The Diagnosis and Treatment Approach for Oligo-Recurrent and Oligo-Progressive Renal Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Metastatic Patterns in RCC
3. Classifications and Definition of Oligo-Metastatic Diseases
4. Novel Imaging to Detect Oligo-Recurrence
5. Current Options in Treating Oligo-Metastatic RCC
5.1. Active Surveillance
5.2. Surgery
5.3. Ablation Therapy
5.4. Radiation Therapy
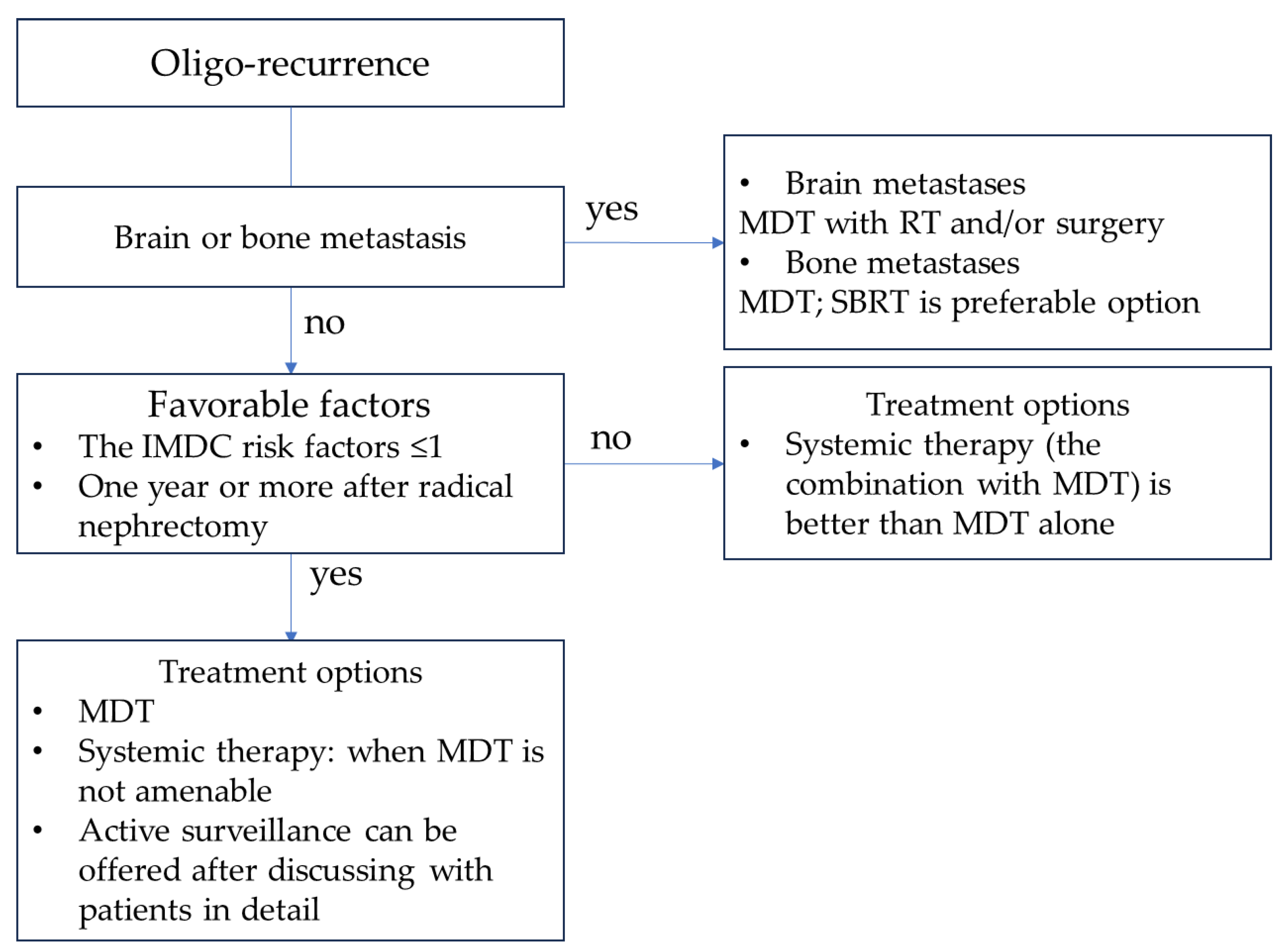
6. Treatment Strategy for Metachronous Oligo-Recurrent RCC
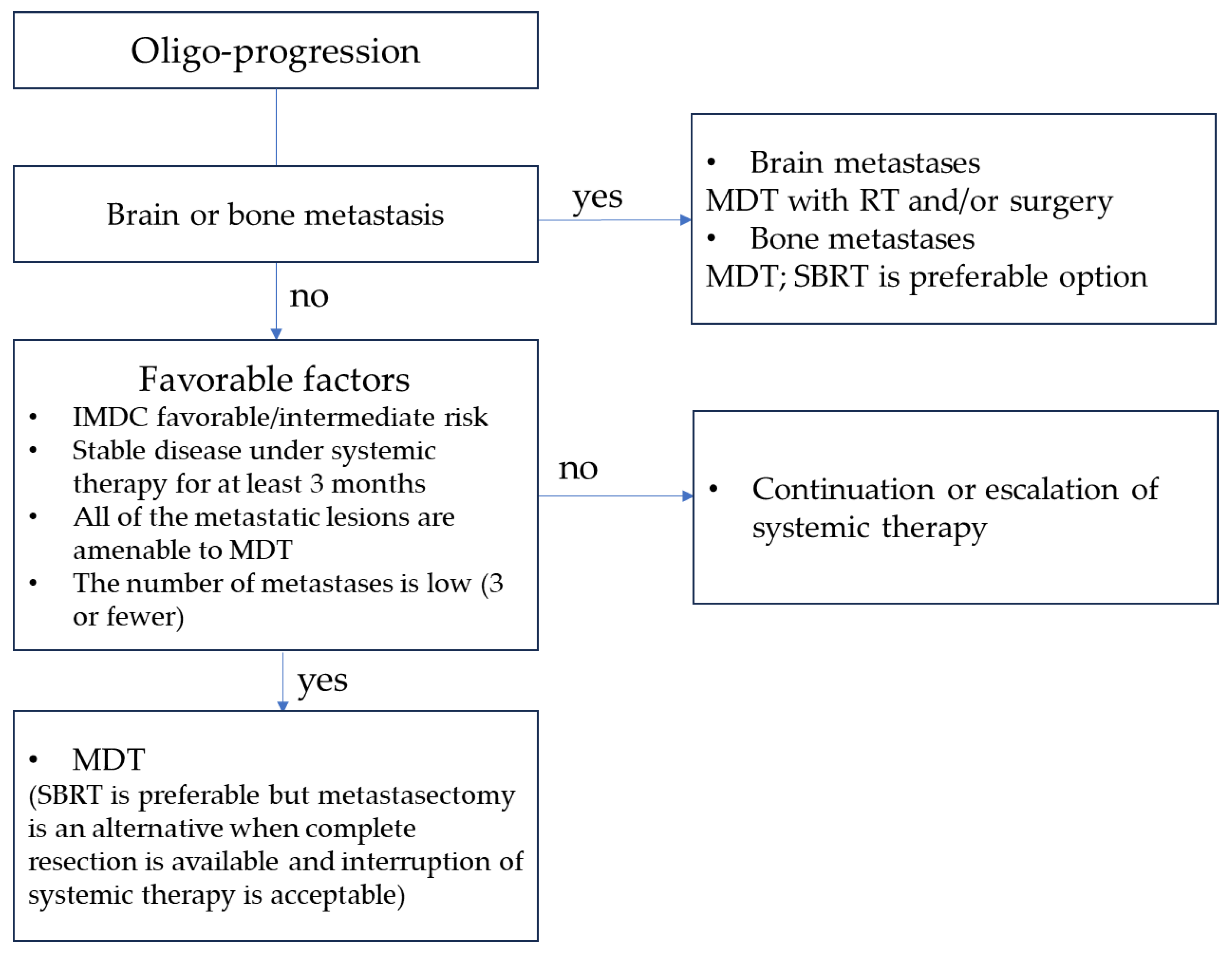
7. Treatment Strategy for Metachronous Oligo-Progressive RCC
8. MDT with Adjuvant Systemic Therapies for Treatment-Naïve Oligo-Metastatic RCC
8.1. SBRT with Adjuvant Systemic Therapies
8.2. Metastasectomy with Adjuvant Systemic Therapies
9. Brain and Bone Metastases
10. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Christensen, M.; Hannan, R. The Emerging Role of Radiation Therapy in Renal Cell Carcinoma. Cancers 2022, 14, 4693. [Google Scholar] [CrossRef]
- All, S.; Garant, A.; Hannan, R. Stereotactic Ablative Radiation (SAbR) for Oligometastatic RCC. Semin. Radiat. Oncol. 2021, 31, 227–234. [Google Scholar] [CrossRef]
- Gupta, K.; Miller, J.D.; Li, J.Z.; Russell, M.W.; Charbonneau, C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): A literature review. Cancer Treat. Rev. 2008, 34, 193–205. [Google Scholar] [CrossRef]
- Kim, S.P.; Weight, C.J.; Leibovich, B.C.; Thompson, R.H.; Costello, B.A.; Cheville, J.C.; Lohse, C.M.; Boorjian, S.A. Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Urology 2011, 78, 1101–1106. [Google Scholar] [CrossRef]
- Alt, A.L.; Boorjian, S.A.; Lohse, C.M.; Costello, B.A.; Leibovich, B.C.; Blute, M.L. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 2011, 117, 2873–2882. [Google Scholar] [CrossRef]
- Hao, C.; Liu, J.; Ladbury, C.; Dorff, T.; Sampath, S.; Pal, S.; Dandapani, S. Stereotactic body radiation therapy to the kidney for metastatic renal cell carcinoma: A narrative review of an emerging concept. Cancer Treat. Res. Commun. 2023, 35, 100692. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Motzer, R.J. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2017, 376, 354–366. [Google Scholar] [CrossRef]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Dingemans, A.C.; Hendriks, L.E.L.; Berghmans, T.; Levy, A.; Hasan, B.; Faivre-Finn, C.; Giaj-Levra, M.; Giaj-Levra, N.; Girard, N.; Greillier, L.; et al. Definition of Synchronous Oligometastatic Non-Small Cell Lung Cancer-A Consensus Report. J. Thorac. Oncol. 2019, 14, 2109–2119. [Google Scholar] [CrossRef]
- Jackson, J.C.; Franco, A.; Wang, D.; Autorino, R.; Vourganti, S. Metastasis-directed treatment in kidney cancer. Curr. Opin. Urol. 2023, 33, 375–382. [Google Scholar] [CrossRef]
- Chiou, J.K.; Chang, L.W.; Li, J.R.; Wang, S.S.; Yang, C.K.; Chen, C.S.; Lu, K.; Cheng, C.L.; Chiu, K.Y.; Hung, S.C. Metastasectomy Improves Overall Survival in Metastatic Renal Cell Carcinoma: A Retrospective Cohort Study. Anticancer Res. 2023, 43, 3193–3201. [Google Scholar] [CrossRef]
- Meagher, M.F.; Mir, M.C.; Autorino, R.; Minervini, A.; Kriegmair, M.; Maurer, T.; Porpiglia, F.; Van Bruwaene, S.; Linares, E.; Hevia, V.; et al. Impact of Metastasectomy on Cancer Specific and Overall Survival in Metastatic Renal Cell Carcinoma: Analysis of the REMARCC Registry. Clin. Genitourin. Cancer 2022, 20, 326–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Schoenhals, J.; Christie, A.; Mohamad, O.; Wang, C.; Bowman, I.; Singla, N.; Hammers, H.; Courtney, K.; Bagrodia, A.; et al. Stereotactic Ablative Radiation Therapy (SAbR) Used to Defer Systemic Therapy in Oligometastatic Renal Cell Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 367–375. [Google Scholar] [CrossRef]
- Bianchi, M.; Sun, M.; Jeldres, C.; Shariat, S.F.; Trinh, Q.D.; Briganti, A.; Tian, Z.; Schmitges, J.; Graefen, M.; Perrotte, P.; et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann. Oncol. 2012, 23, 973–980. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; de Souza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Ishihara, H.; Takagi, T.; Kondo, T.; Fukuda, H.; Tachibana, H.; Yoshida, K.; Iizuka, J.; Kobayashi, H.; Ishida, H.; Tanabe, K. Prognostic impact of metastasectomy in renal cell carcinoma in the postcytokine therapy era. Urol. Oncol. 2021, 39, 77.e17–77.e25. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Dabestani, S.; Thorstenson, A.; Lindblad, P.; Harmenberg, U.; Ljungberg, B.; Lundstam, S. Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: A population-based study. World J. Urol. 2016, 34, 1081–1086. [Google Scholar] [CrossRef]
- Chandrasekar, T.; Klaassen, Z.; Goldberg, H.; Kulkarni, G.S.; Hamilton, R.J.; Fleshner, N.E. Metastatic renal cell carcinoma: Patterns and predictors of metastases-A contemporary population-based series. Urol. Oncol. 2017, 35, 661.e7–661.e14. [Google Scholar] [CrossRef]
- Wei, H.; Miao, J.; Cui, J.; Zheng, W.; Chen, X.; Zhang, Q.; Liu, F.; Mao, Z.; Qiu, S.; Zhang, D. The prognosis and clinicopathological features of different distant metastases patterns in renal cell carcinoma: Analysis based on the SEER database. Sci. Rep. 2021, 11, 17822. [Google Scholar] [CrossRef]
- Fallara, G.; Larcher, A.; Dabestani, S.; Fossati, N.; Järvinen, P.; Nisen, H.; Gudmundsson, E.; Lam, T.B.; Marconi, L.; Fernandéz-Pello, S.; et al. Recurrence pattern in localized RCC: Results from a European multicenter database (RECUR). Urol. Oncol. 2022, 40, 494.e11–494.e17. [Google Scholar] [CrossRef]
- Niibe, Y.; Hayakawa, K. Oligometastases and oligo-recurrence: The new era of cancer therapy. Jpn. J. Clin. Oncol. 2010, 40, 107–111. [Google Scholar] [CrossRef]
- Niibe, Y.; Chang, J.Y. Novel insights of oligometastases and oligo-recurrence and review of the literature. Pulm. Med. 2012, 2012, 261096. [Google Scholar] [CrossRef]
- Gebbia, V.; Girlando, A.D.I.; Girlando, A.; Fazio, I.; Borsellino, N.; Piazza, D.; Serretta, V.; Pergolizzi, S.; Pontoriero, A.; Firenze, A.; et al. Stereotactic Radiotherapy for the Treatment of Patients With Oligo-progressive Metastatic Renal Cell Carcinoma Receiving Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitor: Data From the Real World. Anticancer Res. 2020, 40, 7037–7043. [Google Scholar] [CrossRef]
- Foster, C.C.; Pitroda, S.P.; Weichselbaum, R.R. Definition, Biology, and History of Oligometastatic and Oligoprogressive Disease. Cancer J. 2020, 26, 96–99. [Google Scholar] [CrossRef]
- Rizzo, A.; Racca, M.; Dall’Armellina, S.; Rescigno, P.; Banna, G.L.; Albano, D.; Dondi, F.; Bertagna, F.; Annunziata, S.; Treglia, G. The Emerging Role of PET/CT with PSMA-Targeting Radiopharmaceuticals in Clear Cell Renal Cancer: An Updated Systematic Review. Cancers 2023, 15, 355. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.Y.; Lee, S. Role of F-18 FDG PET/CT in the follow-up of asymptomatic renal cell carcinoma patients for postoperative surveillance: Based on conditional survival analysis. J. Cancer Res. Clin. Oncol. 2022, 148, 215–224. [Google Scholar] [CrossRef]
- Muselaers, S.; Erdem, S.; Bertolo, R.; Ingels, A.; Kara, Ö.; Pavan, N.; Roussel, E.; Pecoraro, A.; Marchioni, M.; Carbonara, U.; et al. PSMA PET/CT in Renal Cell Carcinoma: An Overview of Current Literature. J. Clin. Med. 2022, 11, 1829. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Rathmell, W.K.; Rumble, R.B.; Van Veldhuizen, P.J.; Al-Ahmadie, H.; Emamekhoo, H.; Hauke, R.J.; Louie, A.V.; Milowsky, M.I.; Molina, A.M.; Rose, T.L.; et al. Management of Metastatic Clear Cell Renal Cell Carcinoma: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2957–2995. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Dorff, T.B.; Elson, P.; Rodriguez, C.S.; Shepard, D.; Wood, L.; Humbert, J.; Pyle, L.; Wong, Y.N.; Finke, J.H.; et al. Active surveillance in metastatic renal-cell carcinoma: A prospective, phase 2 trial. Lancet Oncol. 2016, 17, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.R.; Costello, B.A.; Bhavsar, N.A.; Vaishampayan, U.; Pal, S.K.; Zakharia, Y.; Jim, H.S.L.; Fishman, M.N.; Molina, A.M.; Kyriakopoulos, C.E.; et al. Active surveillance of metastatic renal cell carcinoma: Results from a prospective observational study (MaRCC). Cancer 2021, 127, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Colas, S.; Beauval, J.B.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Schmidinger, M.; Kawada, T.; Bekku, K.; Kimura, T.; Shariat, S.F. Radical Nephrectomy After Immune Checkpoint Inhibitors for Metastatic Renal Cell Carcinoma. Eur. Urol. Focus 2023, 9, 275–277. [Google Scholar] [CrossRef]
- Hsieh, P.Y.; Hung, S.C.; Li, J.R.; Wang, S.S.; Yang, C.K.; Chen, C.S.; Lu, K.; Cheng, C.L.; Chiu, K.Y. The effect of metastasectomy on overall survival in metastatic renal cell carcinoma: A systematic review and meta-analysis. Urol. Oncol. 2021, 39, 422–430. [Google Scholar] [CrossRef]
- Chanez, B.; Caillol, F.; Ratone, J.P.; Pesenti, C.; Rochigneux, P.; Pignot, G.; Thomassin, J.; Brunelle, S.; Walz, J.; Salem, N.; et al. Endoscopic Ultrasound-Guided Radiofrequency Ablation as an Future Alternative to Pancreatectomy for Pancreatic Metastases from Renal Cell Carcinoma: A Prospective Study. Cancers 2021, 13, 5267. [Google Scholar] [CrossRef]
- Ali, M.; Mooi, J.; Lawrentschuk, N.; McKay, R.R.; Hannan, R.; Lo, S.S.; Hall, W.A.; Siva, S. The Role of Stereotactic Ablative Body Radiotherapy in Renal Cell Carcinoma. Eur. Urol. 2022, 82, 613–622. [Google Scholar] [CrossRef]
- Hannan, R.; Christensen, M.; Christie, A.; Garant, A.; Pedrosa, I.; Robles, L.; Mannala, S.; Wang, C.; Hammers, H.; Arafat, W.; et al. Stereotactic Ablative Radiation for Systemic Therapy-naïve Oligometastatic Kidney Cancer. Eur. Urol. Oncol. 2022, 5, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Msaouel, P.; Hara, K.; Choi, H.; Le, V.; Shah, A.Y.; Wang, J.; Jonasch, E.; Choi, S.; Nguyen, Q.N.; et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: A single-arm, single-centre, feasibility, phase 2 trial. Lancet Oncol. 2021, 22, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.W.; Li, H.Z.; Gao, X.S.; Liu, M.Z.; Yin, H.; Yang, K.W.; Chen, J.Y.; Ren, X.Y.; Wang, D. Outcomes of High-Dose Stereotactic Ablative Radiotherapy to All/Multiple Sites for Oligometastatic Renal Cell Cancer Patients. Curr. Oncol. 2022, 29, 7832–7841. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.; Patel, S.; North, S.A.; Sahgal, A.; Chu, W.; Soliman, H.; Ahmad, B.; Winquist, E.; Niazi, T.; Patenaude, F.; et al. Stereotactic Radiotherapy for Oligoprogression in Metastatic Renal Cell Cancer Patients Receiving Tyrosine Kinase Inhibitor Therapy: A Phase 2 Prospective Multicenter Study. Eur. Urol. 2021, 80, 693–700. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Venkatesan, A.M.; Msaouel, P.; Ghia, A.J.; Li, J.; Yeboa, D.N.; Nguyen, Q.N.; Bishop, A.J.; Jonasch, E.; Shah, A.Y.; et al. Definitive radiotherapy for extracranial oligoprogressive metastatic renal cell carcinoma as a strategy to defer systemic therapy escalation. BJU Int. 2022, 129, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Pasquier, D.; Bernadou, G.; Calais, G.; Maroun, P.; Bossi, A.; Theodore, C.; Albiges, L.; Stefan, D.; de Crevoisier, R.; et al. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: A study of the Getug group. Eur. J. Cancer 2018, 98, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Masini, C.; Iotti, C.; De Giorgi, U.; Bellia, R.S.; Buti, S.; Salaroli, F.; Zampiva, I.; Mazzarotto, R.; Mucciarini, C.; Vitale, M.G.; et al. Nivolumab in Combination with Stereotactic Body Radiotherapy in Pretreated Patients with Metastatic Renal Cell Carcinoma. Results of the Phase II NIVES Study. Eur. Urol. 2022, 81, 274–282. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Plimack, E.R.; Procopio, G.; McDermott, D.F.; et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020, 126, 4156–4167. [Google Scholar] [CrossRef]
- Marconi, R.; Strolin, S.; Bossi, G.; Strigari, L. A meta-analysis of the abscopal effect in preclinical models: Is the biologically effective dose a relevant physical trigger? PLoS ONE 2017, 12, e0171559. [Google Scholar] [CrossRef]
- Hannan, R.; Christensen, M.; Hammers, H.; Christie, A.; Paulman, B.; Lin, D.; Garant, A.; Arafat, W.; Courtney, K.; Bowman, I.; et al. Phase II Trial of Stereotactic Ablative Radiation for Oligoprogressive Metastatic Kidney Cancer. Eur. Urol. Oncol. 2022, 5, 216–224. [Google Scholar] [CrossRef]
- Ingrosso, G.; Becherini, C.; Francolini, G.; Lancia, A.; Alì, E.; Caini, S.; Teriaca, M.A.; Marchionni, A.; Filippi, A.R.; Livi, L.; et al. Stereotactic body radiotherapy (SBRT) in combination with drugs in metastatic kidney cancer: A systematic review. Crit. Rev. Oncol. Hematol. 2021, 159, 103242. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, S.G.C.; Pavic, M.; Stellamans, K.; Lievens, Y.; Becherini, C.; Scorsetti, M.; Alongi, F.; Ricardi, U.; Jereczek-Fossa, B.A.; Westhoff, P.; et al. Metastases-directed stereotactic body radiotherapy in combination with targeted therapy or immunotherapy: Systematic review and consensus recommendations by the EORTC-ESTRO OligoCare consortium. Lancet Oncol. 2023, 24, e121–e132. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Schoenhals, J.E.; Mohamad, O.; Christie, A.; Zhang, Y.; Li, D.; Singla, N.; Bowman, I.; Arafat, W.; Hammers, H.; Courtney, K.; et al. Stereotactic Ablative Radiation Therapy for Oligoprogressive Renal Cell Carcinoma. Adv. Radiat. Oncol. 2021, 6, 100692. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Lalani, A.K.; Swaminath, A. Cytoreductive stereotactic body radiotherapy (SBRT) and combination SBRT with immune checkpoint inhibitors in metastatic renal cell carcinoma. Can. Urol. Assoc. J. 2021, 15, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.D.; Chang, J.Y. Time to abandon single-site irradiation for inducing abscopal effects. Nat. Rev. Clin. Oncol. 2019, 16, 123–135. [Google Scholar] [CrossRef]
- Siva, S.; Bressel, M.; Wood, S.T.; Shaw, M.G.; Loi, S.; Sandhu, S.K.; Tran, B.A.; Azad, A.; Lewin, J.H.; Cuff, K.E.; et al. Stereotactic Radiotherapy and Short-course Pembrolizumab for Oligometastatic Renal Cell Carcinoma-The RAPPORT Trial. Eur. Urol. 2022, 81, 364–372. [Google Scholar] [CrossRef]
- Harshman, L.C.; Drake, C.G.; Haas, N.B.; Manola, J.; Puligandla, M.; Signoretti, S.; Cella, D.; Gupta, R.T.; Bhatt, R.; Van Allen, E.; et al. Transforming the Perioperative Treatment Paradigm in Non-Metastatic RCC-A Possible Path Forward. Kidney Cancer 2017, 1, 31–40. [Google Scholar] [CrossRef]
- Pal, S.K.; Uzzo, R.; Karam, J.A.; Master, V.A.; Donskov, F.; Suarez, C.; Albiges, L.; Rini, B.; Tomita, Y.; Kann, A.G.; et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2022, 400, 1103–1116. [Google Scholar] [CrossRef]
- Powles, T.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Gurney, H.; Chang, Y.H.; Lee, J.L.; et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1133–1144. [Google Scholar] [CrossRef]
- Kotecha, R.R.; Flippot, R.; Nortman, T.; Guida, A.; Patil, S.; Escudier, B.; Motzer, R.J.; Albiges, L.; Voss, M.H. Prognosis of Incidental Brain Metastases in Patients With Advanced Renal Cell Carcinoma. J. Natl. Compr. Cancer Netw. 2021, 19, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Huang, Z.; Huang, X. Bone Metastasis in Renal Cell Carcinoma Patients: Risk and Prognostic Factors and Nomograms. J. Oncol. 2021, 2021, 5575295. [Google Scholar] [CrossRef] [PubMed]
- Franzese, C.; Navarria, P.; Bellu, L.; Marzo, M.A.; Clerici, E.; Badalamenti, M.; Baldaccini, D.; Franceschini, D.; Comito, T.; Teriaca, A.; et al. Risk-group Classification by Recursive Partitioning Analysis of Patients Affected by Oligometastatic Renal Cancer Treated with Stereotactic Radiotherapy. Clin. Oncol. R. Coll. Radiol. 2022, 34, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Huntoon, K.; Damante, M.; Wang, J.; Olencki, T.; Elder, J.B. Survival benefit with resection of brain metastases from renal cell carcinoma in the setting of molecular targeted therapy and/or immune therapy. Curr. Probl. Cancer 2022, 46, 100805. [Google Scholar] [CrossRef]
- Navarria, P.; Pessina, F.; Minniti, G.; Franzese, C.; Marini, B.; D’Agostino, G.; Badalamenti, M.; Raspagliesi, L.; Reggiori, G.; Lobefalo, F.; et al. Multimodal Treatments for Brain Metastases from Renal Cell Carcinoma: Results of a Multicentric Retrospective Study. Cancers 2023, 15, 1393. [Google Scholar] [CrossRef]
- Liu, Y.; Long, W.; Zhang, Z.; Zhang, Z.; Mai, L.; Huang, S.; Han, H.; Zhou, F.; Dong, P.; He, L. Metastasis-directed stereotactic body radiotherapy for oligometastatic renal cell carcinoma: Extent of tumor burden eradicated by radiotherapy. World J. Urol. 2021, 39, 4183–4190. [Google Scholar] [CrossRef]
- Onal, C.; Guler, O.C.; Hurmuz, P.; Yavas, G.; Tilki, B.; Oymak, E.; Yavas, C.; Ozyigit, G. Bone-only oligometastatic renal cell carcinoma patients treated with stereotactic body radiotherapy: A multi-institutional study. Strahlenther. Onkol. 2022, 198, 940–948. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekku, K.; Kawada, T.; Sekito, T.; Yoshinaga, K.; Maruyama, Y.; Yamanoi, T.; Tominaga, Y.; Sadahira, T.; Katayama, S.; Iwata, T.; et al. The Diagnosis and Treatment Approach for Oligo-Recurrent and Oligo-Progressive Renal Cell Carcinoma. Cancers 2023, 15, 5873. https://doi.org/10.3390/cancers15245873
Bekku K, Kawada T, Sekito T, Yoshinaga K, Maruyama Y, Yamanoi T, Tominaga Y, Sadahira T, Katayama S, Iwata T, et al. The Diagnosis and Treatment Approach for Oligo-Recurrent and Oligo-Progressive Renal Cell Carcinoma. Cancers. 2023; 15(24):5873. https://doi.org/10.3390/cancers15245873
Chicago/Turabian StyleBekku, Kensuke, Tatsushi Kawada, Takanori Sekito, Kasumi Yoshinaga, Yuki Maruyama, Tomoaki Yamanoi, Yusuke Tominaga, Takuya Sadahira, Satoshi Katayama, Takehiro Iwata, and et al. 2023. "The Diagnosis and Treatment Approach for Oligo-Recurrent and Oligo-Progressive Renal Cell Carcinoma" Cancers 15, no. 24: 5873. https://doi.org/10.3390/cancers15245873
APA StyleBekku, K., Kawada, T., Sekito, T., Yoshinaga, K., Maruyama, Y., Yamanoi, T., Tominaga, Y., Sadahira, T., Katayama, S., Iwata, T., Nishimura, S., Edamura, K., Kobayashi, T., Kobayashi, Y., Araki, M., & Niibe, Y. (2023). The Diagnosis and Treatment Approach for Oligo-Recurrent and Oligo-Progressive Renal Cell Carcinoma. Cancers, 15(24), 5873. https://doi.org/10.3390/cancers15245873






