Multidimensional Predictors of Cancer-Related Fatigue Based on the Predisposing, Precipitating, and Perpetuating (3P) Model: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
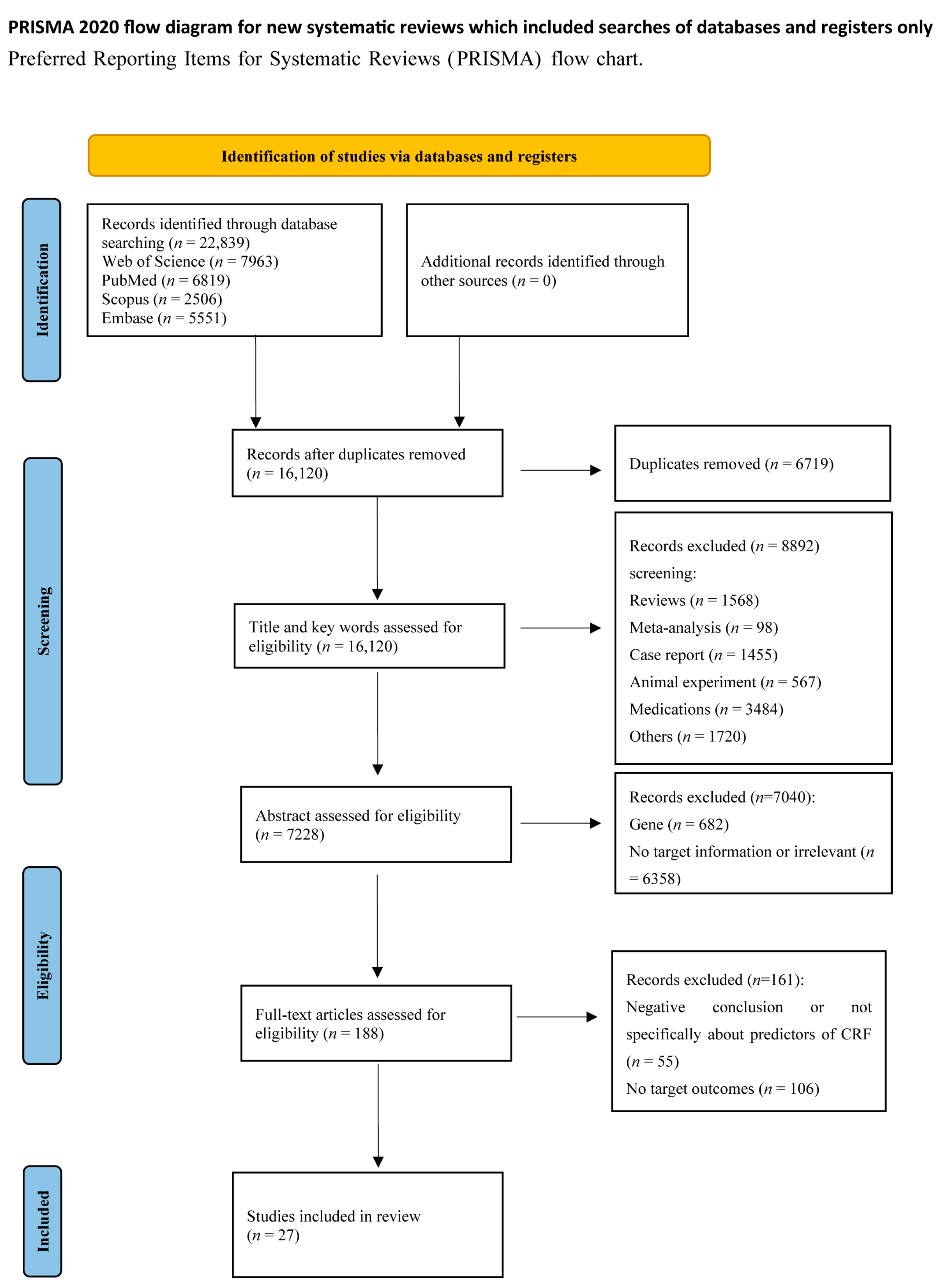
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- (1)
- Study participants were cancer patients with fatigue due to various cancer or cancer treatment (including solid and liquid tumors).
- (2)
- Studies which focused on the association of biomarker or risk factor with cancer-related fatigue.
- (3)
- Studies in which the primary outcome or secondary outcome was cancer-related fatigue.
- (1)
- Studies in which the outcome indicator was chronic fatigue syndrome or other disease-related fatigue rather than CRF.
- (2)
- Studies which did not report any correlation between biomarkers or risk factors and CRF.
- (3)
- Biomarker or risk factor of CRF was any gene polymorphism.
- (4)
- Studies that were not published in English or were not available in full text.
- (5)
- Reviews, meta-analyses, protocols, animal experiments, conference reports, medications, case reports, and non-human studies.
- (6)
- Duplication.
2.3. Data Extraction
2.4. Quality Assessment
3. Results
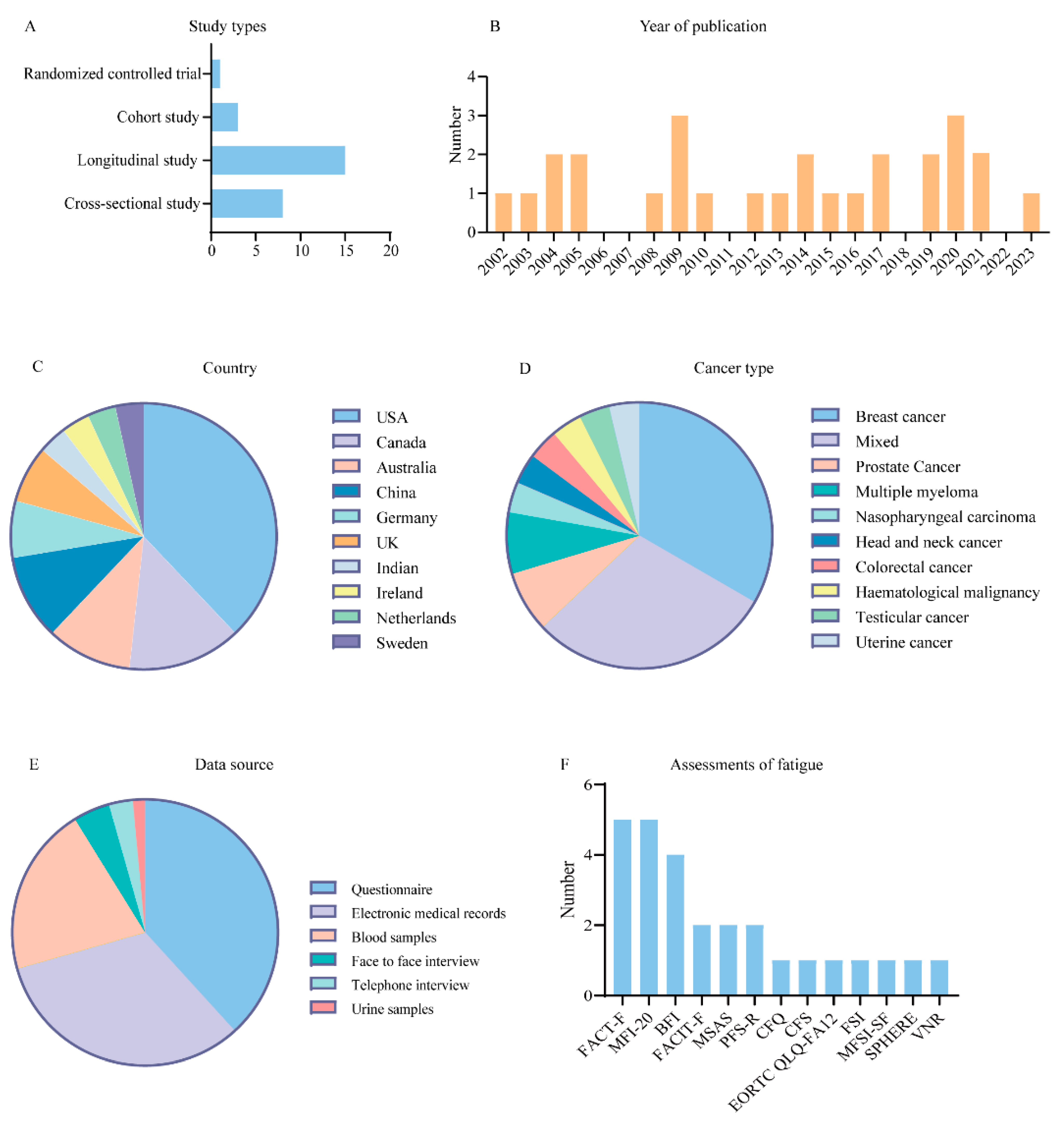
3.1. Overview
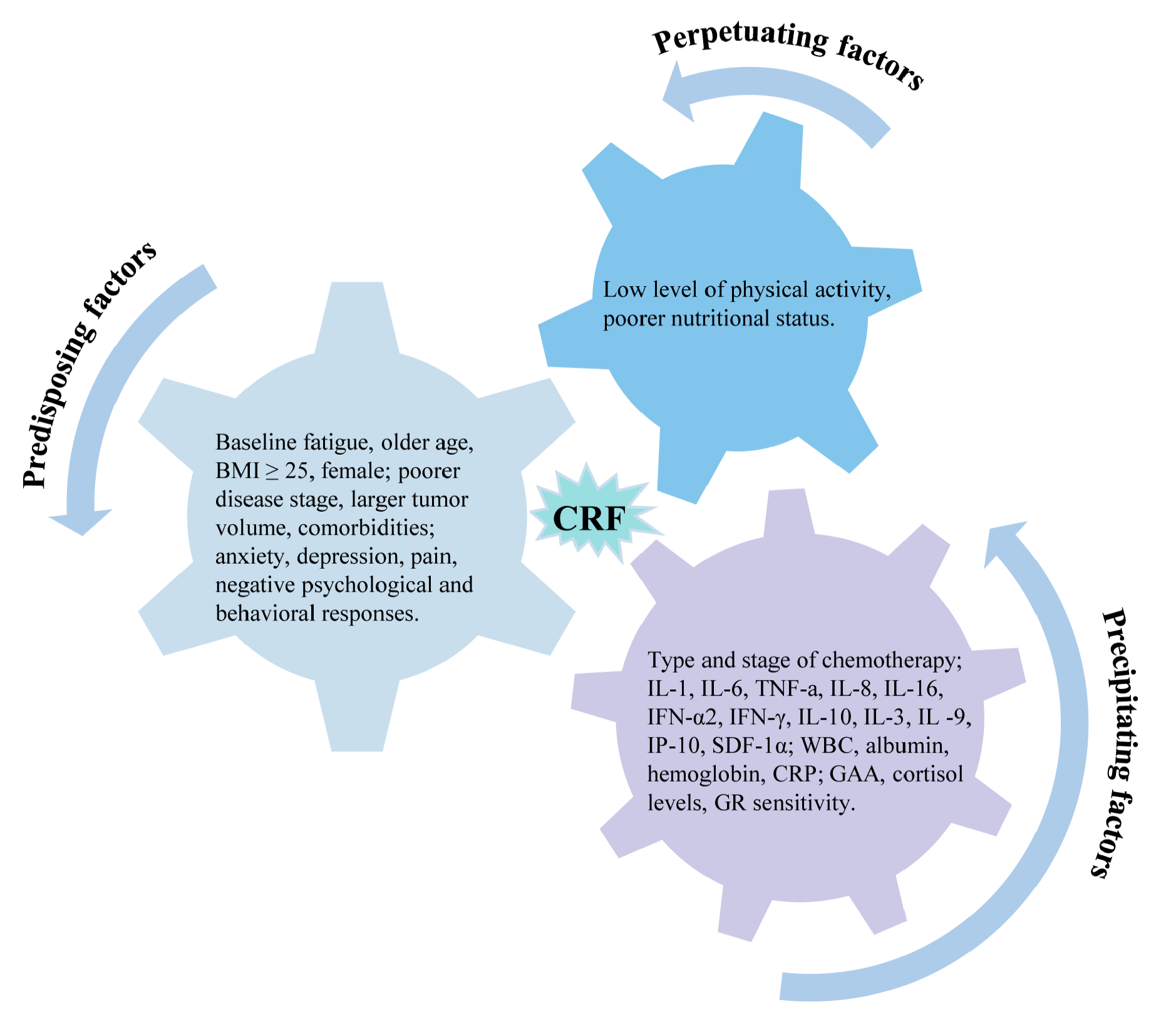
3.2. Predisposing Factors
3.2.1. Baseline Fatigue
3.2.2. Demographic Characteristics
3.2.3. Clinical Characteristics
3.2.4. Psychosocial Traits
3.2.5. Physical Symptoms
3.3. Precipitating Factors
3.3.1. Radiotherapy and Chemotherapy
3.3.2. Inflammatory Factors
3.3.3. Laboratory Indicators and Metabolites
3.4. Perpetuating Factors
4. Discussion
4.1. Predisposing Factors
4.2. Precipitating Factors
4.3. Perpetuating Factors
4.4. Limitations and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cella, D.; Davis, K.; Breitbart, W.; Curt, G. Fatigue Coalition Cancer-Related Fatigue: Prevalence of Proposed Diagnostic Criteria in a United States Sample of Cancer Survivors. J. Clin. Oncol. 2001, 19, 3385–3391. [Google Scholar] [CrossRef]
- Minnella, E.M.; Awasthi, R.; Loiselle, S.-E.; Agnihotram, R.V.; Ferri, L.E.; Carli, F. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg. 2018, 153, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Zhao, F.; Fisch, M.J.; O’Mara, A.M.; Cella, D.; Mendoza, T.R.; Cleeland, C.S. Prevalence and Characteristics of Moderate to Severe Fatigue: A Multicenter Study in Cancer Patients and Survivors. Cancer 2014, 120, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Al Maqbali, M.; Al Sinani, M.; Al Naamani, Z.; Al Badi, K.; Tanash, M.I. Prevalence of Fatigue in Patients with Cancer: A Systematic Review and Meta-Analysis. J. Pain. Symptom Manag. 2021, 61, 167–189.e14. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.; Hong, S.; Natarajan, L.; Mills, P.J.; Dimsdale, J.E. The Association between Fatigue and Inflammatory Marker Levels in Cancer Patients: A Quantitative Review. Brain Behav. Immun. 2007, 21, 413–427. [Google Scholar] [CrossRef]
- Yang, S.; Chu, S.; Gao, Y.; Ai, Q.; Liu, Y.; Li, X.; Chen, N. A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells 2019, 8, 738. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Michaelis, K.A.; Marks, D.L. The Central Role of Hypothalamic Inflammation in the Acute Illness Response and Cachexia. Semin. Cell Dev. Biol. 2016, 54, 42–52. [Google Scholar] [CrossRef]
- Raudonis, B.M.; Kelley, I.H.; Rowe, N.; Ellis, J. A Pilot Study of Proinflammatory Cytokines and Fatigue in Women with Breast Cancer During Chemotherapy. Cancer Nurs. 2017, 40, 323–331. [Google Scholar] [CrossRef]
- Bower, J.E. The Role of Neuro-immune Interactions in Cancer-related Fatigue: Biobehavioral Risk Factors and Mechanisms. Cancer 2019, 125, 353–364. [Google Scholar] [CrossRef]
- Dhruva, A.; Aouizerat, B.E.; Cooper, B.; Paul, S.M.; Dodd, M.; West, C.; Wara, W.; Lee, K.; Dunn, L.B.; Langford, D.J.; et al. Cytokine Gene Associations with Self-Report Ratings of Morning and Evening Fatigue in Oncology Patients and Their Family Caregivers. Biol. Res. Nurs. 2015, 17, 175–184. [Google Scholar] [CrossRef]
- Tian, T.; Qin, W.; Liu, B.; Wang, D.; Wang, J.; Jiang, T.; Yu, C. Catechol-O-Methyltransferase Val158Met Polymorphism Modulates Gray Matter Volume and Functional Connectivity of the Default Mode Network. PLoS ONE 2013, 8, e78697. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.M.; Bruner, D.W.; Mitchell, S.A.; Minasian, L.M.; Basch, E.; Dueck, A.C.; Cella, D.; Reeve, B.B. A Literature Synthesis of Symptom Prevalence and Severity in Persons Receiving Active Cancer Treatment. Support. Care Cancer 2013, 21, 1525–1550. [Google Scholar] [CrossRef]
- Rutherford, C.; Campbell, R.; White, K.; King, M. Patient-Reported Outcomes as Predictors of Survival in Patients with Bowel Cancer: A Systematic Review. Qual. Life Res. 2019, 28, 2871–2887. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Mitchell, S.A.; Jacobsen, P.B.; Pirl, W.F. Screening, Evaluation, and Management of Cancer-Related Fatigue: Ready for Implementation to Practice? CA A Cancer J. Clin. 2015, 65, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef]
- Hilarius, D.L.; Kloeg, P.H.; van der Wall, E.; Komen, M.; Gundy, C.M.; Aaronson, N.K. Cancer-Related Fatigue: Clinical Practice versus Practice Guidelines. Support. Care Cancer 2011, 19, 531–538. [Google Scholar] [CrossRef]
- Tolotti, A.; Bonetti, L.; Pedrazzani, C.; Bianchi, M.; Moser, L.; Pagnucci, N.; Sari, D.; Valcarenghi, D. Nursing Management of Fatigue in Cancer Patients and Suggestions for Clinical Practice: A Mixed Methods Study. BMC Nurs. 2021, 20, 182. [Google Scholar] [CrossRef]
- Piper, B.F.; Borneman, T.; Sun, V.C.-Y.; Koczywas, M.; Uman, G.; Ferrell, B.; James, R.L. Cancer-Related Fatigue: Role of Oncology Nurses in Translating National Comprehensive Cancer Network Assessment Guidelines into Practice. Clin. J. Oncol. Nurs. 2008, 12, 37–47. [Google Scholar] [CrossRef]
- Scott, J.A.; Lasch, K.E.; Barsevick, A.M.; Piault-Louis, E. Patients’ Experiences with Cancer-Related Fatigue: A Review and Synthesis of Qualitative Research. Oncol. Nurs. Forum 2011, 38, E191–E203. [Google Scholar] [CrossRef]
- Greener, J.G.; Kandathil, S.M.; Moffat, L.; Jones, D.T. A Guide to Machine Learning for Biologists. Nat. Rev. Mol. Cell Biol. 2022, 23, 40–55. [Google Scholar] [CrossRef]
- Satheeshkumar, P.S.; Pili, R.; Epstein, J.B.; Thazhe, S.B.K.; Sukumar, R.; Mohan, M.P. Characteristics and Predictors Associated with Cancer-Related Fatigue among Solid and Liquid Tumors. J. Cancer Res. Clin. Oncol. 2023, 149, 13875–13888. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Du, J.; Yang, M.; Xu, Q.; Huang, J.; Tan, W.; Xu, T.; Wang, L.; Nie, W.; Zhao, L. Development and External Validation of a Machine Learning-Based Prediction Model for the Cancer-Related Fatigue Diagnostic Screening in Adult Cancer Patients: A Cross-Sectional Study in China. Support. Care Cancer 2023, 31, 106. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kors, J.A.; Ioannou, S.; John, L.H.; Markus, A.F.; Rekkas, A.; de Ridder, M.A.J.; Seinen, T.M.; Williams, R.D.; Rijnbeek, P.R. Trends in the Conduct and Reporting of Clinical Prediction Model Development and Validation: A Systematic Review. J. Am. Med. Inform. Assoc. 2022, 29, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.A.; Navar, A.M.; Pencina, M.J.; Ioannidis, J.P.A. Opportunities and Challenges in Developing Risk Prediction Models with Electronic Health Records Data: A Systematic Review. J. Am. Med. Inform. Assoc. 2017, 24, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Sleight, A.G.; Crowder, S.L.; Skarbinski, J.; Coen, P.; Parker, N.H.; Hoogland, A.I.; Gonzalez, B.D.; Playdon, M.C.; Cole, S.; Ose, J.; et al. A New Approach to Understanding Cancer-Related Fatigue: Leveraging the 3P Model to Facilitate Risk Prediction and Clinical Care. Cancers 2022, 14, 1982. [Google Scholar] [CrossRef]
- Wang, T.; Yin, J.; Miller, A.H.; Xiao, C. A Systematic Review of the Association between Fatigue and Genetic Polymorphisms. Brain Behav. Immun. 2017, 62, 230–244. [Google Scholar] [CrossRef]
- Susanne, K.; Michael, F.; Thomas, S.; Peter, E.; Andreas, H. Predictors of Fatigue in Cancer Patients: A Longitudinal Study. Support. Care Cancer 2019, 27, 3463–3471. [Google Scholar] [CrossRef]
- Stobäus, N.; Müller, M.J.; Küpferling, S.; Schulzke, J.-D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hwang, S.S.; Chang, V.T.; Rue, M.; Kasimis, B. Multidimensional Independent Predictors of Cancer-Related Fatigue. J. Pain Symptom Manag. 2003, 26, 604–614. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Godfrey, C.; McInerney, P.; Soares, C.B.; Khalil, H.; Parker, D. Methodology for Jbi Scoping Reviews. In The Joanna Briggs Institute Reviewers Manual 2015; Joanna Briggs Institute: Adelaide, Australia, 2015; pp. 3–24. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.R.; Juneau, P.; Regan, J.M.; Liwang, J.; Alshawi, S.; Wang, A.; Saligan, L.N. Brain-Derived Neurotrophic Factor Polymorphism Val66Met Protects against Cancer-Related Fatigue. Transl. Psychiatry 2020, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Agarwal, S.; Minhas, V.; Bhatnagar, S.; Mishra, S.; Kumar, V.; Bharati, S.; Gupta, N.; Khan, M. To Assess the Prevalence and Predictors of Cancer-Related Fatigue and Its Impact on Quality of Life in Advanced Cancer Patients Receiving Palliative Care in a Tertiary Care Hospital: A Cross-Sectional Descriptive Study. Indian. J. Palliat. Care 2020, 26, 523. [Google Scholar] [CrossRef] [PubMed]
- Vardy, J.L.; Dhillon, H.M.; Pond, G.R.; Renton, C.; Dodd, A.; Zhang, H.; Clarke, S.J.; Tannock, I.F. Fatigue in People with Localized Colorectal Cancer Who Do and Do Not Receive Chemotherapy: A Longitudinal Prospective Study. Ann. Oncol. 2016, 27, 1761–1767. [Google Scholar] [CrossRef]
- Feng, L.R.; Wolff, B.S.; Lukkahatai, N.; Espina, A.; Saligan, L.N. Exploratory Investigation of Early Biomarkers for Chronic Fatigue in Prostate Cancer Patients Following Radiation Therapy. Cancer Nurs. 2017, 40, 184–193. [Google Scholar] [CrossRef]
- Feng, L.R.; Fernández-Martínez, J.L.; Zaal, K.J.M.; deAndrés-Galiana, E.J.; Wolff, B.S.; Saligan, L.N. mGluR5 Mediates Post-Radiotherapy Fatigue Development in Cancer Patients. Transl. Psychiatry 2018, 8, 110. [Google Scholar] [CrossRef]
- Feng, L.R.; Fuss, T.; Dickinson, K.; Ross, A.; Saligan, L.N. Co-Occurring Symptoms Contribute to Persistent Fatigue in Prostate Cancer. Oncology 2019, 96, 183–191. [Google Scholar] [CrossRef]
- Hughes, A.; Suleman, S.; Rimes, K.A.; Marsden, J.; Chalder, T. Cancer-Related Fatigue and Functional Impairment–Towards an Understanding of Cognitive and Behavioural Factors. J. Psychosom. Res. 2020, 134, 110127. [Google Scholar] [CrossRef]
- Gerber, L.H.; Stout, N.; McGarvey, C.; Soballe, P.; Shieh, C.; Diao, G.; Springer, B.A.; Pfalzer, L.A. Factors Predicting Clinically Significant Fatigue in Women Following Treatment for Primary Breast Cancer. Support. Care Cancer 2011, 19, 1581–1591. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, H.; Xiao, H.; Zhang, Z.; Huang, C.; Huang, M. Serum Metabolomics Reveals the Effects of Accompanying Treatment on Fatigue in Patients with Multiple Myeloma. Support. Care Cancer 2023, 31, 43. [Google Scholar] [CrossRef]
- Kleckner, A.S.; Culakova, E.; Kleckner, I.R.; Belcher, E.K.; Demark-Wahnefried, W.; Parker, E.A.; Padula, G.D.A.; Ontko, M.; Janelsins, M.C.; Mustian, K.M.; et al. Nutritional Status Predicts Fatty Acid Uptake from Fish and Soybean Oil Supplements for Treatment of Cancer-Related Fatigue: Results from a Phase II Nationwide Study. Nutrients 2021, 14, 184. [Google Scholar] [CrossRef]
- Chen, L.-M.; Yang, Q.-L.; Duan, Y.-Y.; Huan, X.-Z.; He, Y.; Wang, C.; Fan, Y.-Y.; Cai, Y.-C.; Li, J.-M.; Chen, L.-P.; et al. Multidimensional Fatigue in Patients with Nasopharyngeal Carcinoma Receiving Concurrent Chemoradiotherapy: Incidence, Severity, and Risk Factors. Support. Care Cancer 2021, 29, 5009–5019. [Google Scholar] [CrossRef]
- Xiao, C.; Eldridge, R.C.; Beitler, J.J.; Higgins, K.A.; Chico, C.E.; Felger, J.C.; Wommack, E.C.; Knobf, T.; Saba, N.F.; Shin, D.M.; et al. Association Among Glucocorticoid Receptor Sensitivity, Fatigue, and Inflammation in Patients with Head and Neck Cancer. Psychosom. Med. 2020, 82, 508–516. [Google Scholar] [CrossRef]
- Zordan, R.; Manitta, V.; Nandurkar, H.; Cole-Sinclair, M.; Philip, J.; Saligan, L.N.; Fernández-Martínez, J.L.; deAndrés-Galiana, E.J.; Sonis, S. Prevalence and Predictors of Fatigue in Haemo-Oncological Patients. Cancer Inform. 2014, 44, 1013–1017. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, D.; Peng, Y.; Yang, Y.; Zhuang, X.; Li, Z.; Wang, M.; Chen, L.; Zhang, H. Cancer-Related Fatigue and Chemotherapy-Associated Adverse Effects: Correlation with TNF-α, IL-1 and 17-Hydroxycorticosteroids. Future Oncol. 2014, 10, 1619–1626. [Google Scholar] [CrossRef]
- Pertl, M.M.; Hevey, D.; Boyle, N.T.; Hughes, M.M.; Collier, S.; O’Dwyer, A.M.; Harkin, A.; Kennedy, M.J.; Connor, T.J. C-Reactive Protein Predicts Fatigue Independently of Depression in Breast Cancer Patients Prior to Chemotherapy. Brain Behav. Immun. 2013, 34, 108–119. [Google Scholar] [CrossRef]
- Goldstein, D.; Bennett, B.K.; Webber, K.; Boyle, F.; de Souza, P.L.; Wilcken, N.R.C.; Scott, E.M.; Toppler, R.; Murie, P.; O’Malley, L.; et al. Cancer-Related Fatigue in Women with Breast Cancer: Outcomes of a 5-Year Prospective Cohort Study. J. Clin. Oncol. 2012, 30, 1805–1812. [Google Scholar] [CrossRef]
- Hoffman, A.J.; von Eye, A.; Gift, A.G.; Given, B.A.; Given, C.W.; Rothert, M. Testing a Theoretical Model of Perceived Self-Efficacy for Cancer-Related Fatigue Self-Management and Optimal Physical Functional Status. Nurs. Res. 2009, 58, 32–41. [Google Scholar] [CrossRef]
- Luctkar-Flude, M.; Groll, D.; Woodend, K.; Tranmer, J. Fatigue and Physical Activity in Older Patients with Cancer: A Six-Month Follow-Up Study. Oncol. Nurs. Forum 2009, 36, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Booker, R.; Olson, K.; Pilarski, L.M.; Noon, J.P.; Bahlis, N.J. The Relationships Among Physiologic Variables, Quality of Life, and Fatigue in Patients with Multiple Myeloma. Oncol. Nurs. Forum 2009, 36, 209–216. [Google Scholar] [CrossRef]
- Von Ah, D.M.; Kang, D.-H.; Carpenter, J.S. Predictors of Cancer-Related Fatigue in Women with Breast Cancer Before, during, and after Adjuvant Therapy. Cancer Nurs. 2008, 31, 134–144. [Google Scholar] [CrossRef]
- Fleer, J.; Sleijfer, D.T.; Hoekstra, H.J.; Tuinman, M.A. Prevalence, Changes in and Correlates of Fatigue in the First Year after Diagnosis of Testicular Cancer. Anticancer Res. 2005, 25, 4647–4653. [Google Scholar]
- Andrykowski, M.A.; Schmidt, J.E.; Salsman, J.M.; Beacham, A.O.; Jacobsen, P.B.; Dupont, A.; Bower, J.E.; Stanton, A.L.; Ganz, P.A. Use of a Case Definition Approach to Identify Cancer-Related Fatigue in Women Undergoing Adjuvant Therapy for Breast Cancer. J. Clin. Oncol. 2005, 23, 6613–6622. [Google Scholar] [CrossRef]
- Gélinas, C.; Fillion, L. Factors Related to Persistent Fatigue Following Completion of Breast Cancer Treatment. Oncol. Nurs. Forum 2004, 31, 269–278. [Google Scholar] [CrossRef]
- Ahlberg, K.; Ekman, T.; Gaston-Johansson, F. Levels of Fatigue Compared to Levels of Cytokines and Hemoglobin during Pelvic Radiotherapy: A Pilot Study. Biol. Res. Nurs. 2004, 5, 203–210. [Google Scholar] [CrossRef]
- Cella, D.; Lai, J.; Chang, C.-H.; Peterman, A.; Slavin, M. Fatigue in Cancer Patients Compared with Fatigue in the General United States Population. Cancer 2002, 94, 528–538. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Bennett, J.A.; Nail, L.; Schwartz, A. Strength, Physical Activity, and Age Predict Fatigue in Older Breast Cancer Survivors. Oncol. Nurs. Forum 2008, 35, 815–821. [Google Scholar] [CrossRef]
- Álvarez-Bustos, A.; de Pedro, C.G.; Romero-Elías, M.; Ramos, J.; Osorio, P.; Cantos, B.; Maximiano, C.; Méndez, M.; Fiuza-Luces, C.; Méndez-Otero, M.; et al. Prevalence and Correlates of Cancer-Related Fatigue in Breast Cancer Survivors. Support. Care Cancer 2021, 29, 6523–6534. [Google Scholar] [CrossRef] [PubMed]
- van Baar, H.; Bours, M.J.L.; Beijer, S.; van Zutphen, M.; van Duijnhoven, F.J.B.; Kok, D.E.; Wesselink, E.; de Wilt, J.H.W.; Kampman, E.; Winkels, R.M. Body Composition and Its Association with Fatigue in the First 2 Years after Colorectal Cancer Diagnosis. J. Cancer Surviv. 2021, 15, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Champion, V.L.; Wagner, L.I.; Monahan, P.O.; Daggy, J.; Smith, L.; Cohee, A.; Ziner, K.W.; Haase, J.E.; Miller, K.; Pradhan, K.; et al. Comparison of Younger and Older Breast Cancer Survivors and Age-Matched Controls on Specific and Overall QoL Domains. Cancer 2014, 120, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Carreira, H.; Williams, R.; Dempsey, H.; Stanway, S.; Smeeth, L.; Bhaskaran, K. Quality of Life and Mental Health in Breast Cancer Survivors Compared with Non-Cancer Controls: A Study of Patient-Reported Outcomes in the United Kingdom. J. Cancer Surviv. 2021, 15, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.; Yeo, W.; Mo, K.F.; Tse, K.Y.; Lee, Y.M.; Tse, S.M.; Ho, F.P.; Kwan, W.H. A Case Control Study on Risk Factors of Lymphedema after Axillary Lymph Node Dissection for Breast Cancer in Hong Kong. J. Clin. Oncol. 2007, 25, 9048. [Google Scholar] [CrossRef]
- Harris, C.S.; Kober, K.M.; Cooper, B.; Conley, Y.P.; Dhruva, A.A.; Hammer, M.J.; Paul, S.; Levine, J.D.; Miaskowski, C.A. Symptom Clusters in Outpatients with Cancer Using Different Dimensions of the Symptom Experience. Support. Care Cancer 2022, 30, 6889–6899. [Google Scholar] [CrossRef] [PubMed]
- Miaskowski, C.; Dodd, M.; Lee, K. Symptom Clusters: The New Frontier in Symptom Management Research. J. Natl. Cancer Inst. Monogr. 2004, 2004, 17–21. [Google Scholar] [CrossRef]
- Kroenke, K.; Theobald, D.; Wu, J.; Loza, J.K.; Carpenter, J.S.; Tu, W. The Association of Depression and Pain with Health-Related Quality of Life, Disability, and Health Care Use in Cancer Patients. J. Pain Symptom Manag. 2010, 40, 327–341. [Google Scholar] [CrossRef]
- Reyes, C.C.; Anderson, K.O.; Gonzalez, C.E.; Ochs, H.C.; Wattana, M.; Acharya, G.; Todd, K.H. Depression and Survival Outcomes after Emergency Department Cancer Pain Visits. BMJ Support. Palliat. Care 2019, 9, e36. [Google Scholar] [CrossRef]
- Li, J.-X. Pain and Depression Comorbidity: A Preclinical Perspective. Behav. Brain Res. 2015, 276, 92–98. [Google Scholar] [CrossRef]
- Ruiz-Casado, A.; Álvarez-Bustos, A.; de Pedro, C.G.; Méndez-Otero, M.; Romero-Elías, M. Cancer-Related Fatigue in Breast Cancer Survivors: A Review. Clin. Breast Cancer 2021, 21, 10–25. [Google Scholar] [CrossRef]
- Fox, R.S.; Ancoli-Israel, S.; Roesch, S.C.; Merz, E.L.; Mills, S.D.; Wells, K.J.; Sadler, G.R.; Malcarne, V.L. Sleep Disturbance and Cancer-Related Fatigue Symptom Cluster in Breast Cancer Patients Undergoing Chemotherapy. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2020, 28, 845–855. [Google Scholar] [CrossRef]
- Wang, S.-H.; He, G.-P.; Jiang, P.-L.; Tang, L.-L.; Feng, X.-M.; Zeng, C.; Wang, G.-F. Relationship between Cancer-Related Fatigue and Personality in Patients with Breast Cancer after Chemotherapy. Psychooncology 2013, 22, 2386–2390. [Google Scholar] [CrossRef] [PubMed]
- Hofer, F.; Koinig, K.A.; Nagl, L.; Borjan, B.; Stauder, R. Fatigue at Baseline Is Associated with Geriatric Impairments and Represents an Adverse Prognostic Factor in Older Patients with a Hematological Malignancy. Ann. Hematol. 2018, 97, 2235–2243. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Port, J.D.; Kaufman, K.R.; Jatoi, A.; Hart, C.R.; Gries, K.J.; Lanza, I.R.; Kumar, R. Skeletal Muscle Mitochondrial Dysfunction and Muscle and Whole Body Functional Deficits in Cancer Patients with Weight Loss. J. Appl. Physiol. 2022, 132, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Sanoff, H.K.; Deal, A.M.; Krishnamurthy, J.; Torrice, C.; Dillon, P.; Sorrentino, J.; Ibrahim, J.G.; Jolly, T.A.; Williams, G.; Carey, L.A.; et al. Effect of Cytotoxic Chemotherapy on Markers of Molecular Age in Patients with Breast Cancer. J. Natl. Cancer Inst. 2014, 106, dju057. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-Related Fatigue--Mechanisms, Risk Factors, and Treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Lamkin, D.M. Inflammation and Cancer-Related Fatigue: Mechanisms, Contributing Factors, and Treatment Implications. Brain Behav. Immun. 2013, 30, S48–S57. [Google Scholar] [CrossRef]
- Brownstein, C.G.; Twomey, R.; Temesi, J.; Wrightson, J.G.; Martin, T.; Medysky, M.E.; Culos-Reed, S.N.; Millet, G.Y. Physiological and Psychosocial Correlates of Cancer-Related Fatigue. J. Cancer Surviv. 2022, 16, 1339–1354. [Google Scholar] [CrossRef]
- Koornstra, R.H.T.; Peters, M.; Donofrio, S.; van den Borne, B.; de Jong, F.A. Management of Fatigue in Patients with Cancer—A Practical Overview. Cancer Treat. Rev. 2014, 40, 791–799. [Google Scholar] [CrossRef]
- Aapro, M.; Scotte, F.; Bouillet, T.; Currow, D.; Vigano, A. A Practical Approach to Fatigue Management in Colorectal Cancer. Clin. Colorectal Cancer 2017, 16, 275–285. [Google Scholar] [CrossRef]
- Maurer, T.; Jaskulski, S.; Behrens, S.; Jung, A.Y.; Obi, N.; Johnson, T.; Becher, H.; Chang-Claude, J. Tired of Feeling Tired–The Role of Circulating Inflammatory Biomarkers and Long-Term Cancer Related Fatigue in Breast Cancer Survivors. Breast 2021, 56, 103–109. [Google Scholar] [CrossRef]
- Yennu, S.; Urbauer, D.L.; Bruera, E. Factors Associated with the Severity and Improvement of Fatigue in Patients with Advanced Cancer Presenting to an Outpatient Palliative Care Clinic. BMC Palliat. Care 2012, 11, 16. [Google Scholar] [CrossRef]
- Wang, X.S.; Giralt, S.A.; Mendoza, T.R.; Engstrom, M.C.; Johnson, B.A.; Peterson, N.; Broemeling, L.D.; Cleeland, C.S. Clinical Factors Associated with Cancer-Related Fatigue in Patients Being Treated for Leukemia and Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2002, 20, 1319–1328. [Google Scholar] [CrossRef]
- Ghoshal, A.; Damani, A.; Muckaden, M. Association of Cancer-Related Fatigue with Other Symptoms and Impact on Quality of Life of Palliative Care Patients in a Tertiary Cancer Institute: A Prospective Observational Study (S751). J. Pain Symptom Manag. 2016, 51, 435. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Semik, J.; Habermann, N.; Wiskemann, J.; Ulrich, C.M.; Steindorf, K. Cancer-Related Fatigue Shows a Stable Association with Diurnal Cortisol Dysregulation in Breast Cancer Patients. Brain Behav. Immun. 2016, 52, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Luecken, L.J.; Dausch, B.; Gulla, V.; Hong, R.; Compas, B.E. Alterations in Morning Cortisol Associated with PTSD in Women with Breast Cancer. J. Psychosom. Res. 2004, 56, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.K. Altered Circadian Rhythms and Cancer-Related Fatigue Outcomes. Integr. Cancer Ther. 2011, 10, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in Cancer Research and Emerging Applications in Clinical Oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. Metabolic Features of Chronic Fatigue Syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E3749. [Google Scholar] [CrossRef]
- Surowiec, I.; Gjesdal, C.G.; Jonsson, G.; Norheim, K.B.; Lundstedt, T.; Trygg, J.; Omdal, R. Metabolomics Study of Fatigue in Patients with Rheumatoid Arthritis Naïve to Biological Treatment. Rheumatol. Int. 2016, 36, 703–711. [Google Scholar] [CrossRef]
- Twomey, R.; Martin, T.; Temesi, J.; Culos-Reed, S.N.; Millet, G.Y. Tailored Exercise Interventions to Reduce Fatigue in Cancer Survivors: Study Protocol of a Randomized Controlled Trial. BMC Cancer 2018, 18, 757. [Google Scholar] [CrossRef]
- Sharour, L.A. Cancer-Related Fatigue, Laboratory Markers as Indicators for Nutritional Status among Patients with Colorectal Cancer. Nutr. Cancer 2020, 72, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.J.; Skinner, T.L.; Wright, O.R.L. Nutrition Therapy for the Management of Cancer-Related Fatigue and Quality of Life: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2019, 122, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, B.; Jiang, M.; Yang, Y.; Wang, C.; Huang, C.; Han, L. Prevalence and Risk Factors of Cancer-Related Fatigue: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2020, 111, 103707. [Google Scholar] [CrossRef] [PubMed]
- Afari, N.; Buchwald, D. Chronic Fatigue Syndrome: A Review. Am. J. Psychiatry 2003, 160, 221–236. [Google Scholar] [CrossRef]
- Servaes, P.; Prins, J.; Verhagen, S.; Bleijenberg, G. Fatigue after Breast Cancer and in Chronic Fatigue Syndrome: Similarities and Differences. J. Psychosom. Res. 2002, 52, 453–459. [Google Scholar] [CrossRef]
| No. | Author, Year | Country | Study Type | Data Source | Sample Size | Cancer | Predictors | Assessments of Fatigue | Definition of Fatigue |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Zhang, 2023 [42] | China | A cross-sectional study | A, B, C | 30 | Multiple myeloma | GAA | BFI-C | N/A |
| 2 | Kleckner, 2021 [43] | USA | RCT | A, B, C | 85 | Breast cancer | Serum omega-3s | MFSI-SF | N/A |
| 3 | Chen, 2021 [44] | China | A longitudinal study | A, B | 79 | Nasopharyngeal carcinoma | Cancer stage IVB, 3–6 courses of treatment | MFI-20 | N/A |
| 4 | Xiao, 2020 [45] | USA | A longitudinal study | A, B, C | 77 | Head and neck cancer | GR sensitivity | MFI-20 | N/A |
| 5 | Agarwal, 2020 [35] | India | A cross-sectional study | A | 110 | Mixed | Pain, physical functioning, performance status, albumin | FACIT-F | A score < 30 indicates severe fatigue |
| 6 | Hughes, 2020 [40] | UK | A longitudinal study | A, B | 159 | Breast cancer | Cancer-related catastrophizing, all-or-nothing behaviors, perceived punishing responses, anxiety | CFQ | A score > 4 |
| 7 | Susanne, 2019 [27] | Germany | A longitudinal study | A, B | 948 | Mixed | Baseline fatigue, depression | EORTC QLQ-FA12 | N/A |
| 8 | Feng, 2019 [39] | USA | A longitudinal study | A, B | 47 | Prostate Cancer | Urinary dysfunction, pain, depressive symptoms | FACT-F | A clinically significant decrease in the FACT-F score of ≥3 points |
| 9 | Raudonis, 2017 [8] | USA | A longitudinal study | A, B, C | 11 | Breast cancer | Chemotherapy type, time (sequence of visit), IL-6 | PFS-R | Levels of fatigue range from 0 (absent), 0.1 to 3.99 (mild), 4 to 6.99 (moderate), or 7.0 or greater (severe) |
| 10 | Feng, 2017 [37] | USA | A longitudinal study | A, B, C | 34 | Prostate Cancer | IL-3, IL-8, IL-9, IL-10, IL-16, IP-10, IFN-α2, IFN-γ, SDF-1α | FACT-F | A score change of ≥3 is considered clinically significant |
| 11 | Vardy, 2016 [36] | Australia, Canada | A cohort study | A, B, C | 361 | Colorectal cancer | Baseline fatigue, cognitive and affective symptoms, quality of life, comorbidities, chemotherapy | FACT-F | Standardized score ≤ 68/100 |
| 12 | Stobäus, 2015 [28] | Germany | A cross-sectional study | A, B, C, D | 285 | Mixed | Low recent protein intake | BFI | A score ≥ 4 |
| 13 | Zordan, 2014 [46] | Australia | A cross-sectional study | A, B | 180 | Hematological malignancy | Performance status, stage of disease, feeling sad, feeling irritable | MSAS-SF | N/A |
| 14 | Zhang, 2014 [47] | China | A longitudinal study | A, C, E | 200 | Mixed | TNF-a, IL-1 | CFS | A score ≥ 5 |
| 15 | Pertl, 2013 [48] | Ireland | A longitudinal study | A, B, C | 61 | Breast cancer | CRP | FACT-F | N/A |
| 16 | Goldstein, 2012 [49] | Australia | A cohort study | A, B, C, D | 218 | Breast cancer | Tumor size | Fatigue subscale of SPHERE | A score ≥ 3 |
| 17 | Gerber, 2011 [41] | USA | A longitudinal study | A, B, C | 44 | Breast cancer | BMI, WBC, upper limb volume, physical activity levels | VNR | A score ≥ 4 |
| 18 | Hoffman, 2009 [50] | USA | A cross-sectional study | A, B | 298 | Mixed | Older age, comorbidity, female | BFI | N/A |
| 19 | Luctkar-Flude, 2009 [51] | Canada | A longitudinal study | A, B | 440 | Mixed | Physical activity levels | MSAS | N/A |
| 20 | Booker, 2009 [52] | Canada | A cross-sectional study | A, B | 56 | Multiple myeloma | CRP | FACT-F | N/A |
| 21 | Von Ah, 2008 [53] | USA | A longitudinal study | A, C | 44 | Breast cancer | Morning cortisol, IL-1β, mood disturbance | PFS-R | N/A |
| 22 | Fleer, 2005 [54] | Netherlands | A longitudinal study | A, B, C | 52 | Testicular cancer | Older age, trait anxiety, baseline fatigue | MFI-20 | N/A |
| 23 | Andrykowski, 2005 [55] | USA, UK | A longitudinal study | A, B, D, E | 288 | Breast cancer | Chemotherapy, fatigue catastrophizing | FSI | N/A |
| 24 | Gélinas, 2004 [56] | Canada | A cross-sectional study | A, B | 103 | Breast cancer | Cancer-related stressors, passive and active coping, pain | MFI-20 | N/A |
| 25 | Ahlberg, 2004 [57] | Sweden | A longitudinal study | A, B, C | 15 | Uterine cancer | IL-6 | MFI-20 | N/A |
| 26 | Hwang, 2003 [30] | USA | A cross-sectional study | A | 180 | Mixed | Physical symptoms (pain, lack of appetite, feeling drowsy, dyspnea); psychological symptoms (feeling sad and feeling irritable) | BFI, FACT-F | BFI usual fatigue ≥ 3/10 |
| 27 | Cella, 2002 [58] | USA | A cohort study | E | 3492 | Mixed | Hemoglobin | FACIT-F | N/A |
| Author, Year | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|
| Zhang, 2023 [42] | yes | yes | yes | yes | yes | no | yes | yes |
| Chen, 2021 [44] | yes | yes | yes | yes | yes | yes | yes | yes |
| Xiao, 2020 [45] | yes | yes | yes | yes | yes | yes | yes | yes |
| Agarwal, 2020 [35] | yes | yes | yes | yes | yes | yes | yes | yes |
| Hughes, 2020 [40] | yes | yes | yes | yes | yes | yes | yes | yes |
| Susanne, 2019 [27] | yes | yes | yes | yes | yes | yes | yes | yes |
| Feng, 2019 [39] | yes | yes | yes | yes | yes | yes | yes | yes |
| Raudonis, 2017 [8] | yes | yes | yes | yes | yes | yes | yes | yes |
| Feng, 2017 [37] | yes | yes | yes | yes | yes | no | yes | yes |
| Stobäus, 2015 [28] | yes | yes | yes | yes | yes | yes | yes | yes |
| Zordan, 2014 [46] | yes | yes | yes | yes | yes | yes | yes | yes |
| Zhang, 2014 [47] | yes | yes | yes | yes | yes | no | yes | yes |
| Pertl, 2013 [48] | yes | yes | yes | yes | yes | yes | yes | yes |
| Gerber, 2011 [41] | yes | yes | yes | yes | yes | no | yes | yes |
| Hoffman, 2009 [50] | yes | yes | yes | yes | yes | yes | yes | yes |
| Luctkar-Flude, 2009 [51] | yes | yes | yes | yes | yes | yes | yes | yes |
| Booker, 2009 [52] | yes | yes | yes | yes | yes | yes | yes | yes |
| Von Ah, 2008 [53] | yes | yes | yes | yes | yes | yes | yes | yes |
| Fleer, 2005 [54] | yes | yes | yes | yes | yes | no | yes | yes |
| Andrykowski, 2005 [55] | yes | yes | yes | yes | yes | yes | yes | yes |
| Gélinas, 2004 [56] | yes | yes | yes | yes | yes | yes | yes | yes |
| Ahlberg, 2004 [57] | yes | yes | yes | yes | no | no | yes | yes |
| Hwang, 2003 [30] | yes | yes | yes | yes | yes | yes | yes | yes |
| Author, Year | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Exposed Cohort | Non-Exposed Cohort | Ascertainment of Exposure | Outcome of Interest | Assessment of Outcome | Length of Follow-Up | Adequacy of Follow-Up | |||
| Vardy, 2016 [36] | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Goldstein, 2012 [49] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Cella, 2002 [58] | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Author, Year | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Bias | |
|---|---|---|---|---|---|---|---|
| Random Sequence Generation | Allocation Concealment | ||||||
| Kleckner, 2021 [43] | low | low | low | unclear | low | low | low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tian, L.; Liu, X.; Zhang, H.; Tang, Y.; Zhang, H.; Nie, W.; Wang, L. Multidimensional Predictors of Cancer-Related Fatigue Based on the Predisposing, Precipitating, and Perpetuating (3P) Model: A Systematic Review. Cancers 2023, 15, 5879. https://doi.org/10.3390/cancers15245879
Wang Y, Tian L, Liu X, Zhang H, Tang Y, Zhang H, Nie W, Wang L. Multidimensional Predictors of Cancer-Related Fatigue Based on the Predisposing, Precipitating, and Perpetuating (3P) Model: A Systematic Review. Cancers. 2023; 15(24):5879. https://doi.org/10.3390/cancers15245879
Chicago/Turabian StyleWang, Yiming, Lv Tian, Xia Liu, Hao Zhang, Yongchun Tang, Hong Zhang, Wenbo Nie, and Lisheng Wang. 2023. "Multidimensional Predictors of Cancer-Related Fatigue Based on the Predisposing, Precipitating, and Perpetuating (3P) Model: A Systematic Review" Cancers 15, no. 24: 5879. https://doi.org/10.3390/cancers15245879
APA StyleWang, Y., Tian, L., Liu, X., Zhang, H., Tang, Y., Zhang, H., Nie, W., & Wang, L. (2023). Multidimensional Predictors of Cancer-Related Fatigue Based on the Predisposing, Precipitating, and Perpetuating (3P) Model: A Systematic Review. Cancers, 15(24), 5879. https://doi.org/10.3390/cancers15245879







