Diagnostics and Treatment of Extrameningeal Solitary Fibrous Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Epidemiology
3. Anatomical Location
4. Diagnosis
5. Clinical and Radiological Differential Diagnosis
6. Pathology
6.1. Histology
6.2. Immunohistochemistry
6.3. Lymphocytic Infiltration
6.4. Differential Diagnosis
6.5. Genetics
7. Treatment of Localized Disease
7.1. Surgical Resection
7.2. Perioperative Radiotherapy
7.3. Perioperative Chemotherapy
7.4. Definitive Radiotherapy
8. Treatment of an Advanced and Metastatic Disease
9. Antiangiogenic Treatment
10. Other Treatment Possibilities
11. Risk Stratification Models of Poor Outcomes
11.1. Factors Predicting Local Recurrence
11.2. Factors Predicting Metastases
11.3. Survival, Prognostic Factors of SFT-Tumor Death, and Overall Survival
12. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Freiser, M.E.; Castaño, J.E.; Whittington, E.E.; Arnold, D.J.; Sidani, C.A. Solitary fibrous tumor of the infratemporal fossa. J. Radiol. Case Rep. 2014, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kinslow, C.J.; Wang, T.J.C. Incidence of extrameningeal solitary fibrous tumors. Cancer 2020, 126, 4067. [Google Scholar] [CrossRef] [PubMed]
- Ginat, D.T.; Bokhari, A.; Bhatt, S.; Dogra, V. Imaging Features of Solitary Fibrous Tumors. Am. J. Roentgenol. 2011, 196, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Klemperer, P.; Coleman, B.R. Primary neoplasms of the pleura. A report of five cases. Am. J. Ind. Med. 1992, 22, 4–31. [Google Scholar] [CrossRef] [PubMed]
- Stout, A.P. Localized pleural mesothelioma. Investigation of its characteristics and histogenesis by the method of tissue culture. Arch. Pathol. 1942, 34, 951–964. [Google Scholar]
- Stout, A.P.; Murray, M.R. Hemangiopericytoma: A Vascular Tumor Featuring Zimmermann’s Pericytes. Ann. Surg. 1942, 116, 26–33. [Google Scholar] [CrossRef]
- Goodlad, J.R.; Fletcher, C.D. Solitary fibrous tumour arising at unusual sites: Analysis of a series. Histopathology 1991, 19, 515–522. [Google Scholar] [CrossRef]
- Gengler, C.; Guillou, L. Solitary fibrous tumour and haemangiopericytoma: Evolution of a concept. Histopathology 2006, 48, 63–74. [Google Scholar] [CrossRef]
- Park, M.S.; Araujo, D.M. New insights into the hemangiopericytoma/solitary fibrous tumor spectrum of tumors. Curr. Opin. Oncol. 2009, 21, 327–331. [Google Scholar] [CrossRef]
- Smrke, A.; Thway, K.; Huang, P.H.; Jones, R.L.; Hayes, A.J. Solitary fibrous tumor: Molecular hallmarks and treatment for a rare sarcoma. Future Oncol. 2021, 17, 3627–3636. [Google Scholar] [CrossRef]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2020, 113, 70. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.A. Solitary Fibrous Tumor of the Pleura. Cancer Control 2006, 13, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ozaniak, A.; Hladik, P.; Lischke, R.; Strizova, Z. Diagnostic challenges and treatment options in patients with solitary fibrous tumor: A single-center observational study. Front. Surg. 2022, 9, 952463. [Google Scholar] [CrossRef] [PubMed]
- Keser, B.N.; Kırman, Ü.N.; Aktemur, G.; Alimoĝlu, O. A rare solitary fibrous tumour of the ascending mesocolon: A case report. Ann. R. Coll. Surg. Engl. 2019, 101, e108–e110. [Google Scholar] [CrossRef]
- Nunes, F.B.; Sant’Ana, M.S.P.; Silva, A.M.B.; Agostini, M.; Silva Canedo, N.H.; de Andrade, B.A.B.; Romañach, M.J.; Corrêa, D.L.; Tomasi, R.A.; Radhakrishnan, R.; et al. Solitary fibrous tumour of the oral cavity: An update. J. Oral Pathol. Med. 2020, 49, 14–20. [Google Scholar] [CrossRef]
- Thway, K.; Ng, W.; Noujaim, J.; Jones, R.L.; Fisher, C. The Current Status of Solitary Fibrous Tumor: Diagnostic Features, Variants, and Genetics. Int. J. Surg. Pathol. 2016, 24, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Musyoki, F.N.; Nahal, A.; Powell, T.I. Solitary fibrous tumor: An update on the spectrum of extrapleural manifestations. Skelet. Radiol. 2012, 41, 5–13. [Google Scholar] [CrossRef]
- White, G.Z.; Cox, E.L.; Schwartz, E.J.; Korkigian, S.A. Rare Solitary Fibrous Tumor in the Pediatric Neck: A Case Report and Review of the Literature. Cureus 2017, 9, e1140. [Google Scholar] [CrossRef]
- Vu, A.F.; Chundury, R.V.; Blandford, A.D.; Perry, J.D. Recurrent Orbital Solitary Fibrous Tumor in a 12-Year-Old. Ocul. Oncol. Pathol. 2017, 3, 83–86. [Google Scholar] [CrossRef]
- Rizk, T.; Awada, A.; Sebaaly, A.; Hourani, R. Solitary fibrous tumor of the scalp in a child. J. Neurosurg. Pediatr. 2013, 11, 79–81. [Google Scholar] [CrossRef]
- Tan, S.Y.; Szymanski, L.J.; Galliani, C.; Parham, D.; Zambrano, E. Solitary Fibrous Tumors in Pediatric Patients: A Rare and Potentially Overdiagnosed Neoplasm, Confirmed by STAT6 Immunohistochemistry. Pediatr. Dev. Pathol. 2018, 21, 389–400. [Google Scholar] [CrossRef]
- Wang, H.; Shen, D.; Hou, Y. Malignant solitary tumor in a child: A case report and review of the literature. J. Pediatr. Surg. 2011, 46, e5–e9. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Xu, W.; Liu, J.; Shen, B.; Deng, X.; Wu, Y.; Wu, W.; Yu, S.; Wang, X.; Lv, Z. Pancreatic solitary fibrous tumor in a toddler managed by pancreaticoduodenectomy: A case report and review of the literature. OncoTargets Ther. 2017, 10, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Gebresellassie, H.W.; Mohammed, Y.; Kotiso, B.; Amare, B.; Kebede, A. A giant solitary fibrous tumor of the adrenal gland in a 13-year old: A case report and review of the literature. J. Med. Case Rep. 2019, 13, 246. [Google Scholar] [CrossRef]
- Croti, U.A.; Braile, D.M.; Moscardini, A.C.; Cury, P.M. Solitary fibrous tumor in a child’s heart. Braz. J. Cardiovasc. Surg. 2008, 23, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Friis, R.B.; Safwat, A.; Baad-Hansen, T.; Aggerholm-Pedersen, N. Solitary Fibrous Tumour: A Single Institution Retrospective Study and Further Validation of a Prognostic Risk Assessment System. Clin. Oncol. R. Coll. Radiol. 2018, 30, 798–804. [Google Scholar] [CrossRef]
- Demicco, E.G.; Park, M.S.; Araujo, D.M.; Fox, P.S.; Bassett, R.L.; Pollock, R.E.; Lazar, A.J.; Wang, W.L. Solitary fibrous tumor: A clinicopathological study of 110 cases and proposed risk assessment model. Mod. Pathol. 2012, 25, 1298–1306. [Google Scholar] [CrossRef]
- Chick, J.F.; Chauhan, N.R.; Madan, R. Solitary fibrous tumors of the thorax: Nomenclature, epidemiology, radiologic and pathologic findings, differential diagnoses, and management. Am. J. Roentgenol. 2013, 200, W238–W248. [Google Scholar] [CrossRef]
- Kallen, M.E.; Hornick, J.L. The 2020 WHO Classification: What’s New in Soft Tissue Tumor Pathology? Am. J. Surg. Pathol. 2021, 45, e1–e23. [Google Scholar] [CrossRef]
- Zhang, W.D.; Chen, J.Y.; Cao, Y.; Liu, Q.Y.; Luo, R.G. Computed tomography and magnetic resonance imaging findings of solitary fibrous tumors in the pelvis: Correlation with histopathological findings. Eur. J. Radiol. 2011, 78, 65–70. [Google Scholar] [CrossRef]
- Bratton, L.; Salloum, R.; Cao, W.; Huber, A.R. Solitary Fibrous Tumor of the Sigmoid Colon Masquerading as an Adnexal Neoplasm. Case Rep. Pathol. 2016, 2016, 4182026. [Google Scholar] [CrossRef]
- Thompson, L.D.; Karamurzin, Y.; Wu, M.L.; Kim, J.H. Solitary fibrous tumor of the larynx. Head Neck Pathol. 2008, 2, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Zhang, L.; Wang, G.; Adhikari, B.K.; Liu, Q.; Zhang, W. Pericardial malignant solitary fibrous tumour with right atrial invasion—A case report and literature review. J. Int. Med. Res. 2019, 47, 2716–2722. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.U.; Din, N.U.; Abdul-Ghafar, J.; Park, Y.K. The many faces of solitary fibrous tumor; diversity of histological features, differential diagnosis and role of molecular studies and surrogate markers in avoiding misdiagnosis and predicting the behavior. Diagn. Pathol. 2021, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; Teixeira, R.; Miller, N.F.; Raj, M.; Sheikh, H.; Sharma, R. Extrapleural Superficial Solitary Fibrous Tumor on the Posterior Shoulder: A Case Report and Review of the Literature. Eplasty 2018, 18, e31. [Google Scholar]
- Gholami, S.; Cassidy, M.R.; Kirane, A.; Kuk, D.; Zanchelli, B.; Antonescu, C.R.; Singer, S.; Brennan, M. Size and Location are the Most Important Risk Factors for Malignant Behavior in Resected Solitary Fibrous Tumors. Ann. Surg. Oncol. 2017, 24, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, A.; Cozzolino, I.; Zito Marino, F.; Accardo, M.; Montella, M.; Panarese, I.; Roccuzzo, G.; Toni, G.; Franco, R.; De Chiara, A. Extrapleural solitary fibrous tumor: A distinct entity from pleural solitary fibrous tumor. An update on clinical, molecular and diagnostic features. Ann. Diagn. Pathol. 2018, 34, 142–150. [Google Scholar] [CrossRef]
- Davanzo, B.; Emerson, R.E.; Lisy, M.; Koniaris, L.G.; Kays, J.K. Solitary fibrous tumor. Transl. Gastroenterol. Hepatol. 2018, 3, 94. [Google Scholar] [CrossRef]
- Thompson, L.D.R.; Lau, S.K. Sinonasal Tract Solitary Fibrous Tumor: A Clinicopathologic Study of Six Cases with a Comprehensive Review of the Literature. Head Neck Pathol. 2018, 12, 471–480. [Google Scholar] [CrossRef]
- You, Y.H.; Liu, R.T.; Zhang, Y. A large solitary fibrous tumour of the pleura: A case report and review of the literature. J. Int. Med. Res. 2018, 46, 1672–1677. [Google Scholar] [CrossRef]
- Kawamura, J.; Tani, M.; Kida, Y.; Sumida, K.; Ogawa, R.; Kawasoe, J.; Yazawa, T.; Yamada, M.; Yamamoto, M.; Harada, H.; et al. Successful laparoscopic treatment of a giant solitary fibrous tumor of the mesorectum: A case report and literature review. Asian J. Endosc. Surg. 2017, 10, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Demicco, E.G.; Wagner, M.J.; Maki, R.G.; Gupta, V.; Iofin, I.; Lazar, A.J.; Wang, W.L. Risk assessment in solitary fibrous tumors: Validation and refinement of a risk stratification model. Mod. Pathol. 2017, 30, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, B.; Redmond, K.C. Largest known malignant solitary fibrous tumour of the pleura-extended resection warranting cardiopulmonary bypass support. Ir. J. Med. Sci. 2019, 188, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.S.; Antonescu, C.R.; Hajdu, C.; Ferrone, C.R.; Hussain, M.; Lewis, J.J.; Brennan, M.F.; Coit, D.G. Clinicopathologic correlates of solitary fibrous tumors. Cancer 2002, 94, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Salas, S.; Resseguier, N.; Blay, J.Y.; Le Cesne, A.; Italiano, A.; Chevreau, C.; Rosset, P.; Isambert, N.; Soulie, P.; Cupissol, D.; et al. Prediction of local and metastatic recurrence in solitary fibrous tumor: Construction of a risk calculator in a multicenter cohort from the French Sarcoma Group (FSG) database. Ann. Oncol. 2017, 28, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Silvanto, A.; Karanjia, N.D.; Bagwan, I.N. Primary hepatic solitary fibrous tumor with histologically benign and malignant areas. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Slater, K. Solitary fibrous tumour of the liver-report on metastasis and local recurrence of a malignant case and review of literature. World J. Surg. Oncol. 2017, 15, 27. [Google Scholar] [CrossRef]
- Sammoud, S.; Ferjani, S.; Hamdani, M.; Toumi, A. Malignant Renal Solitary Fibrous Tumor With Two Local Recurrences and Distant Pulmonary Metastasis. Urology 2019, 127, 9–12. [Google Scholar] [CrossRef]
- Bacalbasa, N.; Balescu, I.; Jinescu, G.; Marcu, M.; Contolenco, A.; Pop, D.; Dobritoiu, D.; Ionescu, O.; Ionescu, P.; Stoica, C. Fat-forming Solitary Fibrous Tumor of the Kidney—A Case Report and Literature Review. In Vivo 2018, 32, 649–652. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, D.; Chen, K.; Zhang, H.; Huang, B. Malignant solitary fibrous tumor of the kidney with liver metastasis: A case report and literature review. J. Cancer Res. Ther. 2018, 14, S1217–S1219. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Xiong, Y.; Xu, T.; Xu, J.; Li, Q.; Yang, G. Atypical/malignant solitary fibrous tumor of the pancreas with spleen vein invasion: Case report and literature review. Medicine 2020, 99, e19783. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Fujino, Y.; Ohara, T.; Kakinoki, K.; Sugimoto, T.; Kajimoto, K.; Tominaga, M. A rare case of metastatic solitary fibrous tumor of the pancreas manifesting as a cystic neoplasm: A case report. Surg. Case Rep. 2019, 5, 142. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Chen, P.; Wang, G.Y.; Zhu, Q.S. Giant solitary fibrous tumor arising from greater omentum. World J. Gastroenterol. 2012, 18, 6515–6520. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.Y.; Bae, J.M. Primary omental malignant solitary fibrous tumour, an extremely rare malignancy: A case report and review of the literature. Arab. J. Gastroenterol. 2019, 20, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Kratiras, Z.; Spapis, V.; Koniaris, E.; Kozyrakis, D.; Skriapas, K. Malignant solitary fibrous tumor of urinary bladder: A rare clinical entity. Arch. Ital. Urol. Androl. 2019, 91. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.N.; Tavares, A.B.; Viveiros, F.A.; Baldaia, H. Solitary fibrous tumour of caecum wall: An unlikely cause of low gastrointestinal haemorrhage. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Ronchi, A.; La Mantia, E.; Gigantino, V.; Perdonà, S.; De Sio, M.; Facchini, G.; Franco, R.; De Chiara, A. A rare case of malignant solitary fibrous tumor in prostate with review of the literature. Diagn. Pathol. 2017, 12, 50. [Google Scholar] [CrossRef]
- Hu, S.; Yi, L.; Yang, L.; Wang, Y. Solitary fibrous tumor of the spermatic cord: A case report and literature review. Exp. Ther. Med. 2015, 9, 55–58. [Google Scholar] [CrossRef]
- Zhou, Y.; Gong, G.; Tang, Y.; Tang, J.; Gan, Y.; Dai, Y. Paratesticular solitary fibrous tumor: A case report and review of literature. Int. J. Clin. Exp. Pathol. 2015, 8, 3358–3361. [Google Scholar]
- Chang, T.H.; Chen, M.; Lee, C.C. Solitary fibrous tumor of the scrotum: A case report and review of the literature. BMC Urol. 2019, 19, 138. [Google Scholar] [CrossRef]
- Yang, E.J.; Howitt, B.E.; Fletcher, C.D.M.; Nucci, M.R. Solitary fibrous tumour of the female genital tract: A clinicopathological analysis of 25 cases. Histopathology 2018, 72, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Biedrzycki, O.J.; Singh, N.; Habeeb, H.; Wathen, N.; Faruqi, A. Solitary fibrous tumor of the female genital tract a case report and review of the literature. Int. J. Gynecol. Pathol. 2007, 26, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Vadmal, M.S.; Pellegrini, A.E. Solitary fibrous tumor of the vagina. Am. J. Dermatopathol. 2000, 22, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Hsu, C.Y.; Chou, Y.H.; Lai, Y.C.; Lin, Y.H.; Wang, H.K.; Chiou, H.J.; Wang, J.; Tiu, C.M. Solitary fibrous tumor of the breast: A case report and review of the literature. J. Clin. Ultrasound 2017, 45, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Salemis, N.S. Solitary fibrous tumor of the breast: A case report and the review of the literature. Breast J. 2018, 24, 78–81. [Google Scholar] [CrossRef]
- Nitta, T.; Kimura, K.; Tominaga, T.; Ikari, A.; Takashima, Y.; Hirata, A.; Takeshita, A.; Ishibashi, T.; Iwamoto, M. Malignant solitary fibrous tumor of the breast. Breast J. 2021, 27, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Czimbalmos, C.; Csecs, I.; Polos, M.; Bartha, E.; Szucs, N.; Toth, A.; Maurovich-Horvat, P.; Becker, D.; Sapi, Z.; Szabolcs, Z.; et al. Uncommon presentation of a rare tumour—Incidental finding in an asymptomatic patient: Case report and comprehensive review of the literature on intrapericardial solitary fibrous tumours. BMC Cancer 2017, 17, 612. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Deng, Q.; Mi, J. Giant solitary fibrous tumor of the heart: Report of a case. Zhonghua Bing Li Xue Za Zhi 2015, 44, 212–213. [Google Scholar]
- Bianchi, G.; Ferrarini, M.; Matteucci, M.; Monteleone, A.; Aquaro, G.D.; Passino, C.; Pucci, A.; Glauber, M. Giant solitary fibrous tumor of the epicardium causing reversible heart failure. Ann. Thorac. Surg. 2013, 96, e49–e51. [Google Scholar] [CrossRef]
- Li, B.; Mao, M.M.; Adhikari, B.K.; Li, Z.Y.; Zhang, W.H. Primary solitary fibrous tumour in the pulmonary artery: A case report. J. Int. Med. Res. 2020, 48, 300060520911273. [Google Scholar] [CrossRef]
- Kitada, M.; Yasuda, S.; Abe, M.; Yoshida, N.; Okazaki, S.; Ishibashi, K. Solitary fibrous tumor of the trachea: A case report. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tamares, S.; Crawley, B.K. Laryngeal Solitary Fibrous Tumor: A Case Report and Systematic Review. J. Voice 2021, 35, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Alawi, F.; Stratton, D.; Freedman, P.D. Solitary fibrous tumor of the oral soft tissues: A clinicopathologic and immunohistochemical study of 16 cases. Am. J. Surg. Pathol. 2001, 25, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Suster, S.; Nascimento, A.G.; Miettinen, M.; Sickel, J.Z.; Moran, C.A. Solitary fibrous tumors of soft tissue. A clinicopathologic and immunohistochemical study of 12 cases. Am. J. Surg. Pathol. 1995, 19, 1257–1266. [Google Scholar] [CrossRef]
- Okui, T.; Ibaragi, S.; Kawai, H.; Sasaki, A. Solitary Fibrous Tumor Arising in the Buccal Space. Case Rep. Med. 2019, 2019, 9459837. [Google Scholar] [CrossRef]
- Lee, C.K.; Liu, K.L.; Huang, S.K. A dedifferentiated solitary fibrous tumor of the parotid gland: A case report with Cytopathologic findings and review of the literature. Diagn. Pathol. 2019, 14, 20. [Google Scholar] [CrossRef]
- Rizzo, S.; Giunta, A.A.; Pennacchi, A. Sinonasal and rhinopharyngeal solitary fibrous tumour: A case report and review of the literature. Acta Otorhinolaryngol. Ital. 2015, 35, 455–458. [Google Scholar] [CrossRef]
- Smith, S.C.; Gooding, W.E.; Elkins, M.; Patel, R.M.; Harms, P.W.; McDaniel, A.S.; Palanisamy, N.; Uram-Tuculescu, C.; Balzer, B.B.; Lucas, D.R.; et al. Solitary Fibrous Tumors of the Head and Neck: A Multi-Institutional Clinicopathologic Study. Am. J. Surg. Pathol. 2017, 41, 1642–1656. [Google Scholar] [CrossRef]
- Sayit, A.T.; Elmali, M.; Gul, A.; Sullu, Y. Solitary fibrous tumor of the orbit: Computed tomography and histopathological findings. J. Cancer Res. Ther. 2019, 15, 719–721. [Google Scholar] [CrossRef]
- Gigantelli, J.W.; Kincaid, M.C.; Soparkar, C.N.; Lee, A.G.; Carter, S.R.; Yeatts, R.P.; Holck, D.E.; Hartstein, M.E.; Kennerdell, J.S. Orbital solitary fibrous tumor: Radiographic and histopathologic correlations. Ophthalmic Plast. Reconstr. Surg. 2001, 17, 207–214. [Google Scholar] [CrossRef]
- Verdi, D.; Pennelli, G.; Pelizzo, M.R.; Toniato, A. Solitary fibrous tumor of the thyroid gland: A report of two cases with an analysis of their clinical and pathological features. Endocr. Pathol. 2011, 22, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.D.R.; Wei, C.; Rooper, L.M.; Lau, S.K. Thyroid Gland Solitary Fibrous Tumor: Report of 3 Cases and a Comprehensive Review of the Literature. Head Neck Pathol. 2019, 13, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Rad, M.; Wang, K.Y.; Jain, S.; Lincoln, C.M. Solitary fibrous tumor of thyroid: A case report with review of literature. Clin. Imaging 2019, 53, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, R.; Arıkan, Ş.M.; Şimşek, M.A.; Özanlağan, E.; Güngör, B. Management of solitary fibrous tumors localized in extremity: Case series and a review of the literature. Eklem Hastalik. Cerrahisi 2017, 28, 121–127. [Google Scholar] [CrossRef]
- Chandanwale, S.S.; Gore, C.R.; Sammi, A.B.; Shah, K.R.; Kaur, P.R. Recurrent solitary fibrous tumor in distal lower extremity: An extremely rare entity. Int. J. Appl. Basic Med. Res. 2014, 4, 134–136. [Google Scholar] [CrossRef]
- Akisue, T.; Matsumoto, K.; Kizaki, T.; Fujita, I.; Yamamoto, T.; Yoshiya, S.; Kurosaka, M. Solitary fibrous tumor in the extremity: Case report and review of the literature. Clin. Orthop. Relat. Res. 2003, 411, 236–244. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, D.H.; Seo, K.J.; Jung, S.N. A Solitary Fibrous Tumor (Cellular Form) of the Ankle. J. Foot Ankle Surg. 2016, 55, 829–831. [Google Scholar] [CrossRef]
- Mohtarrudin, N.; Nor Hanipah, Z.; Mohd Dusa, N. Solitary fibrous tumour of the chest wall. Malays. J. Pathol. 2016, 38, 61–64. [Google Scholar]
- Hong, J.P.; Chung, Y.K.; Kim, S.W.; Kim, T.H.; Lee, K.G. Solitary fibrous tumour of the face: A rare case report. Br. J. Plast. Surg. 2002, 55, 75–77. [Google Scholar] [CrossRef]
- Feasel, P.; Al-Ibraheemi, A.; Fritchie, K.; Zreik, R.T.; Wang, W.L.; Demicco, E.; Saeb-Lima, M.; Goldblum, J.R.; Rubin, B.P.; McKenney, J.K.; et al. Superficial Solitary Fibrous Tumor: A Series of 26 Cases. Am. J. Surg. Pathol. 2018, 42, 778–785. [Google Scholar] [CrossRef]
- Li, X.M.; Reng, J.; Zhou, P.; Cao, Y.; Cheng, Z.Z.; Xiao, Y.; Xu, G.H. Solitary fibrous tumors in abdomen and pelvis: Imaging characteristics and radiologic-pathologic correlation. World J. Gastroenterol. 2014, 20, 5066–5073. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Nakajima, S.; Futagawa, Y.; Fujioka, S.; Okamoto, T.; Yanaga, K. A solitary fibrous tumor originating from the liver surface. Clin. J. Gastroenterol. 2009, 2, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Ong, S.L.; Richards, C.; Garcea, G.; Pollard, C.; Berry, D.; Dennison, A. Inaccuracy of fine-needle biopsy in the diagnosis of solitary fibrous tumour of the liver. Asian J. Surg. 2008, 31, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Kueht, M.; Masand, P.; Rana, A.; Cotton, R.; Goss, J. Concurrent hepatic hemangioma and solitary fibrous tumor: Diagnosis and management. J. Surg. Case Rep. 2015, 2015, rjv089. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.J.; Kim, Y.D.; Yim, Y.J.; Kim, S.T.; Jeon, P.; Kim, K.H.; Byun, H.S.; Song, H.J. Solitary fibrous tumor of the orbit: CT and MR imaging findings. Am. J. Neuroradiol. 2008, 29, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Changku, J.; Shaohua, S.; Zhicheng, Z.; Shusen, Z. Solitary fibrous tumor of the liver: Retrospective study of reported cases. Cancer Invest. 2006, 24, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Imamura, T.; Tateishi, A.; Park, P.; Nakano, H.; Harasawa, A.; Hashimoto, H.; Matsushita, T. Intramuscular solitary fibrous tumor: A clinicopathological case study. J. Comput. Assist. Tomogr. 1999, 23, 458–462. [Google Scholar] [CrossRef]
- Rena, O.; Filosso, P.L.; Papalia, E.; Molinatti, M.; Di Marzio, P.; Maggi, G.; Oliaro, A. Solitary fibrous tumour of the pleura: Surgical treatment. Eur. J. Cardio Thorac. Surg. 2001, 19, 185–189. [Google Scholar] [CrossRef]
- Mordenti, P.; Di Cicilia, R.; Delfanti, R.; Capelli, P.; Paties, C.; Cavanna, L. Solitary fibrous tumors of the pleura: A case report and review of the literature. Tumori 2013, 99, e177–e183. [Google Scholar] [CrossRef]
- Mosquera, J.M.; Fletcher, C.D. Expanding the spectrum of malignant progression in solitary fibrous tumors: A study of 8 cases with a discrete anaplastic component—is this dedifferentiated SFT? Am. J. Surg. Pathol. 2009, 33, 1314–1321. [Google Scholar] [CrossRef]
- Stanisce, L.; Ahmad, N.; Levin, K.; Deckard, N.; Enriquez, M.; Brody, J.; Koshkareva, Y. Solitary Fibrous Tumors in the Head and Neck: Comprehensive Review and Analysis. Head Neck Pathol. 2020, 14, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, A.K.; Prasad, S.R.; Takahashi, N.; Vikram, R.; Zaheer, A.; Sandrasegaran, K. Somatic and visceral solitary fibrous tumors in the abdomen and pelvis: Cross-sectional imaging spectrum. Radiographics 2011, 31, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Fridlington, J.; Weaver, J.; Kelly, B.; Kelly, E. Secondary hypertrophic osteoarthropathy associated with solitary fibrous tumor of the lung. J. Am. Acad. Dermatol. 2007, 57 (Suppl. S5), S106–S110. [Google Scholar] [CrossRef] [PubMed]
- De Los Santos-Aguilar, R.G.; Chávez-Villa, M.; Contreras, A.G.; García-Herrera, J.S.; Gamboa-Domínguez, A.; Vargas-Sánchez, J.; Almeda-Valdes, P.; Reza-Albarrán, A.A.; Iñiguez-Ariza, N.M. Successful Multimodal Treatment of an IGF2-Producing Solitary Fibrous Tumor With Acromegaloid Changes and Hypoglycemia. J. Endocr. Soc. 2019, 3, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, Y.; Takada, A.; Tateno, M.; Sato, H.; Koizumi, M.; Tanaka, A.; Sato, T. Solitary fibrous tumor of the pleura causing recurrent hypoglycemia by secretion of insulin-like growth factor II. Pathol. Int. 1998, 48, 47–52. [Google Scholar] [CrossRef]
- Steigen, S.E.; Schaeffer, D.F.; West, R.B.; Nielsen, T.O. Expression of insulin-like growth factor 2 in mesenchymal neoplasms. Mod. Pathol. 2009, 22, 914–921. [Google Scholar] [CrossRef]
- Jo, V.Y.; Fletcher, C.D. WHO classification of soft tissue tumours: An update based on the 2013 (4th) edition. Pathology 2014, 46, 95–104. [Google Scholar] [CrossRef]
- Hasegawa, T.; Matsuno, Y.; Shimoda, T.; Hasegawa, F.; Sano, T.; Hirohashi, S. Extrathoracic solitary fibrous tumors: Their histological variability and potentially aggressive behavior. Hum. Pathol. 1999, 30, 1464–1473. [Google Scholar] [CrossRef]
- Cox, D.P.; Daniels, T.; Jordan, R.C. Solitary fibrous tumor of the head and neck. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 79–84. [Google Scholar] [CrossRef]
- Herrmann, B.L.; Saller, B.; Kiess, W.; Morgenroth, K.; Drochner, K.; Schröder, T.; Mann, K. Primary malignant fibrous histiocytoma of the lung: IGF-II producing tumor induces fasting hypoglycemia. Exp. Clin. Endocrinol. Diabetes 2000, 108, 515–518. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Y.; Jing, M.; Qu, W.; Li, J.; Zhao, X.R.; Yu, Y.H. Postoperative Radiotherapy for the Treatment of Solitary Fibrous Tumor With Malignant Transformation of the Pelvic: A Rare Case Report With Literature Review. Medicine 2016, 95, e2433. [Google Scholar] [CrossRef] [PubMed]
- Vennarecci, G.; Ettorre, G.M.; Giovannelli, L.; Del Nonno, F.; Perracchio, L.; Visca, P.; Corazza, V.; Vidiri, A.; Visco, G.; Santoro, E. Solitary fibrous tumor of the liver. J. Hepatobiliary Pancreat. Surg. 2005, 12, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Famà, F.; Le Bouc, Y.; Barrande, G.; Villeneuve, A.; Berry, M.G.; Pidoto, R.R.; Saint Marc, O. Solitary fibrous tumour of the liver with IGF-II-related hypoglycaemia. A case report. Langenbecks Arch. Surg. 2008, 393, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Radunz, S.; Baba, H.A.; Sotiropoulos, G.C. Large tumor of the liver and hypoglycemic shock in an 85-year-old patient. Gastroenterology 2012, 142, e10–e11. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Nogueira, T.S.; Neuville, A.; Riehm, S.; Averous, G.; Weber, J.C.; Veillon, F. Delayed enhancement pattern in a localized fibrous tumor of the liver. Am. J. Roentgenol. 2005, 184, 1578–1580. [Google Scholar] [CrossRef]
- Bae, J.M.; Kim, S.W.; Kim, S.W.; Song, S.K. Malignant solitary fibrous tumor of retroperitoneum mimicking gastric submucosal tumor. Korean J. Gastroenterol. 2011, 57, 47–50. [Google Scholar] [CrossRef]
- Tani, E.; Wejde, J.; Åström, K.; Wingmo, I.L.; Larsson, O.; Haglund, F. FNA cytology of solitary fibrous tumors and the diagnostic value of STAT6 immunocytochemistry. Cancer Cytopathol. 2018, 126, 36–43. [Google Scholar] [CrossRef]
- Von Mehren, M.; Randall, R.L.; Benjamin, R.S.; Boles, S.; Bui, M.M.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; Kane, J.M.; et al. Soft Tissue Sarcoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 536–563. [Google Scholar] [CrossRef]
- Pavlidis, E.T.; Pavlidis, T.E. New trends in the surgical management of soft tissue sarcoma: The role of preoperative biopsy. World J. Clin. Oncol. 2023, 14, 89–98. [Google Scholar] [CrossRef]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Mondaza-Hernandez, J.L.; Moura, D.S.; Hindi, N. A Comprehensive Review on Solitary Fibrous Tumor: New Insights for New Horizons. Cancers 2021, 13, 2913. [Google Scholar] [CrossRef] [PubMed]
- De Perrot, M.; Fischer, S.; Bründler, M.A.; Sekine, Y.; Keshavjee, S. Solitary fibrous tumors of the pleura. Ann. Thorac. Surg. 2002, 74, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Georgiesh, T.; Aggerholm-Pedersen, N.; Schöffski, P.; Zhang, Y.; Napolitano, A.; Bovée, J.; Hjelle, Å.; Tang, G.; Spalek, M.; Nannini, M.; et al. Validation of a novel risk score to predict early and late recurrence in solitary fibrous tumour. Br. J. Cancer 2022, 127, 1793–1798. [Google Scholar] [CrossRef]
- Van Houdt, W.J.; Westerveld, C.M.; Vrijenhoek, J.E.; van Gorp, J.; van Coevorden, F.; Verhoef, C.; van Dalen, T. Prognosis of solitary fibrous tumors: A multicenter study. Ann. Surg. Oncol. 2013, 20, 4090–4095. [Google Scholar] [CrossRef] [PubMed]
- Shishido, Y.; Aoyama, A.; Hara, S.; Hamakawa, H.; Takahashi, Y. Tumor-to-Tumor Metastasis: Pulmonary Carcinoid Metastasizing to Solitary Fibrous Tumor. Am. J. Case Rep. 2019, 20, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tao, X.; Shi, H.; Li, K. MRI findings of solitary fibrous tumours in the head and neck region. Dentomaxillofac. Radiol. 2014, 43, 20130415. [Google Scholar] [CrossRef] [PubMed]
- Keraliya, A.R.; Tirumani, S.H.; Shinagare, A.B.; Zaheer, A.; Ramaiya, N.H. Solitary Fibrous Tumors: 2016 Imaging Update. Radiol. Clin. N. Am. 2016, 54, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Phipps, K.M.; Nichols, F.C.; Schleck, C.D.; Deschamps, C.; Cassivi, S.D.; Schipper, P.H.; Allen, M.S.; Wigle, D.A.; Pairolero, P.C. Solitary fibrous tumors of the pleura: Results of surgical treatment and long-term prognosis. J. Thorac. Cardiovasc. Surg. 2009, 138, 19–25. [Google Scholar] [CrossRef]
- Baneckova, M.; Martinek, P.; Skalova, A.; Mezencev, R.; Hadravsky, L.; Michal, M.; Svajdler, M. Solitary fibrous tumors of the head and neck region revisited: A single-institution study of 20 cases and review of the literature. Hum. Pathol. 2020, 99, 1–12. [Google Scholar] [CrossRef]
- Wiriosuparto, S.; Krassilnik, N.; Bhuta, S.; Rao, J.; Firschowitz, S. Solitary fibrous tumor: Report of a case with an unusual presentation as a spindle cell parotid neoplasm. Acta Cytol. 2005, 49, 309–313. [Google Scholar] [CrossRef]
- Santoro, F.; Linari, A.; Maletta, F.; Parente, R.; Torchio, B.; Rossi, E.D.; Messuti, I.; Borasi, A.; Volante, M.; Papotti, M. Solitary fibrous tumor of the thyroid: Report of three cases with a focus on cytological features and histological clues for malignancy. Virchows Arch. 2023, 483, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Askan, G.; Basturk, O. Mesenchymal Tumors Involving the Pancreas: A Clinicopathologic Analysis and Review of the Literature. Turk. Patoloji Derg. 2022, 38, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Consolo, U.; Diamante, F.; Salgarelli, A.C.; Bellini, P. A rare case of solitary fibrous tumor of the temporal region: 7-year-follow-up clinical-radiographic evaluation and literature review. Oral Maxillofac. Surg. Cases 2022, 8, 100240. [Google Scholar] [CrossRef]
- Vincek, V.; Kallis, P.; Vause, A.; Vincek, E.; Ilkovitch, D.; Motaparthi, K. Cutaneous solitary fibrous tumor: Report of three cases with review of histopathological mimics. J. Cutan. Pathol. 2022, 49, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K. Solitary fibrous tumour—Everywhere, and a diagnosis in vogue. Histopathology 1997, 31, 568–576. [Google Scholar] [CrossRef] [PubMed]
- De Saint Aubain Somerhausen, N.; Rubin, B.P.; Fletcher, C.D. Myxoid solitary fibrous tumor: A study of seven cases with emphasis on differential diagnosis. Mod. Pathol. 1999, 12, 463–471. [Google Scholar]
- Sugita, S.; Segawa, K.; Kikuchi, N.; Takenami, T.; Kido, T.; Emori, M.; Akiyama, Y.; Takada, K.; Hinotsu, S.; Hasegawa, T. Prognostic usefulness of a modified risk model for solitary fibrous tumor that includes the Ki-67 labeling index. World J. Surg. Oncol. 2022, 20, 29. [Google Scholar] [CrossRef]
- Hanau, C. Solitary fibrous tumor: Histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum. Pathol. 1995, 26, 440–449. [Google Scholar] [CrossRef]
- Ali, S.Z.; Hoon, V.; Hoda, S.; Heelan, R.; Zakowski, M.F. Solitary fibrous tumor. Cancer 1997, 81, 116–121. [Google Scholar] [CrossRef]
- Al-Shanawani, B.N.; Al-Qattan, M.M.; Arafah, M.M.; Al-Motairi, M.I. A solitary fibrous tumor of the upper limb. Saudi Med. J. 2015, 36, 236–238. [Google Scholar] [CrossRef]
- Gerykova, L.; Vebr, T.; Kudelka, L.; Poczos, P.; Cesak, T.; Gabalec, F.; Hornychova, H.; Soukup, J. Expression of neuroendocrine markers in meningeal and extrameningeal solitary fibrous tumors: A potential diagnostic pitfall. Hum. Pathol. 2023, 137, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Creytens, D.; Ferdinande, L. Diagnostic Utility of STAT6 Immunohistochemistry in the Diagnosis of Fat-forming Solitary Fibrous Tumors. Appl. Immunohistochem. Mol. Morphol. 2016, 24, e12–e13. [Google Scholar] [CrossRef] [PubMed]
- Vivero, M.; Doyle, L.A.; Fletcher, C.D.; Mertens, F.; Hornick, J.L. GRIA2 is a novel diagnostic marker for solitary fibrous tumour identified through gene expression profiling. Histopathology 2014, 65, 71–80. [Google Scholar] [CrossRef]
- Tazzari, M.; Negri, T.; Rini, F.; Vergani, B.; Huber, V.; Villa, A.; Dagrada, P.; Colombo, C.; Fiore, M.; Gronchi, A.; et al. Adaptive immune contexture at the tumour site and downmodulation of circulating myeloid-derived suppressor cells in the response of solitary fibrous tumour patients to anti-angiogenic therapy. Br. J. Cancer 2014, 111, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Flint, A. CD-34 and keratin expression distinguishes solitary fibrous tumor (Fibrous mesothelioma) of pleura from desmoplastic mesothelioma. Hum. Pathol. 1995, 26, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Naso, J.R.; Chiu, C.G.; Goecke, M.E.; Chang, D.; Shiau, C.J. Benign spindle cell lesions of the breast: A diagnostic approach to solitary fibrous tumour, nodular pseudoangiomatous stromal hyperplasia and nodular fasciitis. J. Clin. Pathol. 2019, 72, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, A.; Tayebwa, J.; Collin, A.; Nilsson, J.; Magnusson, L.; von Steyern, F.V.; Brosjo, O.; Domanski, H.A.; Larsson, O.; Sciot, R.; et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer 2013, 52, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R.; Wu, Y.M.; Kalyana-Sundaram, S.; Cao, X.; Lonigro, R.J.; Sung, Y.S.; Chen, C.L.; Zhang, L.; Wang, R.; Su, F.; et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat. Genet. 2013, 45, 180–185. [Google Scholar] [CrossRef]
- Guseva, N.V.; Tanas, M.R.; Stence, A.A.; Sompallae, R.; Schade, J.C.; Bossler, A.D.; Bellizzi, A.M.; Ma, D. The NAB2-STAT6 gene fusion in solitary fibrous tumor can be reliably detected by anchored multiplexed PCR for targeted next-generation sequencing. Cancer Genet. 2016, 209, 303–312. [Google Scholar] [CrossRef]
- Chmielecki, J.; Crago, A.M.; Rosenberg, M.; O’Connor, R.; Walker, S.R.; Ambrogio, L.; Auclair, D.; McKenna, A.; Heinrich, M.C.; Frank, D.A.; et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat. Genet. 2013, 45, 131–132. [Google Scholar] [CrossRef]
- Park, H.K.; Yu, D.B.; Sung, M.; Oh, E.; Kim, M.; Song, J.Y.; Lee, M.S.; Jung, K.; Noh, K.W.; An, S.; et al. Molecular changes in solitary fibrous tumor progression. J. Mol. Med. 2019, 97, 1413–1425. [Google Scholar] [CrossRef]
- Tai, H.-C.; Chuang, I.C.; Chen, T.-C.; Li, C.-F.; Huang, S.-C.; Kao, Y.-C.; Lin, P.-C.; Tsai, J.-W.; Lan, J.; Yu, S.-C.; et al. NAB2–STAT6 fusion types account for clinicopathological variations in solitary fibrous tumors. Mod. Pathol. 2015, 28, 1324–1335. [Google Scholar] [CrossRef]
- Georgiesh, T.; Namløs, H.M.; Sharma, N.; Lorenz, S.; Myklebost, O.; Bjerkehagen, B.; Meza-Zepeda, L.A.; Boye, K. Clinical and molecular implications of NAB2-STAT6 fusion variants in solitary fibrous tumour. Pathology 2021, 53, 713–719. [Google Scholar] [CrossRef]
- Bieg, M.; Moskalev, E.A.; Will, R.; Hebele, S.; Schwarzbach, M.; Schmeck, S.; Hohenberger, P.; Jakob, J.; Kasper, B.; Gaiser, T.; et al. Gene Expression in Solitary Fibrous Tumors (SFTs) Correlates with Anatomic Localization and NAB2-STAT6 Gene Fusion Variants. Am. J. Pathol. 2021, 191, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, D.; Abdulfatah, E.; Pantanowitz, L. Molecular testing of soft tissue tumors. Diagn. Cytopathol. 2023, 51, 12–25. [Google Scholar] [CrossRef]
- Georgiesh, T.; Boye, K.; Bjerkehagen, B. A novel risk score to predict early and late recurrence in solitary fibrous tumour. Histopathology 2020, 77, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, F.; Bouvier-Labit, C.; Finetti, P.; Metellus, P.; Adelaide, J.; Mokhtari, K.; Figarella-Branger, D.; Decouvelaere, A.V.; Miquel, C.; Coindre, J.M.; et al. Gene expression profiling of solitary fibrous tumors. PLoS ONE 2013, 8, e64497. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, M.; Singer, S.; Maki, R.G.; Schwartz, G.K.; Keohan, M.L.; Antonescu, C.R. IGF2 over-expression in solitary fibrous tumours is independent of anatomical location and is related to loss of imprinting. J. Pathol. 2010, 221, 300–307. [Google Scholar] [CrossRef]
- Miettinen, M.M.; el-Rifai, W.; Sarlomo-Rikala, M.; Andersson, L.C.; Knuutila, S. Tumor size-related DNA copy number changes occur in solitary fibrous tumors but not in hemangiopericytomas. Mod. Pathol. 1997, 10, 1194–1200. [Google Scholar]
- Bahrami, A.; Lee, S.; Schaefer, I.M.; Boland, J.M.; Patton, K.T.; Pounds, S.; Fletcher, C.D. TERT promoter mutations and prognosis in solitary fibrous tumor. Mod. Pathol. 2016, 29, 1511–1522. [Google Scholar] [CrossRef]
- Demicco, E.G.; Wani, K.; Ingram, D.; Wagner, M.; Maki, R.G.; Rizzo, A.; Meeker, A.; Lazar, A.J.; Wang, W.L. TERT promoter mutations in solitary fibrous tumour. Histopathology 2018, 73, 843–851. [Google Scholar] [CrossRef]
- Guo, W.; Ji, Y.; Guo, L.; Che, S.; Huai, Q.; Yang, K.; Tan, F.; Xue, Q.; Gao, S.; He, J. Severe hypoglycemia and finger clubbing in a patient with a BRCA1 mutation in a solitary fibrous tumor: A case report. Ann. Transl. Med. 2021, 9, 1093. [Google Scholar] [CrossRef] [PubMed]
- Chelsky, Z.L.; Obeidin, F.; Alexiev, B.A.; Santana-Santos, L.; Duckett, D.; Parker, S.; Baczkowski, J.; Pollack, S.; Vormittag-Nocito, E.; Jennings, L.J. Methylation classifier array and classification of solitary fibrous tumors. J. Clin. Oncol. 2023, 41, e23522. [Google Scholar] [CrossRef]
- Martin-Broto, J.; Stacchiotti, S.; Lopez-Pousa, A.; Redondo, A.; Bernabeu, D.; de Alava, E.; Casali, P.G.; Italiano, A.; Gutierrez, A.; Moura, D.S.; et al. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, G.; Carbone, L.; Carleo, F.; Masala, N.; Graziano, P.; Bray, A.; Martelli, M. Solitary fibrous tumors of the pleura: An analysis of 110 patients treated in a single institution. Ann. Thorac. Surg. 2009, 88, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.L.; Walraven, I.; Lecointe-Artzner, E.; van Houdt, W.J.; Strauss, D.; Schrage, Y.; Hayes, A.J.; Raut, C.P.; Fairweather, M.; Baldini, E.H.; et al. Extrameningeal solitary fibrous tumors-surgery alone or surgery plus perioperative radiotherapy: A retrospective study from the global solitary fibrous tumor initiative in collaboration with the Sarcoma Patients EuroNet. Cancer 2020, 126, 3002–3012. [Google Scholar] [CrossRef]
- De Bernardi, A.; Dufresne, A.; Mishellany, F.; Blay, J.-Y.; Ray-Coquard, I.; Brahmi, M. Novel Therapeutic Options for Solitary Fibrous Tumor: Antiangiogenic Therapy and Beyond. Cancers 2022, 14, 1064. [Google Scholar] [CrossRef]
- Zhou, C.; Li, W.; Shao, J.; Zhao, J. Thoracic solitary fibrous tumors: An analysis of 70 patients who underwent surgical resection in a single institution. J. Cancer Res. Clin. Oncol. 2020, 146, 1245–1252. [Google Scholar] [CrossRef]
- Aridi, T.; Tawil, A.; Hashem, M.; Khoury, J.; Raad, R.A.; Youssef, P. Unique Presentation and Management Approach of Pleural Solitary Fibrous Tumor. Case Rep. Surg. 2019, 2019, 9706825. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, R.; Ji, T.; Chen, Z.; Guo, W. Surgical treatment of primary solitary fibrous tumors involving the pelvic ring. PLoS ONE 2018, 13, e0207581. [Google Scholar] [CrossRef]
- Imaoka, K.; Nishihara, M.; Yamaguchi, M.; Kawasaki, Y.; Sugino, K. Laparoscopic excision of a solitary fibrous tumor originating from the abdominal wall: A case report. J. Surg. Case Rep. 2021, 2021, rjaa602. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, S.; Cai, Y.; Peng, B. Total laparoscopic duodenum-preserving pancreatic head resection for solitary fibrous tumor: The first case report. Asian J. Surg. 2022, 45, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Ajouz, H.; Sohail, A.H.; Hashmi, H.; Martinez Aguilar, M.; Daoui, S.; Tembelis, M.; Aziz, M.; Zohourian, T.; Brathwaite, C.E.M.; Cerfolio, R.J. Surgical considerations in the resection of solitary fibrous tumors of the pleura. J. Cardiothorac. Surg. 2023, 18, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, X.; Li, X.; Tang, Z.; Shi, C.; Wang, G. Diagnosis and surgical treatment of mediastinal solitary fibrous tumor. Asia Pac. J. Clin. Oncol. 2017, 13, e473–e480. [Google Scholar] [CrossRef] [PubMed]
- Krengli, M.; Cena, T.; Zilli, T.; Jereczek-Fossa, B.A.; De Bari, B.; Villa Freixa, S.; Kaanders, J.; Torrente, S.; Pasquier, D.; Sole, C.V.; et al. Radiotherapy in the treatment of extracranial hemangiopericytoma/solitary fibrous tumor: Study from the Rare Cancer Network. Radiother. Oncol. 2020, 144, 114–120. [Google Scholar] [CrossRef]
- Nishikawa, T.; Takei, Y.; Teshima, S.; Deie, K.; Takano, Y.; Tsuno, N.H.; Maeda, M. A hypervascular malignant solitary fibrous tumor in the mesentery of the rectum with good response to preoperative radiation therapy. Int. Cancer Conf. J. 2013, 2, 30–35. [Google Scholar] [CrossRef]
- Shoji, Y.; Ohuchi, M.; Yoshida, I. Solitary fibrous tumor (SFT) of the thymic area—A case report. Nihon Kyobu Geka Gakkai Zasshi 1993, 41, 1431–1435. [Google Scholar]
- Cardillo, G.; Lococo, F.; Carleo, F.; Martelli, M. Solitary fibrous tumors of the pleura. Curr. Opin. Pulm. Med. 2012, 18, 339–346. [Google Scholar] [CrossRef]
- Li, Z.K.; Liu, J.; Chen, C.; Yang, K.Y.; Deng, Y.T.; Jiang, Y. Locally advanced malignant solitary fibrous tumour successfully treated with conversion chemotherapy, operation and postoperative radiotherapy: A case report. J. Int. Med. Res. 2021, 49, 300060521996940. [Google Scholar] [CrossRef]
- Haas, R.L.; Walraven, I.; Lecointe-Artzner, E.; Scholten, A.N.; van Houdt, W.J.; Griffin, A.M.; Ferguson, P.C.; Miah, A.B.; Zaidi, S.; DeLaney, T.F.; et al. Radiation Therapy as Sole Management for Solitary Fibrous Tumors (SFT): A Retrospective Study From the Global SFT Initiative in Collaboration With the Sarcoma Patients EuroNet. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1226–1233. [Google Scholar] [CrossRef]
- Vanfleteren, L.E.; Peulen, H.M.; Creytens, D.H.; Smulders, N.M.; Utama, I.; de Ruysscher, D.K.; ten Velde, G.P. Complete metabolic remission of an irresectable mediastinal solitary fibrous tumour with concurrent chemoradiation. Thorax 2009, 64, 822–823. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, B.; Dong, L.; Liu, B. Recurrent intrathoracic solitary fibrous tumor: Remarkable response to radiotherapy. Ann. Thorac. Med. 2014, 9, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Endo, K.; Aihara, T.; Matsuoka, Y.; Nishimura, H.; Suzuki, H.; Sawaji, Y.; Yamamoto, K.; Fukami, S.; Tanigawa, M.; et al. Salvage carbon ion radiotherapy for recurrent solitary fibrous tumor: A case report and literature review. J. Orthop. Surg. 2020, 28, 2309499019896099. [Google Scholar] [CrossRef] [PubMed]
- Spałek, M.J.; Teterycz, P.; Borkowska, A.; Poleszczuk, J.; Rutkowski, P. Stereotactic radiotherapy for soft tissue and bone sarcomas: Real-world evidence. Ther. Adv. Med. Oncol. 2022, 14, 17588359211070646. [Google Scholar] [CrossRef] [PubMed]
- Arifi, S. Personalised pharmacotherapy options for soft tissue sarcomas. Expert Rev. Precis. Med. Drug Dev. 2022, 7, 17–28. [Google Scholar] [CrossRef]
- Park, M.S.; Ravi, V.; Conley, A.; Patel, S.R.; Trent, J.C.; Lev, D.C.; Lazar, A.J.; Wang, W.L.; Benjamin, R.S.; Araujo, D.M. The role of chemotherapy in advanced solitary fibrous tumors: A retrospective analysis. Clin. Sarcoma Res. 2013, 3, 7. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Libertini, M.; Negri, T.; Palassini, E.; Gronchi, A.; Fatigoni, S.; Poletti, P.; Vincenzi, B.; Dei Tos, A.P.; Mariani, L.; et al. Response to chemotherapy of solitary fibrous tumour: A retrospective study. Eur. J. Cancer 2013, 49, 2376–2383. [Google Scholar] [CrossRef]
- Levard, A.; Derbel, O.; Méeus, P.; Ranchère, D.; Ray-Coquard, I.; Blay, J.Y.; Cassier, P.A. Outcome of patients with advanced solitary fibrous tumors: The Centre Léon Bérard experience. BMC Cancer 2013, 13, 109. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Saponara, M.; Frapolli, R.; Tortoreto, M.; Cominetti, D.; Provenzano, S.; Negri, T.; Dagrada, G.P.; Gronchi, A.; Colombo, C.; et al. Patient-derived solitary fibrous tumour xenografts predict high sensitivity to doxorubicin/dacarbazine combination confirmed in the clinic and highlight the potential effectiveness of trabectedin or eribulin against this tumour. Eur. J. Cancer 2017, 76, 84–92. [Google Scholar] [CrossRef]
- DeVito, N.; Henderson, E.; Han, G.; Reed, D.; Bui, M.M.; Lavey, R.; Robinson, L.; Zager, J.S.; Gonzalez, R.J.; Sondak, V.K.; et al. Clinical Characteristics and Outcomes for Solitary Fibrous Tumor (SFT): A Single Center Experience. PLoS ONE 2015, 10, e0140362. [Google Scholar] [CrossRef]
- Peixoto Callejo, I. Peritoneal solitary fibrous tumour (SFT): Long-term survival of recurrent and metastasised SFT treated with cytoreductive surgery and intraperitoneal chemotherapy. Clin. Transl. Oncol. 2009, 11, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Boyce-Fappiano, D.; Damron, E.P.; Farooqi, A.; Mitra, D.; Conley, A.P.; Somaiah, N.; Araujo, D.M.; Livingston, J.A.; Ratan, R.; Keung, E.Z.; et al. Hypofractionated Radiation Therapy for Unresectable or Metastatic Sarcoma Lesions. Adv. Radiat. Oncol. 2022, 7, 100913. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Metro, G.; Leonardi, G.C.; Sordo, R.D.; Colella, R.; Puma, F.; Ceccarelli, S.; Potenza, R.; Rebonato, A.; Maiettini, D.; et al. Malignant giant solitary fibrous tumor of the pleura metastatic to the thyroid gland. Tumori J. 2016, 102 (Suppl. S2), S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Orue-Echebarria, M.I.; Garciafília, L.; Rodriguez-Bachiller, L.; Díaz-Zorita, B.; Velasco, E.; Ramón, E.; Agra, C.; Rodríguez, A.C. Solitary extrapleural fibrous tumor with hepatic bilobar metastases: Multimodal approach treatment. Clin. Sarcoma Res. 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Maruzzo, M.; Martin-Liberal, J.; Messiou, C.; Miah, A.; Thway, K.; Alvarado, R.; Judson, I.; Benson, C. Pazopanib as first line treatment for solitary fibrous tumours: The Royal Marsden Hospital experience. Clin. Sarcoma Res. 2015, 5, 5. [Google Scholar] [CrossRef]
- Park, M.S.; Patel, S.R.; Ludwig, J.A.; Trent, J.C.; Conrad, C.A.; Lazar, A.J.; Wang, W.L.; Boonsirikamchai, P.; Choi, H.; Wang, X.; et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer 2011, 117, 4939–4947. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Tortoreto, M.; Bozzi, F.; Tamborini, E.; Morosi, C.; Messina, A.; Libertini, M.; Palassini, E.; Cominetti, D.; Negri, T.; et al. Dacarbazine in solitary fibrous tumor: A case series analysis and preclinical evidence vis-a-vis temozolomide and antiangiogenics. Clin. Cancer Res. 2013, 19, 5192–5201. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Negri, T.; Libertini, M.; Palassini, E.; Marrari, A.; De Troia, B.; Gronchi, A.; Dei Tos, A.P.; Morosi, C.; Messina, A.; et al. Sunitinib malate in solitary fibrous tumor (SFT). Ann. Oncol. 2012, 23, 3171–3179. [Google Scholar] [CrossRef]
- Valentin, T.; Fournier, C.; Penel, N.; Bompas, E.; Chaigneau, L.; Isambert, N.; Chevreau, C. Sorafenib in patients with progressive malignant solitary fibrous tumors: A subgroup analysis from a phase II study of the French Sarcoma Group (GSF/GETO). Investig. New Drugs 2013, 31, 1626–1627. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Baldi, G.G.; Frezza, A.M.; Morosi, C.; Greco, F.G.; Collini, P.; Barisella, M.; Dagrada, G.P.; Zaffaroni, N.; Pasquali, S.; et al. Regorafenib in advanced solitary fibrous tumour: Results from an exploratory phase II clinical study. Eur. J. Cancer 2023, 195, 113391. [Google Scholar] [CrossRef]
- Yamada, Y.; Kohashi, K.; Fushimi, F.; Takahashi, Y.; Setsu, N.; Endo, M.; Yamamoto, H.; Tokunaga, S.; Iwamoto, Y.; Oda, Y. Activation of the Akt-mTOR pathway and receptor tyrosine kinase in patients with solitary fibrous tumors. Cancer 2014, 120, 864–876. [Google Scholar] [CrossRef]
- Prunotto, M.; Bosco, M.; Daniele, L.; Macri, L.; Bonello, L.; Schirosi, L.; Rossi, G.; Filosso, P.; Mussa, B.; Sapino, A. Imatinib inhibits in vitro proliferation of cells derived from a pleural solitary fibrous tumor expressing platelet-derived growth factor receptor-beta. Lung Cancer 2009, 64, 244–246. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Negri, T.; Palassini, E.; Conca, E.; Gronchi, A.; Morosi, C.; Messina, A.; Pastorino, U.; Pierotti, M.A.; Casali, P.G.; et al. Sunitinib malate and figitumumab in solitary fibrous tumor: Patterns and molecular bases of tumor response. Mol. Cancer Ther. 2010, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Simeone, N.; Lo Vullo, S.; Morosi, C.; Greco, F.G.; Gronchi, A.; Barisella, M.; Collini, P.; Zaffaroni, N.; Dagrada, G.P.; et al. Activity of axitinib in progressive advanced solitary fibrous tumour: Results from an exploratory, investigator-driven phase 2 clinical study. Eur. J. Cancer 2019, 106, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Gronchi, A.; Strauss, D.; Bonvalot, S.; Jeys, L.; Stacchiotti, S.; Hayes, A.; Honore, C.; Collini, P.; Renne, S.L.; et al. Resectable extra-pleural and extra-meningeal solitary fibrous tumours: A multi-centre prognostic study. Eur. J. Surg. Oncol. 2016, 42, 1064–1070. [Google Scholar] [CrossRef]
- Reisenauer, J.S.; Mneimneh, W.; Jenkins, S.; Mansfield, A.S.; Aubry, M.C.; Fritchie, K.J.; Allen, M.S.; Blackmon, S.H.; Cassivi, S.D.; Nichols, F.C.; et al. Comparison of Risk Stratification Models to Predict Recurrence and Survival in Pleuropulmonary Solitary Fibrous Tumor. J. Thorac. Oncol. 2018, 13, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Lamghari, M.; Azzaoui, I.; Sabur, M.; Aboulfeth, E.; Najih, M. Solitary Fibrous Tumor of the Peritoneum: An Unusual Location of A Rare Tumor. Austin J. Surg. 2022, 9, 1293. [Google Scholar]
- Demicco, E.G.; Griffin, A.M.; Gladdy, R.A.; Dickson, B.C.; Ferguson, P.C.; Swallow, C.J.; Wunder, J.S.; Wang, W.L. Comparison of published risk models for prediction of outcome in patients with extrameningeal solitary fibrous tumour. Histopathology 2019, 75, 723–737. [Google Scholar] [CrossRef]
- Tapias, L.F.; Mercier, O.; Ghigna, M.R.; Lahon, B.; Lee, H.; Mathisen, D.J.; Dartevelle, P.; Lanuti, M. Validation of a scoring system to predict recurrence of resected solitary fibrous tumors of the pleura. Chest 2015, 147, 216–223. [Google Scholar] [CrossRef]
- Yamada, Y.; Kohashi, K.; Kinoshita, I.; Yamamoto, H.; Iwasaki, T.; Yoshimoto, M.; Ishihara, S.; Toda, Y.; Itou, Y.; Koga, Y.; et al. Clinicopathological review of solitary fibrous tumors: Dedifferentiation is a major cause of patient death. Virchows Arch. 2019, 475, 467–477. [Google Scholar] [CrossRef]
- Hassani, M.; Jung, S.; Ghodsi, E.; Seddigh, L.; Kooner, P.; Aoude, A.; Turcotte, R. Value of Cellular Components and Focal Dedifferentiation to Predict the Risk of Metastasis in a Benign-Appearing Extra-Meningeal Solitary Fibrous Tumor: An Original Series from a Tertiary Sarcoma Center. Cancers 2023, 15, 1441. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Sambri, A.; Pedrini, E.; Pazzaglia, L.; Sangiorgi, L.; Ruengwanichayakun, P.; Donati, D.; Benassi, M.S.; Righi, A. Histological and molecular features of solitary fibrous tumor of the extremities: Clinical correlation. Virchows Arch. 2020, 476, 445–454. [Google Scholar] [CrossRef] [PubMed]
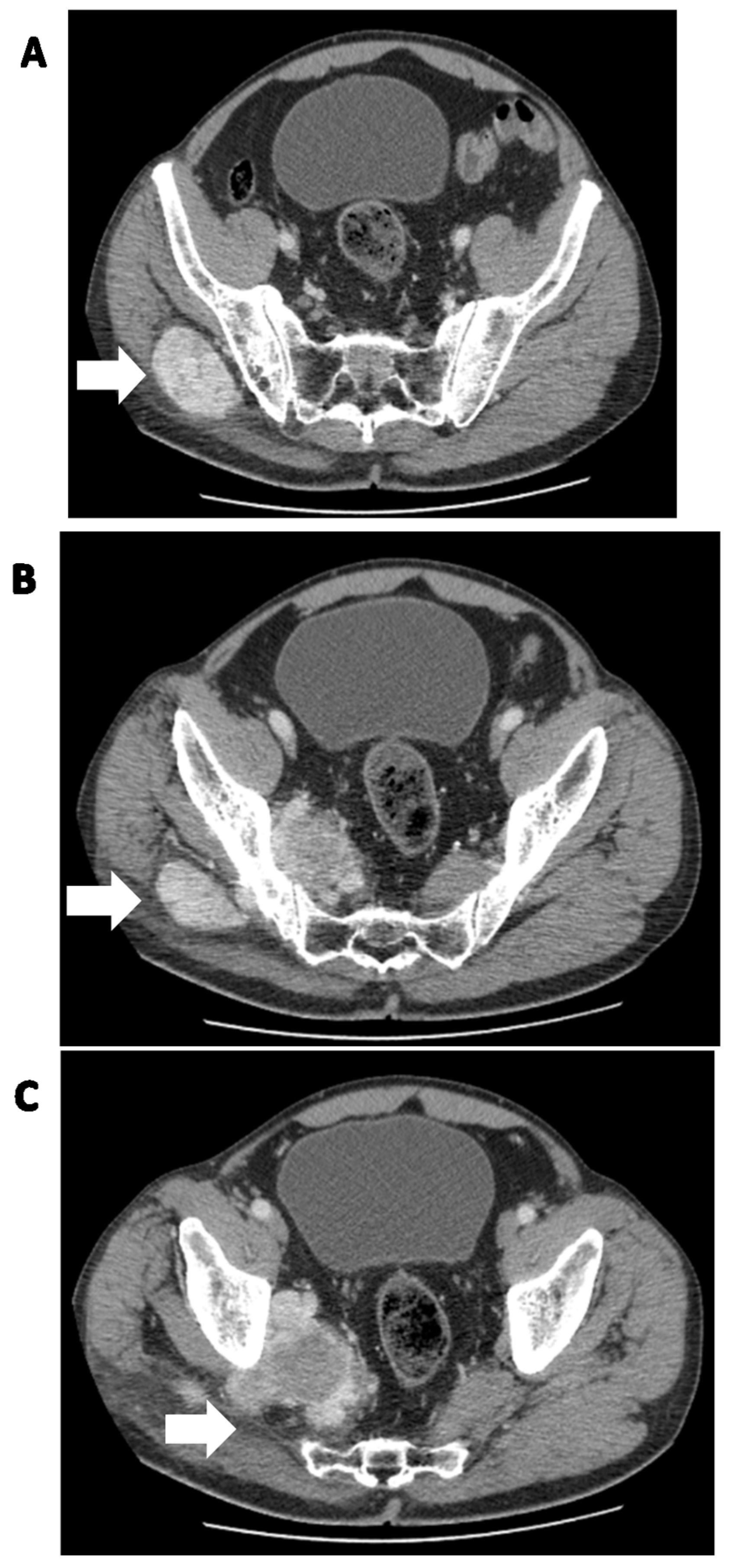



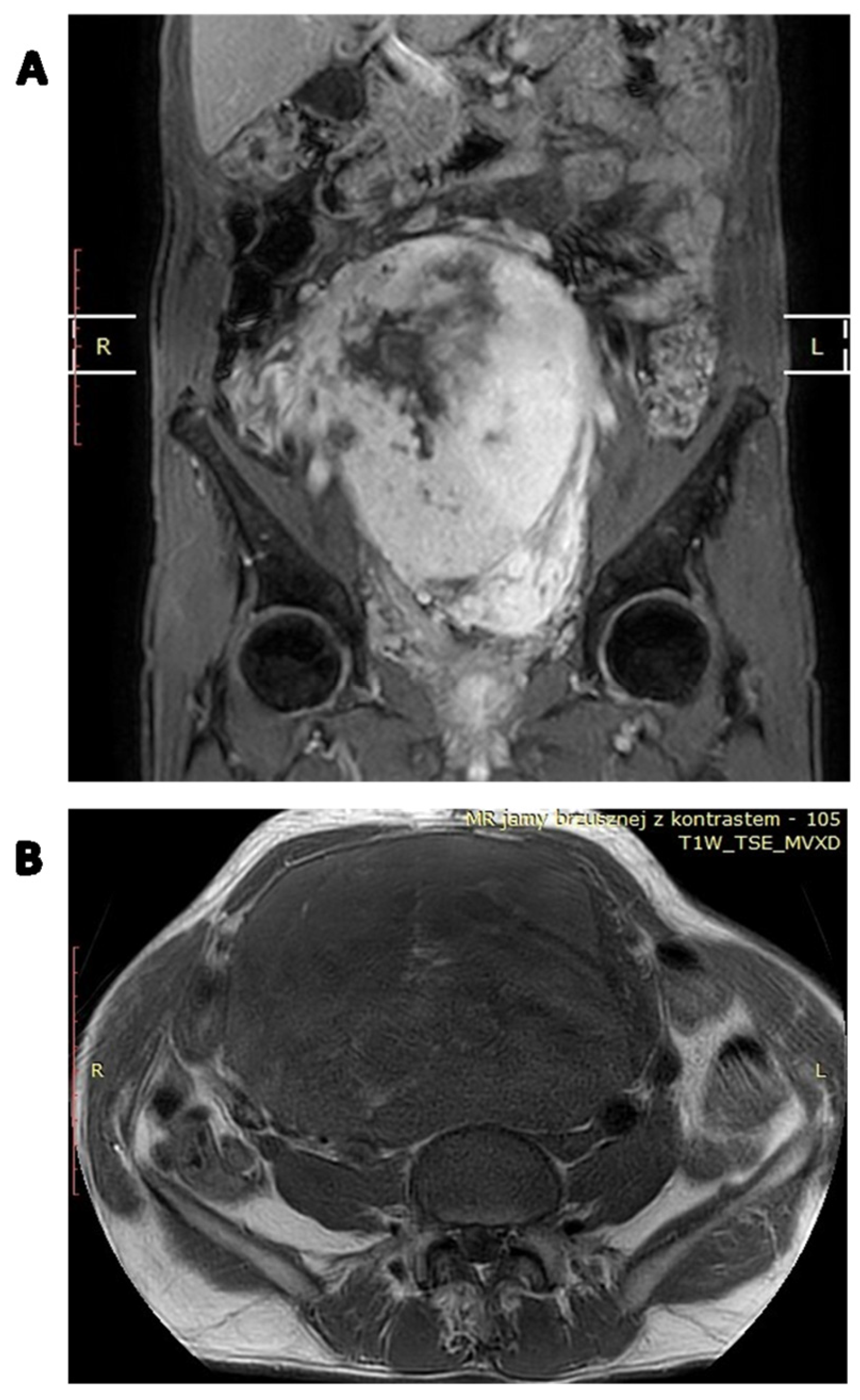
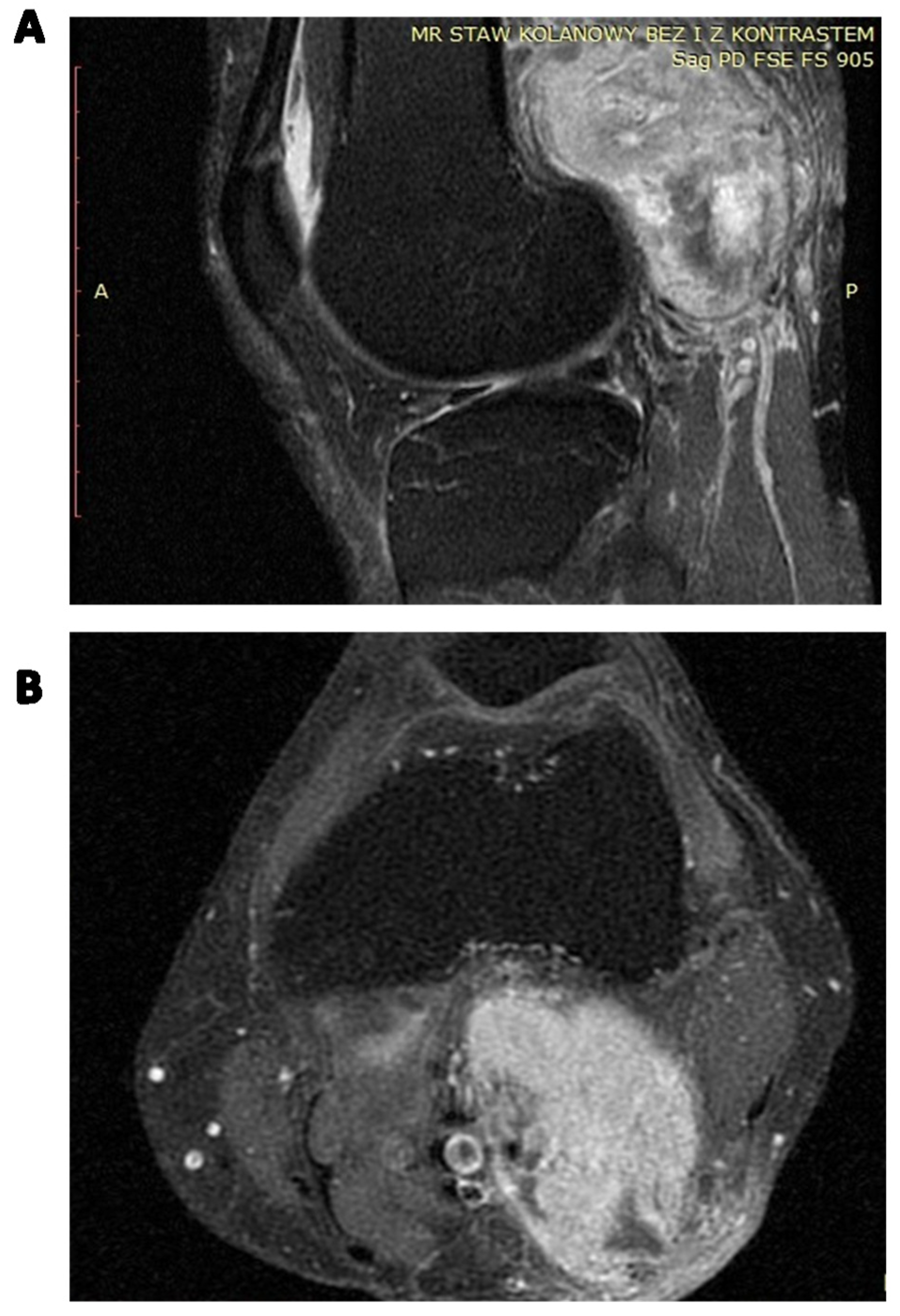



| RISK FACTOR | SCALE | MODEL OF METASTASIS PREDICTION | |
|---|---|---|---|
| Demicco EG 2012 | Demicco EG 2017 | ||
| Age (years) | <55 ≥55 | 0 1 | 0 1 |
| Mitotic count (mitoses/mm2 or mitoses/10 HPFs) | 0 (0) 0.5–1.5 (1–3) ≥2 (≥4) | 0 1 2 | 0 1 2 |
| Tumor size (cm) | 0–4.9 5–9.9 10–14.9 ≥15 | 0 1 2 3 | 0 1 2 3 |
| Necrosis (percentage) | <10% ≥10% | - - | 0 1 |
| POINTS | |||
| RISK | LOW INTERMEDIATE HIGH | 0–2 3–4 5–6 | 0–3 4–5 6–7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik, A.M.; Terlecka, A.; Spałek, M.J.; Boye, K.; Szostakowski, B.; Chmiel, P.; Szumera-Ciećkiewicz, A.; Bobak, K.; Świtaj, T.; Rutkowski, P.; et al. Diagnostics and Treatment of Extrameningeal Solitary Fibrous Tumors. Cancers 2023, 15, 5854. https://doi.org/10.3390/cancers15245854
Janik AM, Terlecka A, Spałek MJ, Boye K, Szostakowski B, Chmiel P, Szumera-Ciećkiewicz A, Bobak K, Świtaj T, Rutkowski P, et al. Diagnostics and Treatment of Extrameningeal Solitary Fibrous Tumors. Cancers. 2023; 15(24):5854. https://doi.org/10.3390/cancers15245854
Chicago/Turabian StyleJanik, Anna Maria, Anna Terlecka, Mateusz J. Spałek, Kjetil Boye, Bartłomiej Szostakowski, Paulina Chmiel, Anna Szumera-Ciećkiewicz, Klaudia Bobak, Tomasz Świtaj, Piotr Rutkowski, and et al. 2023. "Diagnostics and Treatment of Extrameningeal Solitary Fibrous Tumors" Cancers 15, no. 24: 5854. https://doi.org/10.3390/cancers15245854
APA StyleJanik, A. M., Terlecka, A., Spałek, M. J., Boye, K., Szostakowski, B., Chmiel, P., Szumera-Ciećkiewicz, A., Bobak, K., Świtaj, T., Rutkowski, P., & Czarnecka, A. M. (2023). Diagnostics and Treatment of Extrameningeal Solitary Fibrous Tumors. Cancers, 15(24), 5854. https://doi.org/10.3390/cancers15245854







