The Same–Up–Down Staging System for Recurrent Early Glottic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Restaging Systems Used for the Classification of Tumor Recurrence
2.3. Statistical Analyses
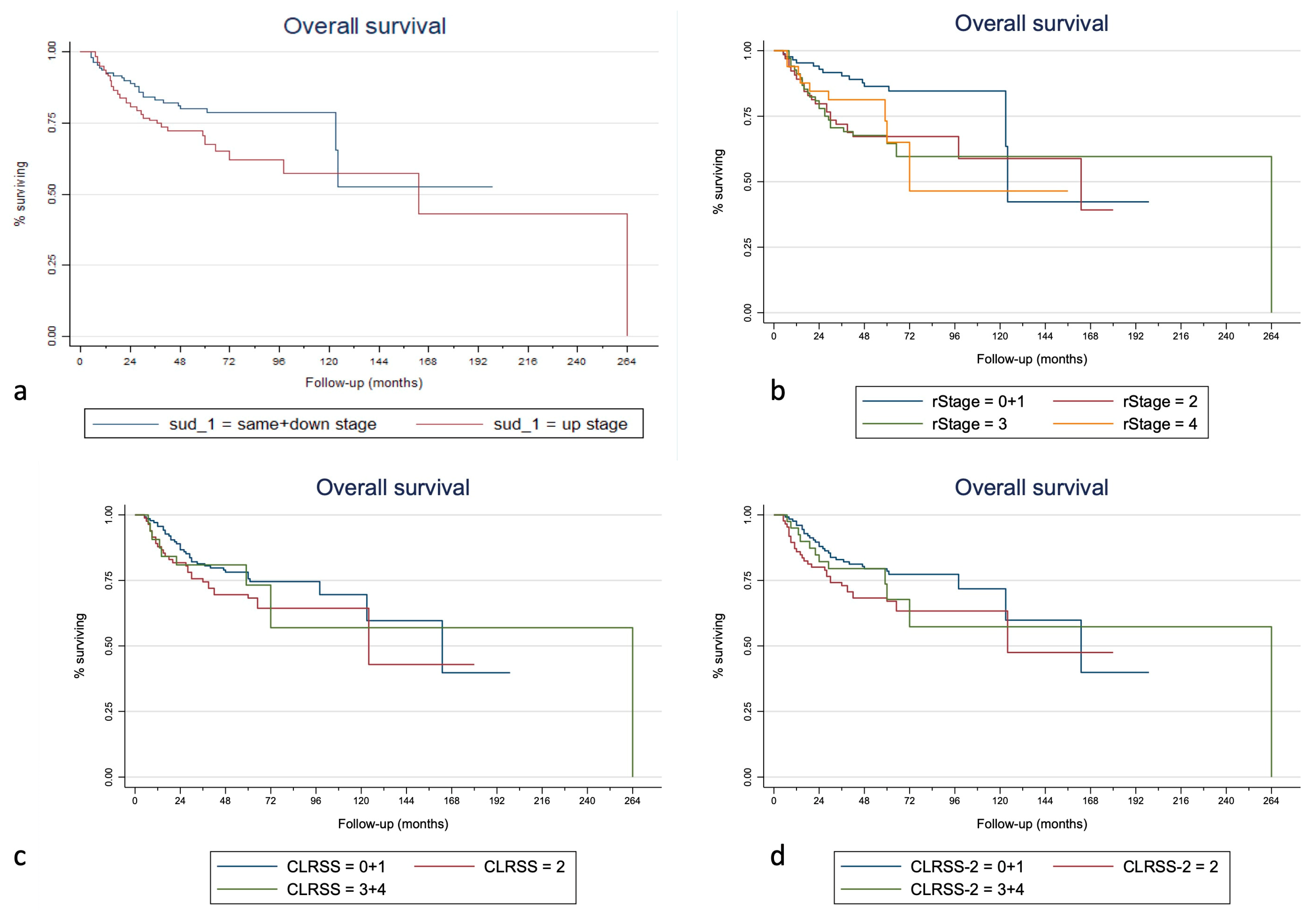
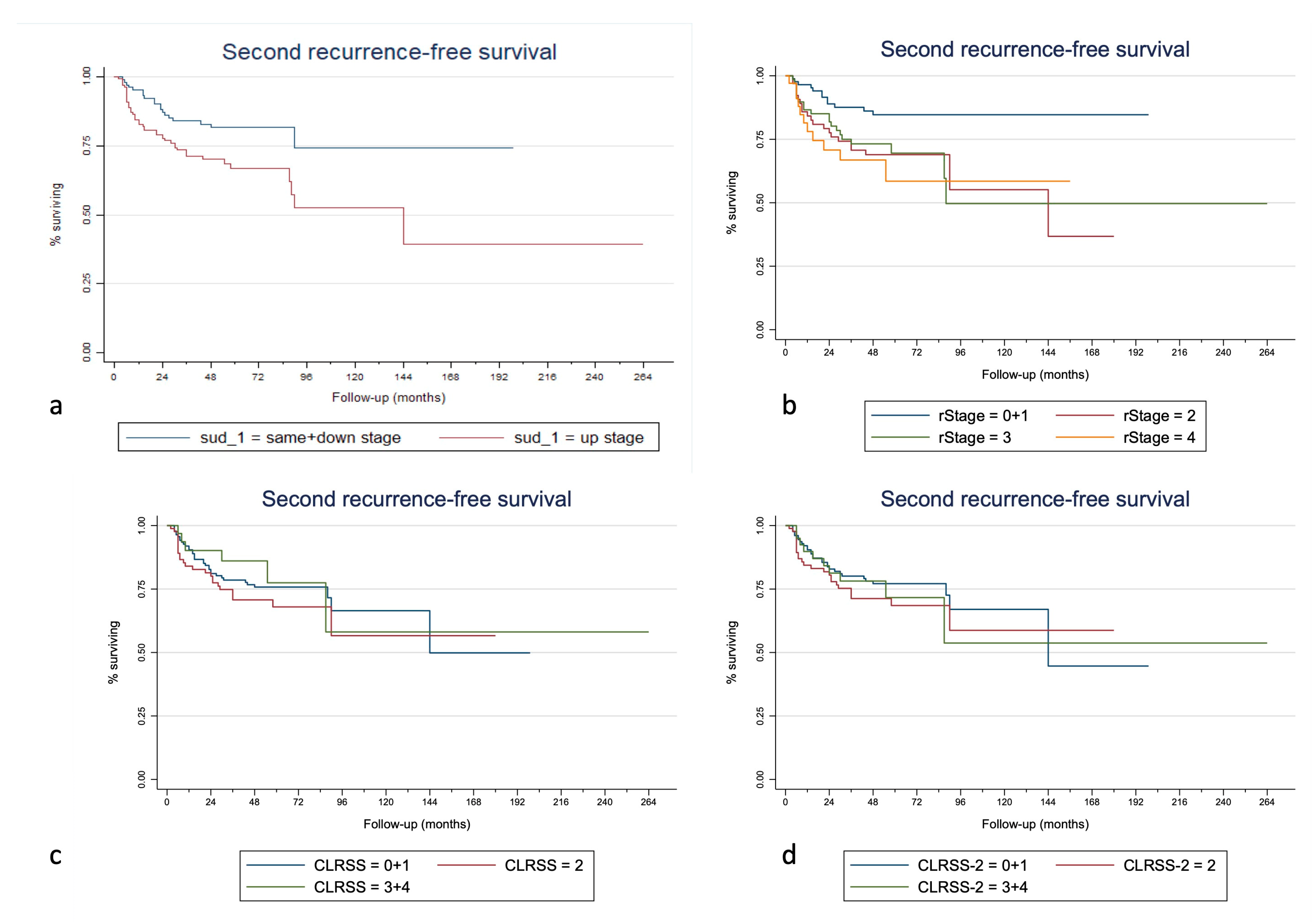
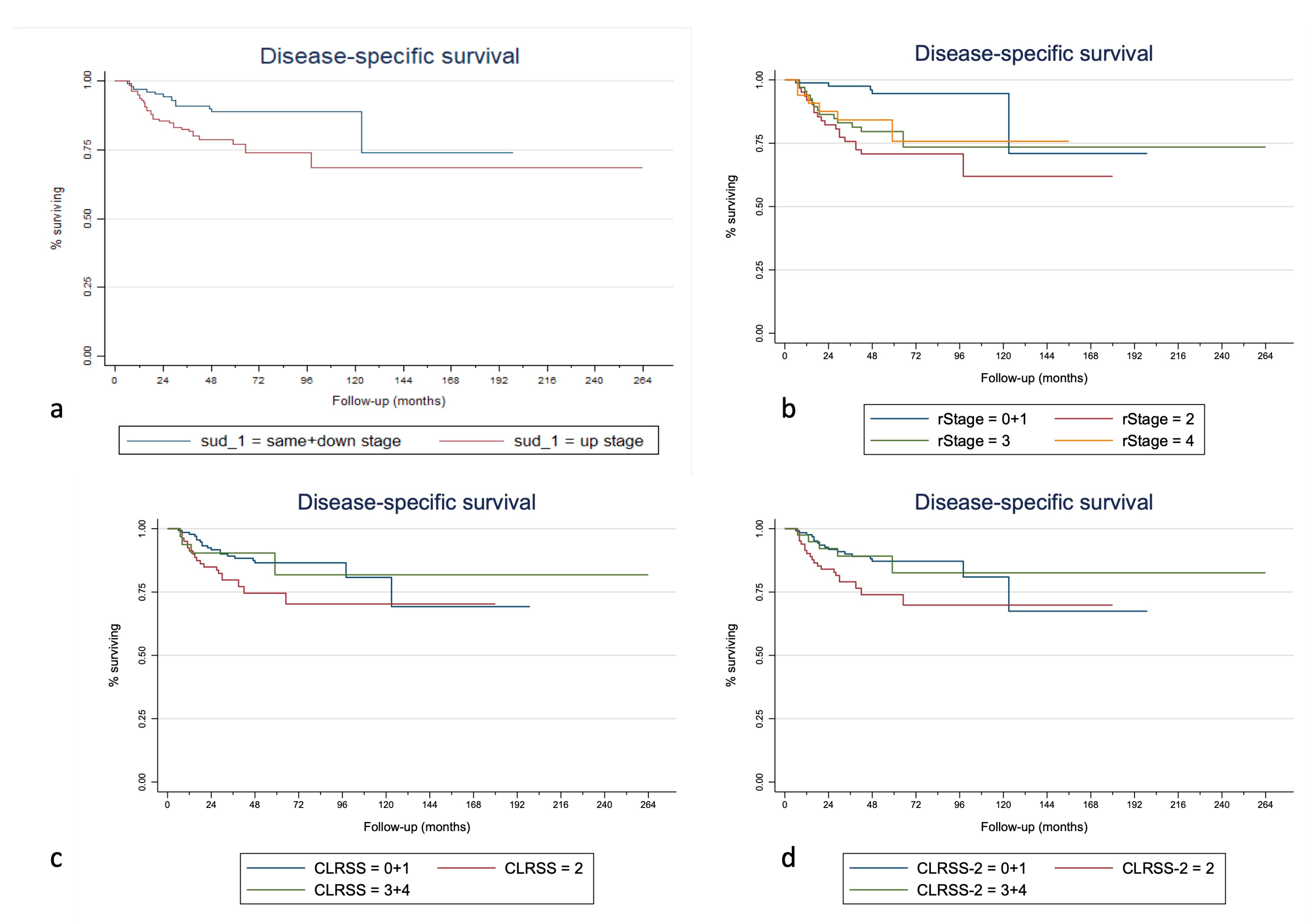
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Locatello, L.G.; Bruno, C.; Gallo, O. Early glottic cancer recurrence: A critical review on its current management. Crit. Rev. Oncol. Hematol. 2021, 160, 103298. [Google Scholar] [CrossRef] [PubMed]
- Gospodarowicz, M.K.; Brierley, J.D.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Baird, B.J.; Sung, C.K.; Beadle, B.M.; Divi, V. Treatment of early-stage laryngeal cancer: A comparison of treatment options. Oral Oncol. 2018, 87, 8–16. [Google Scholar] [CrossRef]
- Brandstorp-Boesen, J.; Sørum Falk, R.; Boysen, M.; Brøndbo, K. Impact of stage, management and recurrence on survival rates in laryngeal cancer. PLoS ONE 2017, 12, e0179371. [Google Scholar] [CrossRef] [PubMed]
- Pakkanen, P.; Irjala, H.; Ilmarinen, T.; Halme, E.; Lindholm, P.; Mäkitie, A.; Wigren, T.; Aaltonen, L.M. Survival and Larynx Preservation in Early Glottic Cancer: A Randomized Trial Comparing Laser Surgery and Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Crevier-Buchman, L.; Circiu, M.P.; Lisan, Q.; Hans, S. Evolution of Voice Quality in Type 1-2 Transoral CO2 Laser Cordectomy: A Prospective Comparative Study. Laryngoscope 2022, 132, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Lazio, M.S.; Vallin, A.; Giannini, C.; Taverna, C.; Maggiore, G.; Saraceno, M.S.; Gallo, O. Phonosurgical Resection Using Submucosal Infusion Technique for Early Glottic Lesions: Diagnostic and Therapeutic Procedure? Ann. Otol. Rhinol. Laryngol. 2019, 128, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Tans, L.; Al-Mamgani, A.; Kwa, S.L.S.; Elbers, J.B.W.; Keskin-Cambay, F.; Sewnaik, A.; Dorr, M.; Nout, R.; Heemsbergen, W. Single vocal cord irradiation for early-stage glottic cancer: Excellent local control and favorable toxicity profile. Oral Oncol. 2022, 127, 105782. [Google Scholar] [CrossRef]
- Sher, D.J.; Timmerman, R.D.; Nedzi, L.; Ding, C.; Pham, N.L.; Zhao, B.; Sumer, B.D. Phase 1 Fractional Dose-Escalation Study of Equipotent Stereotactic Radiation Therapy Regimens for Early-Stage Glottic Larynx Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 110–118. [Google Scholar] [CrossRef]
- Perillo, A.; Landoni, V.; Farneti, A.; Sanguineti, G. Organ motion in linac-based SBRT for glottic cancer. Radiat. Oncol. 2021, 16, 106. [Google Scholar] [CrossRef]
- Chung, S.Y.; Kim, K.H.; Keum, K.C.; Koh, Y.W.; Kim, S.H.; Choi, E.C.; Lee, C.G. Radiotherapy versus cordectomy in the management of early glottic cancer. Cancer Res. Treat. 2018, 50, 156–163. [Google Scholar] [CrossRef]
- Lacy, P.D.; Piccirillo, J.F. Development of a new staging system for patients with recurrent laryngeal squamous cell carcinoma. Cancer 1998, 83, 910–917. [Google Scholar] [CrossRef]
- León, X.; López, M.; García, J.; Viza, I.; Gich, I.; Quer, M. Recurrent laryngeal squamous cell carcinoma: rTNM versus composite laryngeal recurrence staging system. Proposal for a modification of the CLRSS to improve patient classification. Head Neck 2008, 30, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, A.; Shah, J.P.; Medina, J.E.; Olsen, K.D.; Robbins, K.T.; Silver, C.E.; Rodrigo, J.P.; Suárez, C.; Coca-Pelaz, A.; Shaha, A.R.; et al. Incidence of Occult Lymph Node Metastasis in Primary Larynx Squamous Cell Carcinoma, by Subsite, T Classification and Neck Level: A Systematic Review. Cancers 2020, 12, 1059. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; American Joint Commission on Cancer; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Harrell, F.E.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the yield of medical tests. J. Am. Med. Assoc. 1982, 247, 2543–2546. [Google Scholar] [CrossRef]
- Johnson, J.T.; Newman, R.K.; Olson, J.E. Persistent hoarseness: An aggressive approach for early detection of laryngeal cancer. Postgrad. Med. 1980, 67, 122–126. [Google Scholar] [CrossRef]
- Lu, Y.A.; Tsai, M.S.; Lee, L.A.; Lee, S.R.; Lin, L.Y.; Chang, C.F.; Lin, W.N.; Hsin, L.J.; Liao, C.T.; Li, H.Y.; et al. Seeking Medical Assistance for Dysphonia Is Associated with an Improved Survival Rate in Laryngeal Cancer: Real-World Evidence. Diagnostics 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.R.; Eggerstedt, M.; Toor, J.; Tajudeen, B.A.; Husain, I.; Stenson, K.; Al-Khudari, S. Occult Lymph Node Metastasis in Early-Stage Glottic Cancer in the National Cancer Database. Laryngoscope 2021, 131, E1139–E1146. [Google Scholar] [CrossRef] [PubMed]
- Locatello, L.G.; Cannavicci, A.; Gallo, O. Prognostic impact of initial treatment in surgically salvaged recurrences of early glottic cancer. Laryngoscope 2019, 129, 2328–2333. [Google Scholar] [CrossRef]
- Nomura, T.; Ishikawa, J.; Ohki, M.; Ohata, A.; Araki, R.; Kikuchi, S. Multifactorial analysis of local control and survival in patients with early glottic cancer. Laryngoscope 2020, 130, 1701–1706. [Google Scholar] [CrossRef]
- Haapaniemi, A.; Väisänen, J.; Atula, T.; Alho, O.P.; Mäkitie, A.; Koivunen, P. Predictive factors and treatment outcome of laryngeal carcinoma recurrence. Head Neck 2017, 39, 555–563. [Google Scholar] [CrossRef]
- Horschke, S.; Steinmann, D.; Christiansen, H.; de Zwaan, M.; Zimmermann, T. Body image in men with prostate or laryngeal cancer and their female partners. Z. Psychosom. Med. Psychother. 2020, 66, 287–301. [Google Scholar] [CrossRef]
- Gallus, S.; Bosetti, C.; Franceschi, S.; Levi, F.; Negri, E.; La Vecchia, C. Laryngeal cancer in women: Tobacco, alcohol, nutritional, and hormonal factors. Cancer Epidemiol. Biomark. Prev. 2003, 12, 514–517. [Google Scholar]
- Ellis, L.; Rachet, B.; Birchall, M.; Coleman, M.P. Trends and inequalities in laryngeal cancer survival in men and women: England and Wales 1991–2006. Oral Oncol. 2012, 48, 284–289. [Google Scholar] [CrossRef]
- Abrahão, R.; Anantharaman, D.; Gaborieau, V.; Abedi-Ardekani, B.; Lagiou, P.; Lagiou, A.; Ahrens, W.; Holcatova, I.; Betka, J.; Merletti, F.; et al. The influence of smoking, age and stage at diagnosis on the survival after larynx, hypopharynx and oral cavity cancers in Europe: The ARCAGE study. Int. J. Cancer 2018, 143, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Underwood, J.M.; Townsend, J.S.; Tai, E.; White, A.; Davis, S.P.; Fairley, T.L. Persistent cigarette smoking and other tobacco use after a tobacco-related cancer diagnosis. J. Cancer Surviv. 2012, 6, 333–344. [Google Scholar] [CrossRef]
- Mimica, X.; Hanson, M.; Patel, S.G.; McGill, M.; McBride, S.; Lee, N.; Dunn, L.A.; Cracchiolo, J.R.; Shah, J.P.; Wong, R.J.; et al. Salvage surgery for recurrent larynx cancer. Head Neck 2019, 41, 3906–3915. [Google Scholar] [CrossRef] [PubMed]
- Paleri, V.; Thomas, L.; Basavaiah, N.; Drinnan, M.; Mehanna, H.; Jones, T. Oncologic outcomes of open conservation laryngectomy for radiorecurrent laryngeal carcinoma: A systematic review and meta-analysis of English-language literature. Cancer 2011, 117, 2668–2676. [Google Scholar] [CrossRef]
- Cheraghlou, S.; Kuo, P.; Mehra, S.; Yarbrough, W.G.; Judson, B.L. Salvage Surgery after Radiation Failure in T1/T2 Larynx Cancer: Outcomes following Total versus Conservation Surgery. Otolaryngol. Head Neck Surg. 2018, 158, 497–504. [Google Scholar] [CrossRef]
- Bertolin, A.; Lionello, M.; Ghizzo, M.; Cena, I.; Leone, F.; Valerini, S.; Mattioli, F.; Crosetti, E.; Presutti, L.; Succo, G.; et al. Salvage open partial horizontal laryngectomy after failed radiotherapy: A multicentric study. Laryngoscope 2020, 130, 431–436. [Google Scholar] [CrossRef]
- Li, P.; Hu, W.; Zhu, Y.; Liu, J. Treatment and predictive factors in patients with recurrent laryngeal carcinoma: A retrospective study. Oncol. Lett. 2015, 10, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, S.; Held, T.; Lang, K.; Hoerner-Rieber, J.; Zaoui, K.; Forster, T.; Rieken, S.; Plinkert, P.; Debus, J.; Adeberg, S. Salvage radiotherapy for recurrent hypopharyngeal and laryngeal squamous cell carcinoma (SCC) after first-line treatment with surgery alone: A 10-year single-centre experience. Radiat. Oncol. 2019, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Piazza, C.; Paderno, A.; Sjogren, E.V.; Bradley, P.J.; Eckel, H.E.; Mäkitie, A.; Matar, N.; Paleri, V.; Peretti, G.; Puxeddu, R.; et al. Salvage carbon dioxide transoral laser microsurgery for laryngeal cancer after (chemo)radiotherapy: A European Laryngological Society consensus statement. Eur. Arch. Otorhinolaryngol. 2021, 278, 4373–4381. [Google Scholar] [CrossRef] [PubMed]
| Frequency | Percentage | ||
|---|---|---|---|
| Gender | Male | 246 | 95.3 |
| Female | 12 | 4.7 | |
| Smoking status | No | 108 | 41.9 |
| Yes | 150 | 58.1 | |
| Alcohol abuse | No | 206 | 79.8 |
| Yes | 52 | 20.2 | |
| Original treatment | Surgery | 70 | 27.1 |
| Radiotherapy | 188 | 72.9 | |
| Original TNM stage | I | 182 | 70.5 |
| II | 76 | 29.5 | |
| Recurrence TNM stage | I | 89 | 34.5 |
| II | 67 | 26.0 | |
| III | 68 | 26.4 | |
| IV | 34 | 13.2 | |
| CLRSS | I | 140 | 54.3 |
| II | 85 | 32.9 | |
| III | 15 | 5.8 | |
| IV | 18 | 7.0 | |
| CLRSS-2 | I | 129 | 50.4 |
| II | 88 | 34.1 | |
| III | 22 | 8.5 | |
| IV | 19 | 7.4 | |
| Same–up–down stage | Same | 99 | 38.4 |
| Up | 145 | 56.2 | |
| Down | 14 | 5.4 | |
| Salvage treatment | TLM | 49 | 19.0 |
| PL | 31 | 12.0 | |
| TL | 171 | 66.3 | |
| RT | 7 | 2.7 | |
| Distant metastases | yes | 10 | 3.9 |
| no | 248 | 96.1 | |
| TOTAL | 258 | 100 |
| Variable | Overall Survival | Second Recurrence-Free Survival | Disease-Specific Survival | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value Log-Rank Test | Univariate Cox Regression | Multiple Cox Regression | p-Value Log-Rank Test | Univariate Cox Regression | Multiple Cox Regression | p-Value Log-Rank Test | Univariate Cox Regression | Multiple Cox Regression | ||||||||
| HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | |||||
| Age at first | <60 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||
| recurrence | 61–70 | 1.23 | 0.519 | 1.53 | 0.191 | 0.69 | 0.216 | 0.71 | 0.251 | 0.74 | 0.449 | 1.80 | 0.176 | |||
| 71+ | 0.368 | 1.50 | 0.166 | 2.33 | 0.006 * | 0.093 | 0.53 | 0.035 * | 0.65 | 0.155 | 0.648 | 1.03 | 0.923 | 2.55 | 0.032 * | |
| Sex | male | 1.00 | 1.00 | 1.00 | ||||||||||||
| female | 0.029 * | 2.32 | 0.034 * | 0.536 | 1.37 | 0.540 | 0.004 * | 3.33 | 0.006 * | |||||||
| Smoking | no | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| yes | 0.304 | 1.27 | 0.307 | 1.51 | 0.095 | 0.994 | 1.00 | 0.994 | 0.114 | 1.64 | 0.118 | 3.18 | 0.003 * | |||
| Alcool | no | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||||
| yes | 0.804 | 1.07 | 0.804 | 0.414 | 1.26 | 0.417 | 1.20 | 0.538 | 0.830 | 1.08 | 0.830 | |||||
| Initial | rt | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||
| treatment | surgery | 0.005 * | 0.40 | 0.007 * | 0.62 | 0.216 | 0.002 * | 0.33 | 0.003 * | 0.26 | 0.001 * | 0.056 | 0.46 | 0.063 | 0.54 | 0.230 |
| Stage at | 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| diagnosis | 2 | 0.393 | 1.23 | 0.396 | 1.71 | 0.047 * | 0.665 | 0.89 | 0.667 | 0.54 | 0.099 | 0.788 | 1.09 | 0.789 | ||
| Stage at first | 0 + 1 | 1.00 | 1.00 | 1.00 | ||||||||||||
| recurrence | 2 | 2.30 | 0.014 * | 2.51 | 0.011 * | 5.42 | 0.001 * | |||||||||
| 3 | 2.56 | 0.005 * | 2.39 | 0.016 * | 4.15 | 0.006 * | ||||||||||
| 4 | 0.018 * | 2.57 | 0.017 * | 0.013 * | 3.27 | 0.004 * | 0.003 * | 4.10 | 0.016 * | |||||||
| CLRSS | 0 + 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| 2 | 1.44 | 0.147 | 1.35 | 0.247 | 2.21 | 0.020 * | 2.07 | 0.021 * | 3.87 | 0.001 * | ||||||
| 3 + 4 | 0.339 | 1.23 | 0.557 | 0.411 | 0.88 | 0.766 | 1.39 | 0.481 | 0.055 | 1.20 | 0.719 | 3.73 | 0.093 | |||
| CLRSS-2 | 0 + 1 | 1.00 | 1.00 | 1.00 | ||||||||||||
| 2 | 1.66 | 0.048 * | 1.37 | 0.236 | 2.18 | 0.015 * | ||||||||||
| 3 + 4 | 0.116 | 1.51 | 0.202 | 0.486 | 1.20 | 0.611 | 0.035 * | 1.16 | 0.749 | |||||||
| SUD | S + D | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||
| (complete follow-up) | U | 0.029 * | 1.70 | 0.032 * | 1.86 | 0.022 * | 0.005 * | 2.09 | 0.007 * | 1.92 | 0.024 * | 0.012 * | 2.26 | 0.015 * | 2.96 | 0.012 * |
| Salvage at | PL | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||||
| first | TL | 1.30 | 0.480 | 0.84 | 0.604 | 0.98 | 0.972 | 1.10 | 0.852 | |||||||
| recurrence | RT | 3.97 | 0.084 | - | - | 4.85 | 0.055 | 18.95 | 0.007 * | |||||||
| TLM | 0.003 * | 0.29 | 0.041 * | 0.328 | 0.49 | 0.133 | 0.007 * | 0.28 | 0.075 | 1.23 | 0.800 | |||||
| Elective | no | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| neck dissection | yes | 0.460 | 0.80 | 0.463 | 0.65 | 0.219 | 0.517 | 1.21 | 0.520 | 0.595 | 0.81 | 0.597 | 0.34 | 0.062 | ||
| Second | no | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||||
| locoregional recurrence | yes | <0.001 * | 3.59 | <0.001 * | 4.06 | <0.001 * | <0.001 * | 14.75 | <0.001 * | 33.83 | <0.001 * | |||||
| Distant | no | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||||
| metastases | yes | <0.001 * | 8.39 | <0.001 * | 7.78 | <0.001 * | <0.001 * | 14.45 | <0.001 * | 23.99 | <0.001 * | |||||
| Variable | Overall Survival | Second-Recurrence-Free Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| p-Value Log-Rank Test | Univariate Cox Regression | Multiple Cox Regression | p-Value Log-Rank Test | Univariate Cox Regression | Multiple Cox Regression | ||||||
| HR | p-Value | HR | p-Value | HR | p-Value | HR | p-Value | ||||
| SUD-c | S | 1.00 | 1.00 | ||||||||
| U | 1.60 | 0.065 | 1.79 | 0.031 * | |||||||
| D | 0.075 | 0.57 | 0.447 | 0.007 * | - | - | |||||
| SUD-60 | S | 1.00 | 1.00 | ||||||||
| U | 1.40 | 0.218 | 1.78 | 0.033 * | |||||||
| D | 0.159 | 0.32 | 0.227 | 0.008 * | - | - | |||||
| SUD-120 | S | 1.00 | 1.00 | ||||||||
| U | 1.66 | 0.053 | 1.78 | 0.034 * | |||||||
| D | 0.032 * | 0.30 | 0.237 | 0.007 * | - | - | |||||
| SUD-c | S + D | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| U | 0.029 * | 1.70 | 0.032 * | 1.86 | 0.022 * | 0.005 * | 2.09 | 0.007 * | 1.92 | 0.024 * | |
| SUD-60 | S + D | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| U | 0.105 | 1.54 | 0.108 | 1.82 | 0.040 * | 0.006 * | 2.08 | 0.007 * | 1.91 | 0.026 * | |
| SUD-120 | S + D | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| U | 0.016 * | 1.83 | 0.018 * | 1.96 | 0.016 * | 0.005 * | 2.09 | 0.007 * | 1.90 | 0.027 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Licci, G.; Locatello, L.G.; Maggiore, G.; Cozzolino, F.; Caini, S.; Gallo, O. The Same–Up–Down Staging System for Recurrent Early Glottic Cancer. Cancers 2023, 15, 598. https://doi.org/10.3390/cancers15030598
Licci G, Locatello LG, Maggiore G, Cozzolino F, Caini S, Gallo O. The Same–Up–Down Staging System for Recurrent Early Glottic Cancer. Cancers. 2023; 15(3):598. https://doi.org/10.3390/cancers15030598
Chicago/Turabian StyleLicci, Giuseppe, Luca Giovanni Locatello, Giandomenico Maggiore, Flavia Cozzolino, Saverio Caini, and Oreste Gallo. 2023. "The Same–Up–Down Staging System for Recurrent Early Glottic Cancer" Cancers 15, no. 3: 598. https://doi.org/10.3390/cancers15030598
APA StyleLicci, G., Locatello, L. G., Maggiore, G., Cozzolino, F., Caini, S., & Gallo, O. (2023). The Same–Up–Down Staging System for Recurrent Early Glottic Cancer. Cancers, 15(3), 598. https://doi.org/10.3390/cancers15030598








