J.; Lauko, A.; Johnson, S.; Lo, A.; et al. Distinct cell adhesion signature defines glioblastoma myeloid-derived suppressor cell subsets. Cancer Res. 2022, 82, 4274–4287. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the cd56(bright) subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef] [PubMed]
- Kucuksezer, U.C.; Aktas Cetin, E.; Esen, F.; Tahrali, I.; Akdeniz, N.; Gelmez, M.Y.; Deniz, G. The role of natural killer cells in autoimmune diseases. Front. Immunol. 2021, 12, 622306. [Google Scholar] [CrossRef]
- Lamarthee, B.; Callemeyn, J.; Van Herck, Y.; Antoranz, A.; Anglicheau, D.; Boada, P.; Becker, J.U.; Debyser, T.; De Smet, F.; De Vusser, K.; et al. Transcriptional and spatial profiling of the kidney allograft unravels a central role for fcyriii+ innate immune cells in rejection. Nat. Commun. 2023, 14, 4359. [Google Scholar] [CrossRef]
- Waldhauer, I.; Steinle, A. Nk cells and cancer immunosurveillance. Oncogene 2008, 27, 5932–5943. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Pineiro Fernandez, J.; Luddy, K.A.; Harmon, C.; O’Farrelly, C. Hepatic tumor microenvironments and effects on nk cell phenotype and function. Int. J. Mol. Sci. 2019, 20, 4131. [Google Scholar] [CrossRef]
- Zucchini, N.; Crozat, K.; Baranek, T.; Robbins, S.H.; Altfeld, M.; Dalod, M. Natural killer cells in immunodefense against infective agents. Expert Rev. Anti Infect. Ther. 2008, 6, 867–885. [Google Scholar] [CrossRef]
- Burger, M.C.; Zhang, C.; Harter, P.N.; Romanski, A.; Strassheimer, F.; Senft, C.; Tonn, T.; Steinbach, J.P.; Wels, W.S. Car-engineered nk cells for the treatment of glioblastoma: Turning innate effectors into precision tools for cancer immunotherapy. Front. Immunol. 2019, 10, 2683. [Google Scholar] [CrossRef]
- Deng, X.; Terunuma, H.; Nieda, M. Immunosurveillance of cancer and viral infections with regard to alterations of human nk cells originating from lifestyle and aging. Biomedicines 2021, 9, 557. [Google Scholar] [CrossRef]
- Russick, J.; Torset, C.; Hemery, E.; Cremer, I. Nk cells in the tumor microenvironment: Prognostic and theranostic impact. Recent advances and trends. Semin. Immunol. 2020, 48, 101407. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.S.; Ewald, A.J. The changing role of natural killer cells in cancer metastasis. J. Clin. Investig. 2022, 132, e143762. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. The function of nk cells in tumor metastasis and nk cell-based immunotherapy. Cancers 2023, 15, 2323. [Google Scholar] [CrossRef] [PubMed]
- Vyas, M.; Requesens, M.; Nguyen, T.H.; Peigney, D.; Azin, M.; Demehri, S. Natural killer cells suppress cancer metastasis by eliminating circulating cancer cells. Front. Immunol. 2022, 13, 1098445. [Google Scholar] [CrossRef]
- Monjazeb, A.M.; Zamora, A.E.; Grossenbacher, S.K.; Mirsoian, A.; Sckisel, G.D.; Murphy, W.J. Immunoediting and antigen loss: Overcoming the achilles heel of immunotherapy with antigen non-specific therapies. Front. Oncol. 2013, 3, 197. [Google Scholar] [CrossRef]
- Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Kim, S.J.; Lee, K.M. Nk cell-based immunotherapies in cancer. Immune Netw. 2020, 20, e14. [Google Scholar] [CrossRef]
- Campbell, K.S.; Hasegawa, J. Natural killer cell biology: An update and future directions. J. Allergy Clin. Immunol. 2013, 132, 536–544. [Google Scholar] [CrossRef]
- Li, H.; Song, W.; Li, Z.; Zhang, M. Preclinical and clinical studies of car-nk-cell therapies for malignancies. Front. Immunol. 2022, 13, 992232. [Google Scholar] [CrossRef]
- Yilmaz, A.; Cui, H.; Caligiuri, M.A.; Yu, J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 168. [Google Scholar] [CrossRef]
- Han, B.; Mao, F.Y.; Zhao, Y.L.; Lv, Y.P.; Teng, Y.S.; Duan, M.; Chen, W.; Cheng, P.; Wang, T.T.; Liang, Z.Y.; et al. Altered nkp30, nkp46, nkg2d, and dnam-1 expression on circulating nk cells is associated with tumor progression in human gastric cancer. J. Immunol. Res. 2018, 2018, 6248590. [Google Scholar] [CrossRef]
- Ames, E.; Canter, R.J.; Grossenbacher, S.K.; Mac, S.; Smith, R.C.; Monjazeb, A.M.; Chen, M.; Murphy, W.J. Enhanced targeting of stem-like solid tumor cells with radiation and natural killer cells. Oncoimmunology 2015, 4, e1036212. [Google Scholar] [CrossRef]
- Yin, T.; Wang, G.; He, S.; Liu, Q.; Sun, J.; Wang, Y. Human cancer cells with stem cell-like phenotype exhibit enhanced sensitivity to the cytotoxicity of il-2 and il-15 activated natural killer cells. Cell Immunol. 2016, 300, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Breznik, B.; Ko, M.W.; Tse, C.; Chen, P.C.; Senjor, E.; Majc, B.; Habic, A.; Angelillis, N.; Novak, M.; Zupunski, V.; et al. Infiltrating natural killer cells bind, lyse and increase chemotherapy efficacy in glioblastoma stem-like tumorospheres. Commun. Biol. 2022, 5, 436. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Davis, Z.B.; Rechberger, J.S.; Toll, S.A.; Schwartz, J.D.; Daniels, D.J.; Miller, J.S.; Khatua, S. Advances in nk cell therapy for brain tumors. NPJ Precis. Oncol. 2023, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.J.; Ghazanfari, N.; Constantinescu, P.; Mantamadiotis, T.; Barrow, A.D. The role of nk cells and innate lymphoid cells in brain cancer. Front. Immunol. 2020, 11, 1549. [Google Scholar] [CrossRef]
- Lanier, L.L. Nk cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef] [PubMed]
- Moretta, L.; Moretta, A. Unravelling natural killer cell function: Triggering and inhibitory human nk receptors. EMBO J. 2004, 23, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Lu, Y.; Zhang, Y.; Zhan, P.; Chen, Z.; Wang, C.; Yin, Z. Tumor-associated nk cells facilitate tumor growth via nkp46 in immunocompetent murine hepatocellular carcinoma. Immunol. Lett. 2023, 258, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Domagala, J.; Lachota, M.; Klopotowska, M.; Graczyk-Jarzynka, A.; Domagala, A.; Zhylko, A.; Soroczynska, K.; Winiarska, M. The tumor microenvironment-a metabolic obstacle to nk cells’ activity. Cancers 2020, 12, 3542. [Google Scholar] [CrossRef]
- Zalfa, C.; Paust, S. Natural killer cell interactions with myeloid derived suppressor cells in the tumor microenvironment and implications for cancer immunotherapy. Front. Immunol. 2021, 12, 633205. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Sharabi, A. Targeting myeloid-derived suppressor cells to enhance natural killer cell-based immunotherapy. Pharmacol. Ther. 2022, 235, 108114. [Google Scholar] [CrossRef]
- Du, N.; Guo, F.; Wang, Y.; Cui, J. Nk cell therapy: A rising star in cancer treatment. Cancers 2021, 13, 4129. [Google Scholar] [CrossRef]
- Shaim, H.; Shanley, M.; Basar, R.; Daher, M.; Gumin, J.; Zamler, D.B.; Uprety, N.; Wang, F.; Huang, Y.; Gabrusiewicz, K.; et al. Targeting the alphav integrin/tgf-beta axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Investig. 2021, 131, e142116. [Google Scholar] [CrossRef]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. Car-nk cells: A promising cellular immunotherapy for cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhu, J.; Zhang, Y.; Deng, H. Car-nk cell therapy for glioblastoma: What to do next? Front. Oncol. 2023, 13, 1192128. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Toregrosa-Allen, S.; Elzey, B.D.; Utturkar, S.; Lanman, N.A.; Bernal-Crespo, V.; Behymer, M.M.; Knipp, G.T.; Yun, Y.; Veronesi, M.C.; et al. Multispecific targeting of glioblastoma with tumor microenvironment-responsive multifunctional engineered nk cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2107507118. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Zeinabad, H.; Yeoh, W.J.; Arif, M.; Lomora, M.; Banz, Y.; Riether, C.; Krebs, P.; Szegezdi, E. Natural killer cell-mimic nanoparticles can actively target and kill acute myeloid leukemia cells. Biomaterials 2023, 298, 122126. [Google Scholar] [CrossRef]
- Lupo, K.B.; Matosevic, S. Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. Cancers 2019, 11, 769. [Google Scholar] [CrossRef]
- Lamers-Kok, N.; Panella, D.; Georgoudaki, A.M.; Liu, H.; Ozkazanc, D.; Kucerova, L.; Duru, A.D.; Spanholtz, J.; Raimo, M. Natural killer cells in clinical development as non-engineered, engineered, and combination therapies. J. Hematol. Oncol. 2022, 15, 164. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Pollok, K.E.; Shen, J. Unlocking Glioblastoma Secrets: Natural Killer Cell Therapy against Cancer Stem Cells. Cancers 2023, 15, 5836. https://doi.org/10.3390/cancers15245836
Du Y, Pollok KE, Shen J. Unlocking Glioblastoma Secrets: Natural Killer Cell Therapy against Cancer Stem Cells. Cancers. 2023; 15(24):5836. https://doi.org/10.3390/cancers15245836
Chicago/Turabian StyleDu, Yuanning, Karen E. Pollok, and Jia Shen. 2023. "Unlocking Glioblastoma Secrets: Natural Killer Cell Therapy against Cancer Stem Cells" Cancers 15, no. 24: 5836. https://doi.org/10.3390/cancers15245836
APA StyleDu, Y., Pollok, K. E., & Shen, J. (2023). Unlocking Glioblastoma Secrets: Natural Killer Cell Therapy against Cancer Stem Cells. Cancers, 15(24), 5836. https://doi.org/10.3390/cancers15245836






