Peripubertal Nutritional Prevention of Cancer-Associated Gene Expression and Phenotypes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Housing and Husbandry
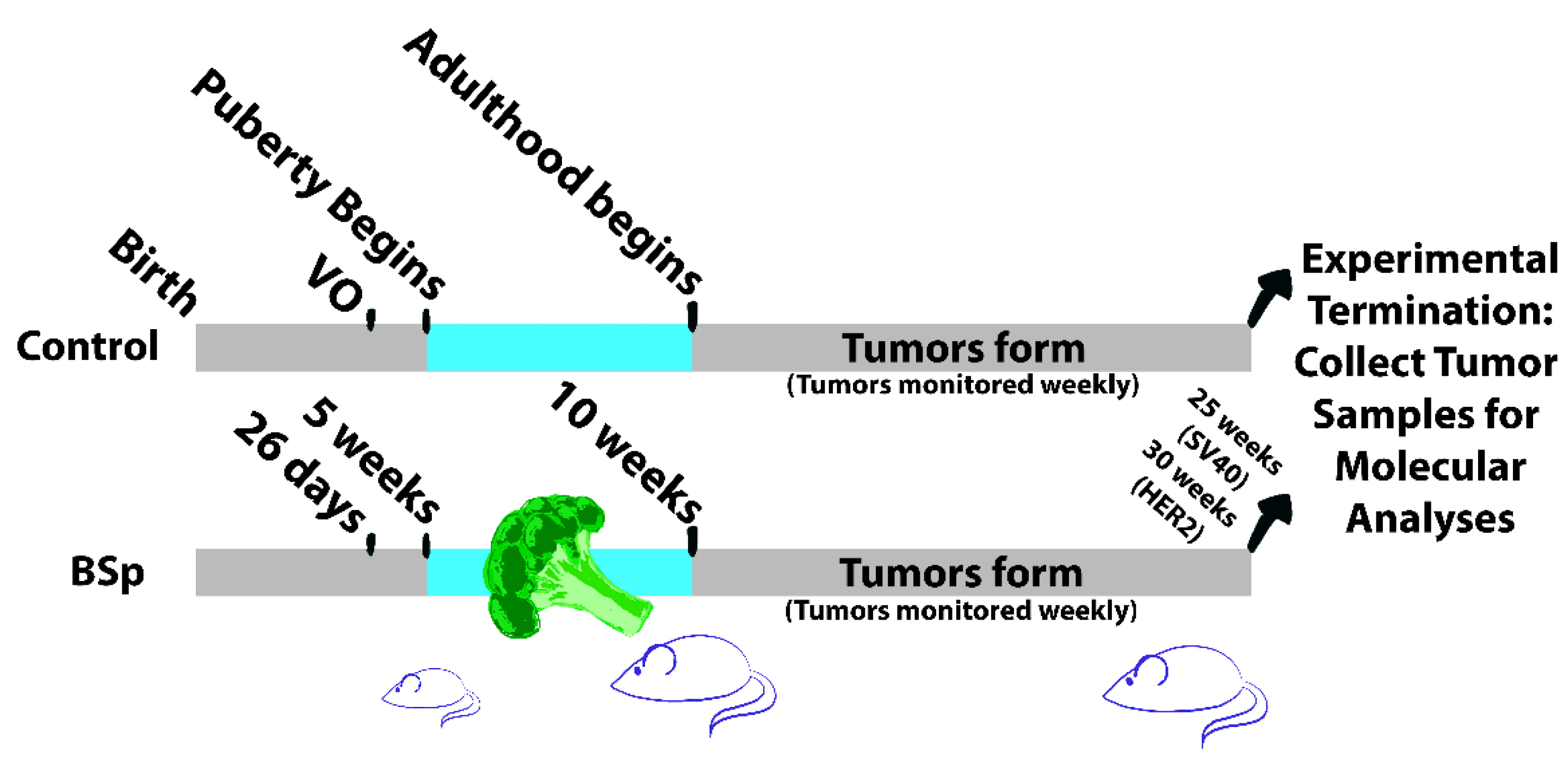
2.1.1. Animal Housing and Experimental Design
2.1.2. Transgenic Mouse Lines
2.1.3. Animal Diet
2.1.4. Tissue Collection
2.2. Nucleic Acid Extraction and Analysis
2.2.1. DNA and RNA Extraction
2.2.2. qPCR
2.2.3. Western Blotting
2.2.4. RNA Sequencing
2.2.5. Reduced Representation Bisulfite Sequencing (RRBS)
2.3. Statistical Analyses
3. Results
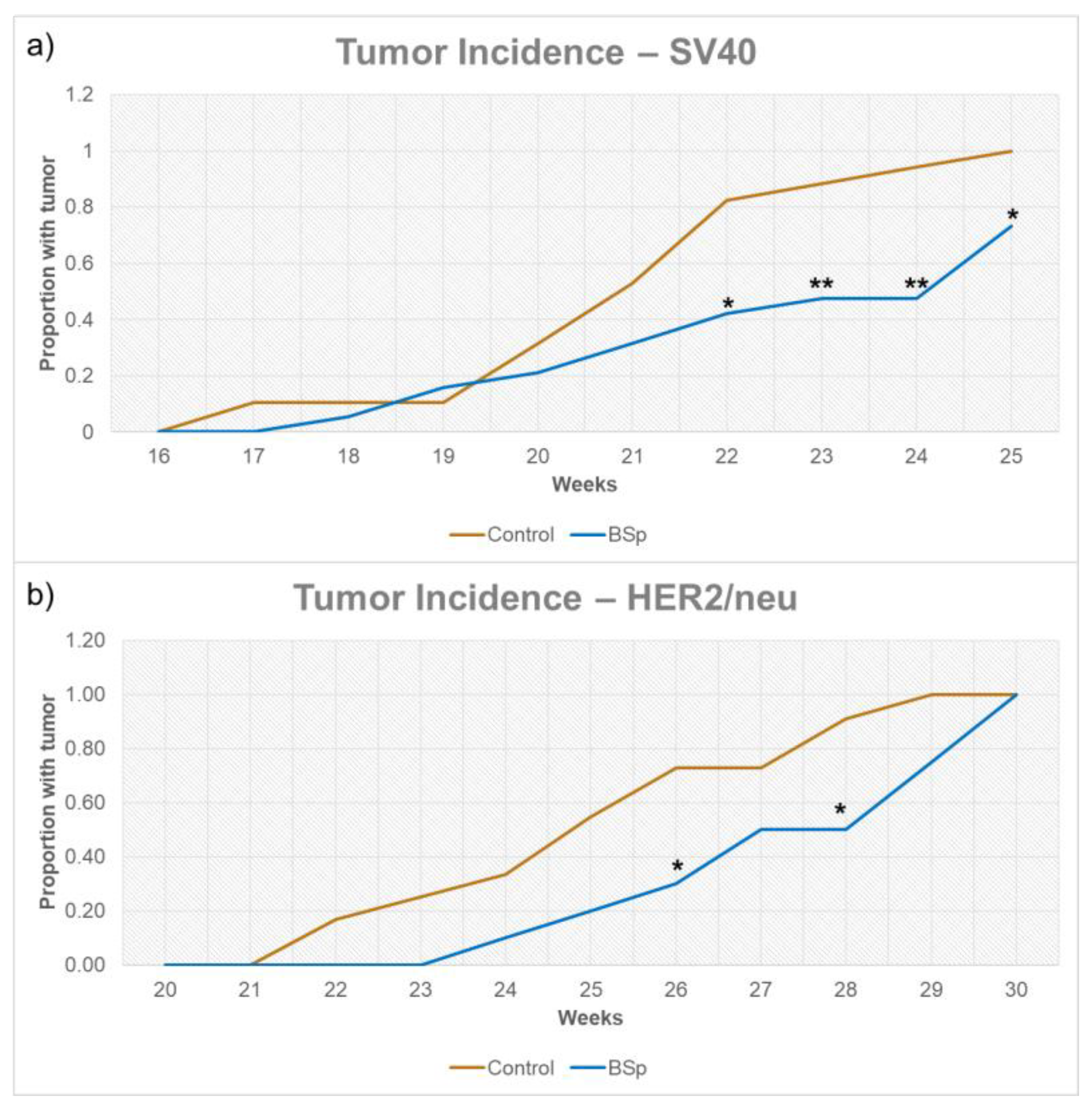
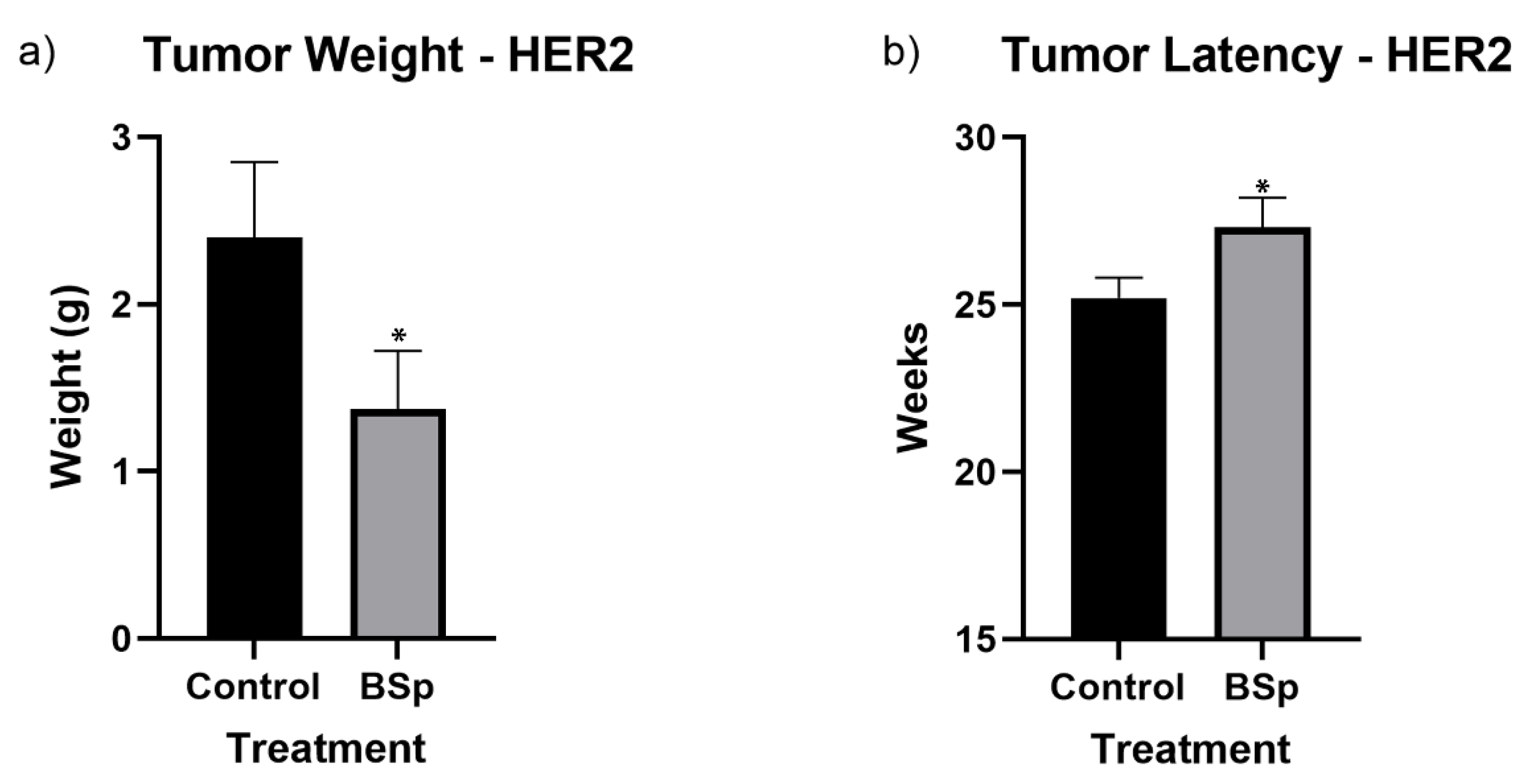
3.1. BSp Administration during the Peripubertal Period Resulted in a Decrease in Mammary Tumor Formation in Both SV40 and HER2/neu Mice
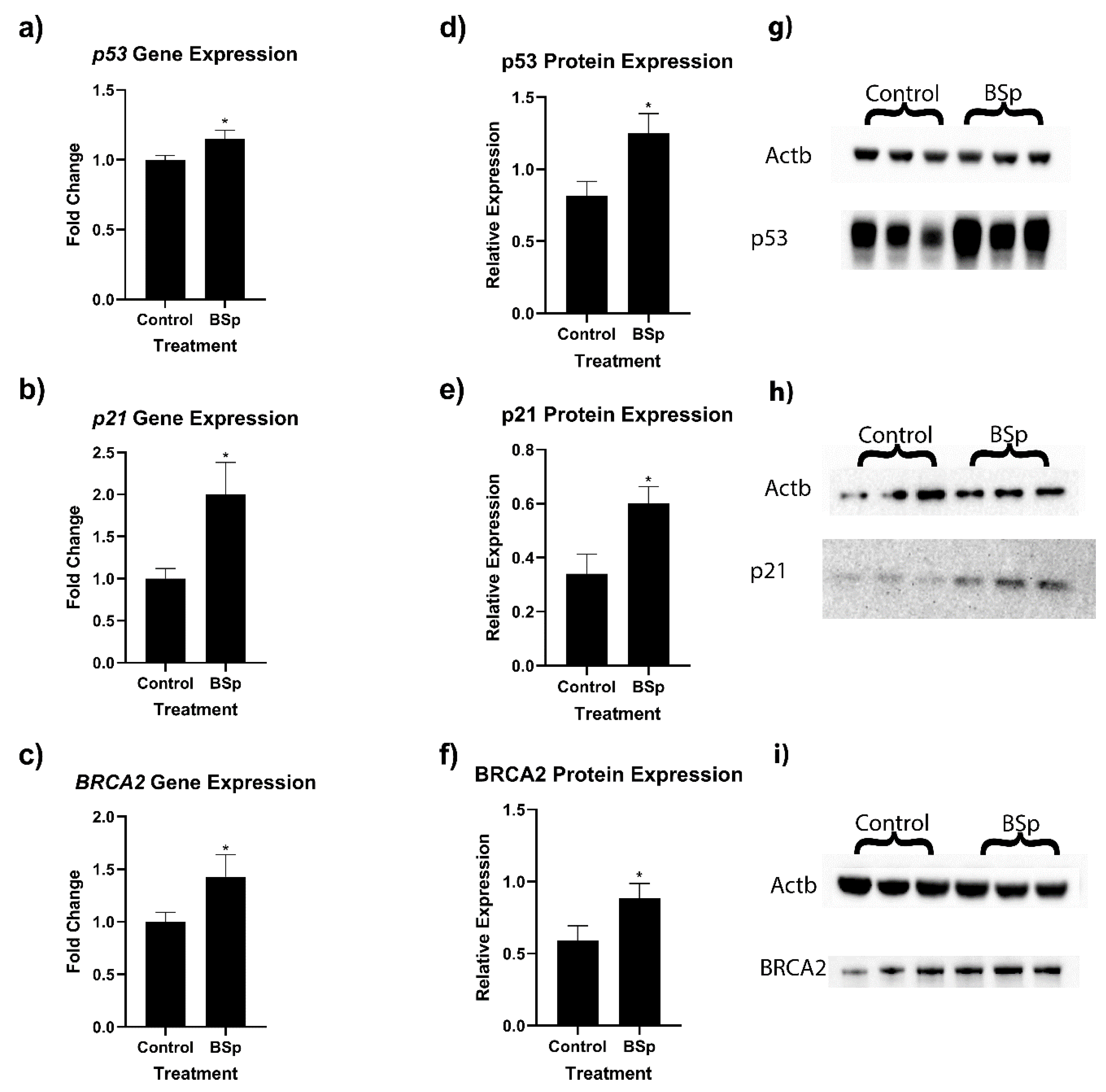
3.2. BSp Administration during the Peripubertal Period Resulted in an Increase in Gene Expression of Key Cancer-Associated Genes in HER2/neu Mice
3.3. BSp Administration during the Peripubertal Period in HER2/neu Mice Resulted in Gross Changes to Gene Expression Profiles, with Effects on Many Key Cancer-Associated Genes and Pathways
3.4. BSp Administration during the Peripubertal Period in HER2/neu Mice Had a Lasting Effect on Genome-Wide Methylation, and was Associated with Increases in Expression to the Anti-Proliferation Linked Erich4 Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute. SEER Cancer Stat Facts: Female Breast Cancer. Bethesda, MD. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 1 August 2022).
- American Cancer Society. Cancer Facts & Figures 2022; American Cancer Society: Atlanta, GA, USA, 2022. [Google Scholar]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Hormone Therapy for Breast Cancer Fact Sheet. 12 July 2022. Available online: https://www.cancer.gov/types/breast/breast-hormone-therapy-fact-sheet (accessed on 1 August 2022).
- National Cancer Institute. Treatment Clinical Trials for Breast Cancer. 2022. Available online: https://www.cancer.gov/about-cancer/treatment/clinical-trials/disease/breast-cancer/treatment (accessed on 1 August 2022).
- Collins, B.; Mackenzie, J.; Stewart, A.; Bielajew, C.; Verma, S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009, 18, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Mayo Foundation for Medical Education and Research. Chemotherapy for Breast Cancer. Mayo Clinic. 24 February 2021. Available online: https://www.mayoclinic.org/tests-procedures/chemotherapy-for-breast-cancer/about/pac-20384931 (accessed on 1 August 2022).
- Greenlee, H.; DuPont-Reyes, M.J.; Balneaves, L.G.; Carlson, L.E.; Cohen, M.R.; Deng, G.; Johnson, J.A.; Mumber, M.; Seely, D.; Zick, S.M.; et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J. Clin. 2017, 67, 194–232. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Scherer, S.; Wiskemann, J.; Steindorf, K. Return to work after breast cancer: The role of treatment-related side effects and potential impact on quality of life. Eur. J. Cancer Care 2019, 28, e13051. [Google Scholar] [CrossRef] [PubMed]
- Asthana, S.; Labani, S.; Kailash, U.; Sinha, D.N.; Mehrotra, R. Association of Smokeless Tobacco Use and Oral Cancer: A Systematic Global Review and Meta-Analysis. Nicotine Tob. Res. 2019, 21, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, R.F.; Bergmann, A.; Thuler, L.C. Alcohol consumption and risk of cancer: A systematic literature review. Asian Pac. J. Cancer Prev. 2013, 14, 4965–4972. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Breast Cancer Prevention: Tamoxifen and Raloxifene. 16 December 2021. Available online: https://www.cancer.org/cancer/breast-cancer/risk-and-prevention/tamoxifen-and-raloxifene-for-breast-cancer-prevention.html (accessed on 1 August 2022).
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef] [Green Version]
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef]
- Ullah, M.F. Sulforaphane (SFN): An Isothiocyanate in a Cancer Chemoprevention Paradigm. Medicines 2015, 2, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Krisanits, B.; Randise, J.F.; Burton, C.E.; Findlay, V.J.; Turner, D.P. Pubertal mammary development as a "susceptibility window" for breast cancer disparity. Adv. Cancer Res. 2020, 146, 57–82. [Google Scholar] [CrossRef]
- Cohn, B.A.; Cirillo, P.M.; Terry, M.B. DDT and Breast Cancer: Prospective Study of Induction Time and Susceptibility Windows. J. Natl. Cancer Inst. 2019, 111, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Biro, F.M.; Deardorff, J. Identifying opportunities for cancer prevention during preadolescence and adolescence: Puberty as a window of susceptibility. J. Adolesc. Health 2013, 52 (Suppl. 5), S15–S20, Erratum in J. Adolesc. Health 2013, 52, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, A.; Lteif, A. Development of the human breast. Semin. Plast. Surg. 2013, 27, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns Hopkins Medicine. Normal Breast Development and Changes. 20 July 2020. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/normal-breast-development-and-changes (accessed on 1 August 2022).
- Cohn, B.A.; La Merrill, M.; Krigbaum, N.Y.; Yeh, G.; Park, J.S.; Zimmermann, L.; Cirillo, P.M. DDT Exposure in Utero and Breast Cancer. J. Clin. Endocrinol. Metab. 2015, 100, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Michels, K.B.; Brody, J.G.; Byrne, C.; Chen, S.; Jerry, D.J.; Malecki, K.M.C.; Martin, M.B.; Miller, R.L.; Breast Cancer and the Environment Research Program (BCERP); et al. Environmental exposures during windows of susceptibility for breast cancer: A framework for prevention research. Breast Cancer Res. 2019, 21, 96. [Google Scholar] [CrossRef] [Green Version]
- Hilakivi-Clarke, L. Maternal exposure to diethylstilbestrol during pregnancy and increased breast cancer risk in daughters. Breast Cancer Res. 2014, 16, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodicoat, D.H.; Schoemaker, M.J.; Jones, M.E.; McFadden, E.; Griffin, J.; Ashworth, A.; Swerdlow, A.J. Timing of pubertal stages and breast cancer risk: The Breakthrough Generations Study. Breast Cancer Res. 2014, 16, R18, Erratum in Breast Cancer Res. 2020, 22, 19. [Google Scholar] [CrossRef] [Green Version]
- Apter, D. Hormonal events during female puberty in relation to breast cancer risk. Eur. J. Cancer Prev. 1996, 5, 476–482. [Google Scholar]
- Haraldsdottir, A.; Torfadottir, J.E.; Valdimarsdottir, U.A.; Adami, H.O.; Aspelund, T.; Tryggvadottir, L.; Thordardottir, M.; Birgisdottir, B.E.; Harris, T.B.; Launer, L.J.; et al. Dietary habits in adolescence and midlife and risk of breast cancer in older women. PLoS ONE 2018, 13, e0198017, Erratum in PLoS ONE 2018, 13, e0206026. [Google Scholar] [CrossRef]
- Stoll, B.A. Western diet, early puberty, and breast cancer risk. Breast Cancer Res. Treat. 1998, 49, 187–193. [Google Scholar] [CrossRef]
- Caligioni, C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009, 48, A-4I. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.F.; Felicio, L.S.; Randall, P.K.; Sims, C.; Finch, C.E. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol. Reprod. 1982, 27, 327–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brust, V.; Schindler, P.M.; Lewejohann, L. Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front. Zool. 2015, 12 (Suppl. 1), S17. [Google Scholar] [CrossRef] [Green Version]
- Maroulakou, I.G.; Anver, M.; Garrett, L.; Green, J.E. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc. Natl. Acad. Sci. USA 1994, 91, 11236–11240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guy, C.T.; Webster, M.A.; Schaller, M.; Parsons, T.J.; Cardiff, R.D.; Muller, W.J. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. USA 1992, 89, 10578–10582. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Meeran, S.M.; Tollefsbol, T.O. Combinatorial bioactive botanicals re-sensitize tamoxifen treatment in ER-negative breast cancer via epigenetic reactivation of ERα expression. Sci. Rep. 2017, 7, 9345. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, M.; Wu, H.; Li, Y.; Tollefsbol, T.O. Maternal Epigenetic Regulation Contributes to Prevention of Estrogen Receptor-negative Mammary Cancer with Broccoli Sprout Consumption. Cancer Prev. Res. 2020, 13, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Arora, I.; Li, Y.; Sharma, M.; Crowley, M.R.; Crossman, D.K.; Li, S.; Tollefsbol, T.O. Systematic integrated analyses of methylomic and transcriptomic impacts of early combined botanicals on estrogen receptor-negative mammary cancer. Sci. Rep. 2021, 11, 9481. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527, Erratum in Nat. Biotechnol. 2016, 34, 888. [Google Scholar] [CrossRef]
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Research 2015, 4, 1521. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akalin, A.; Kormaksson, M.; Li, S.; Garrett-Bakelman, F.E.; Figueroa, M.E.; Melnick, A.; Mason, C.E. methylKit: A comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 2012, 13, R87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickey, G.L.; Grant, S.W.; Dunning, J.; Siepe, M. Statistical primer: Sample size and power calculations-why, when and how? Eur. J. Cardiothorac. Surg. 2018, 54, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Buckhaults, P.; Li, S.; Tollefsbol, T. Temporal Efficacy of a Sulforaphane-Based Broccoli Sprout Diet in Prevention of Breast Cancer through Modulation of Epigenetic Mechanisms. Cancer Prev. Res. 2018, 11, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Haslam, S.Z.; Schwartz, R.C. Is there a link between a high-fat diet during puberty and breast cancer risk? Women’s Health 2011, 7, 1–3. [Google Scholar] [CrossRef]
- Linos, E.; Willett, W.C.; Cho, E.; Frazier, L. Adolescent diet in relation to breast cancer risk among premenopausal women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Farvid, M.S.; Holmes, M.D.; Chen, W.Y.; Rosner, B.A.; Tamimi, R.M.; Willett, W.C.; Eliassen, A.H. Postdiagnostic Fruit and Vegetable Consumption and Breast Cancer Survival: Prospective Analyses in the Nurses’ Health Studies. Cancer Res. 2020, 80, 5134–5143. [Google Scholar] [CrossRef]
- Diab, S.G.; Yu, Y.Y.; Hilsenbeck, S.G.; Allred, D.C.; Elledge, R.M. WAF1/CIP1 protein expression in human breast tumors. Breast Cancer Res. Treat. 1997, 43, 99–103. [Google Scholar] [CrossRef]
- Shamloo, B.; Usluer, S. p21 in Cancer Research. Cancers 2019, 11, 1178. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, J.; Zeng, B.; Yang, D.; Sun, J.; Yin, X.; Lu, M.; Qiu, Z.; Peng, W.; Xiang, T.; et al. PSMD2 regulates breast cancer cell proliferation and cell cycle progression by modulating p21 and p27 proteasomal degradation. Cancer Lett. 2018, 430, 109–122. [Google Scholar] [CrossRef]
- Duffy, M.J.; Synnott, N.C.; Crown, J. Mutant p53 in breast cancer: Potential as a therapeutic target and biomarker. Breast Cancer Res. Treat. 2018, 170, 213–219. [Google Scholar] [CrossRef]
- Mehrgou, A.; Akouchekian, M. The importance of BRCA1 and BRCA2 genes mutations in breast cancer development. Med. J. Islam. Repub. Iran 2016, 30, 369. [Google Scholar]
- Tereschenko, I.V.; Basham, V.M.; Ponder, B.A.; Pharoah, P.D. BRCA1 and BRCA2 mutations in Russian familial breast cancer. Hum. Mutat. 2002, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Bracci, M.; Ciarapica, V.; Zabaleta, M.E.; Tartaglione, M.F.; Pirozzi, S.; Giuliani, L.; Piva, F.; Valentino, M.; Ledda, C.; Rapisarda, V.; et al. BRCA1 and BRCA2 Gene Expression: Diurnal Variability and Influence of Shift Work. Cancers 2019, 11, 1146. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Liu, X.; Gao, H.; Zhang, L.; Ji, Q.; Wang, Z.; Zhou, L.; Wang, Y.; Sui, H.; Fan, Z.; et al. Overexpression of colorectal cancer oncogene CHRDL2 predicts a poor prognosis. Oncotarget 2017, 8, 11489–11506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Pan, R.; Li, H.; Zhang, W.; Ren, C.; Lu, Q.; Chen, H.; Zhang, X.; Nie, Y. CHRDL2 promotes osteosarcoma cell proliferation and metastasis through the BMP-9/PI3K/AKT pathway. Cell Biol. Int. 2021, 45, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Demoures, B.; Siegfried, G.; Khatib, A.-M. PCSK1 (Proprotein Convertase Subtilisin/Kexin Type 1) Atlas Genet Cytogenet Oncol Haematol. 1 September 2017. Available online: http://atlasgeneticsoncology.org/gene/41671/pcsk1 (accessed on 24 August 2022).
- Chou, C.L.; Chen, T.J.; Lin, C.Y.; Lee, S.W.; Wang, S.C.; Chu, S.S.; Yang, C.C. PCSK1 Overexpression in Rectal Cancer Correlates with Poor Response to Preoperative Chemoradiotherapy and Prognosis. OncoTargets Ther. 2020, 13, 3141–3150. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Liu, Y.; Xiao, W.; Zhao, N.; Zhu, C.; Yu, D.; Zhao, Y. Prognostic SLC family genes promote cell proliferation, migration, and invasion in hepatocellular carcinoma. Acta Biochim. Biophys. Sin. 2021, 53, 1065–1075. [Google Scholar] [CrossRef]
- Roeckel, N.; Woerner, S.M.; Kloor, M.; Yuan, Y.P.; Patsos, G.; Gromes, R.; Kopitz, J.; Gebert, J. High frequency of LMAN1 abnormalities in colorectal tumors with microsatellite instability. Cancer Res. 2009, 69, 292–299. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, J.; Mills, I.G.; Klevebring, D.; Liu, W.; Neiman, M.; Xu, J.; Wikström, P.; Wiklund, P.; Wiklund, F.; Egevad, L.; et al. The mitochondrial and autosomal mutation landscapes of prostate cancer. Eur. Urol. 2013, 63, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, H.; Fan, L.; Fang, M.; He, X.; Lu, B.; Pang, Z. CLEC4s as Potential Therapeutic Targets in Hepatocellular Carcinoma Microenvironment. Front. Cell Dev. Biol. 2021, 9, 681372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Hu, Z.Q.; Long, J.H.; Zhu, G.M.; Wang, Y.; Jia, Y.; Zhou, J.; Ouyang, Y.; Zeng, Z. Clinical Implications of Tumor-Infiltrating Immune Cells in Breast Cancer. J. Cancer 2019, 10, 6175–6184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, G.; Kudo, Y.; Tang, B.; Liu, T.; Jin, S.; Liu, J.; Zuo, X.; Mi, S.; Shao, W.; Ma, X.; et al. PARP6 acts as a tumor suppressor via downregulating Survivin expression in colorectal cancer. Oncotarget 2016, 7, 18812–18824. [Google Scholar] [CrossRef] [Green Version]
- Tuncel, H.; Tanaka, S.; Oka, S.; Nakai, S.; Fukutomi, R.; Okamoto, M.; Ota, T.; Kaneko, H.; Tatsuka, M.; Shimamoto, F. PARP6, a mono(ADP-ribosyl) transferase and a negative regulator of cell proliferation, is involved in colorectal cancer development. Int. J. Oncol. 2012, 41, 2079–2086. [Google Scholar] [CrossRef] [Green Version]
- Stransky, L.; Cotter, K.; Forgac, M. The Function of V-ATPases in Cancer. Physiol. Rev. 2016, 96, 1071–1091. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Yi, J.; Yang, X.; Liu, L.; Lou, X.; Zhang, Z.; Qi, H.; Wang, Z.; Zou, J.; Zhu, W.G.; et al. MDM2-mediated degradation of WRN promotes cellular senescence in a p53-independent manner. Oncogene 2019, 38, 2501–2515. [Google Scholar] [CrossRef]
- Hlaváč, V.; Václavíková, R.; Brynychová, V.; Koževnikovová, R.; Kopečková, K.; Vrána, D.; Gatěk, J.; Souček, P. Role of Genetic Variation in ABC Transporters in Breast Cancer Prognosis and Therapy Response. Int. J. Mol. Sci. 2020, 21, 9556. [Google Scholar] [CrossRef]
- Yuan, W.; Chen, S.; Li, B.; Han, X.; Meng, B.; Zou, Y.; Chang, S. The N6-methyladenosine reader protein YTHDC2 promotes gastric cancer progression via enhancing YAP mRNA translation. Transl. Oncol. 2022, 16, 101308. [Google Scholar] [CrossRef]
- Gong, L.B.; Wen, T.; Li, Z.; Xin, X.; Che, X.F.; Wang, J.; Liu, Y.P.; Qu, X.J. DYNC1I1 Promotes the Proliferation and Migration of Gastric Cancer by Up-Regulating IL-6 Expression. Front. Oncol. 2019, 9, 491, Erratum in Front. Oncol. 2022, 12, 819244. [Google Scholar] [CrossRef]
- Yoon, J.; Grinchuk, O.V.; Kannan, S.; Ang, M.J.Y.; Li, Z.; Tay, E.X.Y.; Lok, K.Z.; Lee, B.W.L.; Chuah, Y.H.; Chia, K.; et al. A chemical biology approach reveals a dependency of glioblastoma on biotin distribution. Sci. Adv. 2021, 7, eabf6033. [Google Scholar] [CrossRef] [PubMed]
- Vadlapudi, A.D.; Vadlapatla, R.K.; Pal, D.; Mitra, A.K. Biotin uptake by T47D breast cancer cells: Functional and molecular evidence of sodium-dependent multivitamin transporter (SMVT). Int. J. Pharm. 2013, 441, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Sukjoi, W.; Siritutsoontorn, S.; Chansongkrow, P.; Waiwitlikhit, S.; Polyak, S.W.; Warnnissorn, M.; Charoensawan, V.; Thuwajit, C.; Jitrapakdee, S. Overexpression of Holocarboxylase Synthetase Predicts Lymph Node Metastasis and Unfavorable Prognosis in Breast Cancer. Anticancer Res. 2020, 40, 4557–4565. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Cai, Z.; Dong, W.; Ye, J.; Cai, Z.; Han, Z.; Liang, Y.; Zhuo, Y.; Luo, Y.; et al. Down-regulation of ACACA suppresses the malignant progression of Prostate Cancer through inhibiting mitochondrial potential. J. Cancer 2021, 12, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, L.; Qiu, X.; Zeng, F.; Chen, W.; An, Y.; Hu, B.; Wu, X.; Wu, X. BLCAP arrests G₁/S checkpoint and induces apoptosis through downregulation of pRb1 in HeLa cells. Oncol. Rep. 2016, 35, 3050–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, G.S.; Rajendran, P.; Dashwood, R.H. CCAR1 and CCAR2 as gene chameleons with antagonistic duality: Preclinical, human translational, and mechanistic basis. Cancer Sci. 2020, 111, 3416–3425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mei, Y.; Fu, N.Y.; Guan, L.; Xie, W.; Liu, H.H.; Yu, C.D.; Yin, Z.; Yu, V.C.; You, H. TRIM39 regulates cell cycle progression and DNA damage responses via stabilizing p21. Proc. Natl. Acad. Sci. USA 2012, 109, 20937–20942, Erratum in Proc. Natl. Acad. Sci. USA 2013, 110, 4148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royston, K.J.; Udayakumar, N.; Lewis, K.; Tollefsbol, T.O. A Novel Combination of Withaferin A and Sulforaphane Inhibits Epigenetic Machinery, Cellular Viability and Induces Apoptosis of Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.W.; Wang, M.; Sun, N.X.; Qing, Y.; Yin, T.F.; Li, C.; Wu, D. Sulforaphane-induced epigenetic regulation of Nrf2 expression by DNA methyltransferase in human Caco-2 cells. Oncol. Lett. 2019, 18, 2639–2647. [Google Scholar] [CrossRef] [Green Version]
- Lenburg, M.E.; Liou, L.S.; Gerry, N.P.; Frampton, G.M.; Cohen, H.T.; Christman, M.F. Previously unidentified changes in renal cell carcinoma gene expression identified by parametric analysis of microarray data. BMC Cancer 2003, 3, 31. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, H.J.; Pau, G.; Dijkgraaf, G.J.; Basset-Seguin, N.; Modrusan, Z.; Januario, T.; Tsui, V.; Durham, A.B.; Dlugosz, A.A.; Haverty, P.M.; et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell 2015, 27, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.; Rosen, N.; Twik, M.; BarShir, R.; Iny Stein, T.; Dahary, D.; Fishilevich, S.; Lancet, D. The GeneCards Suite Chapter, Practical Guide to Life Science Databases. 2022, pp. 27–56. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=PPP2R5A (accessed on 24 August 2022).
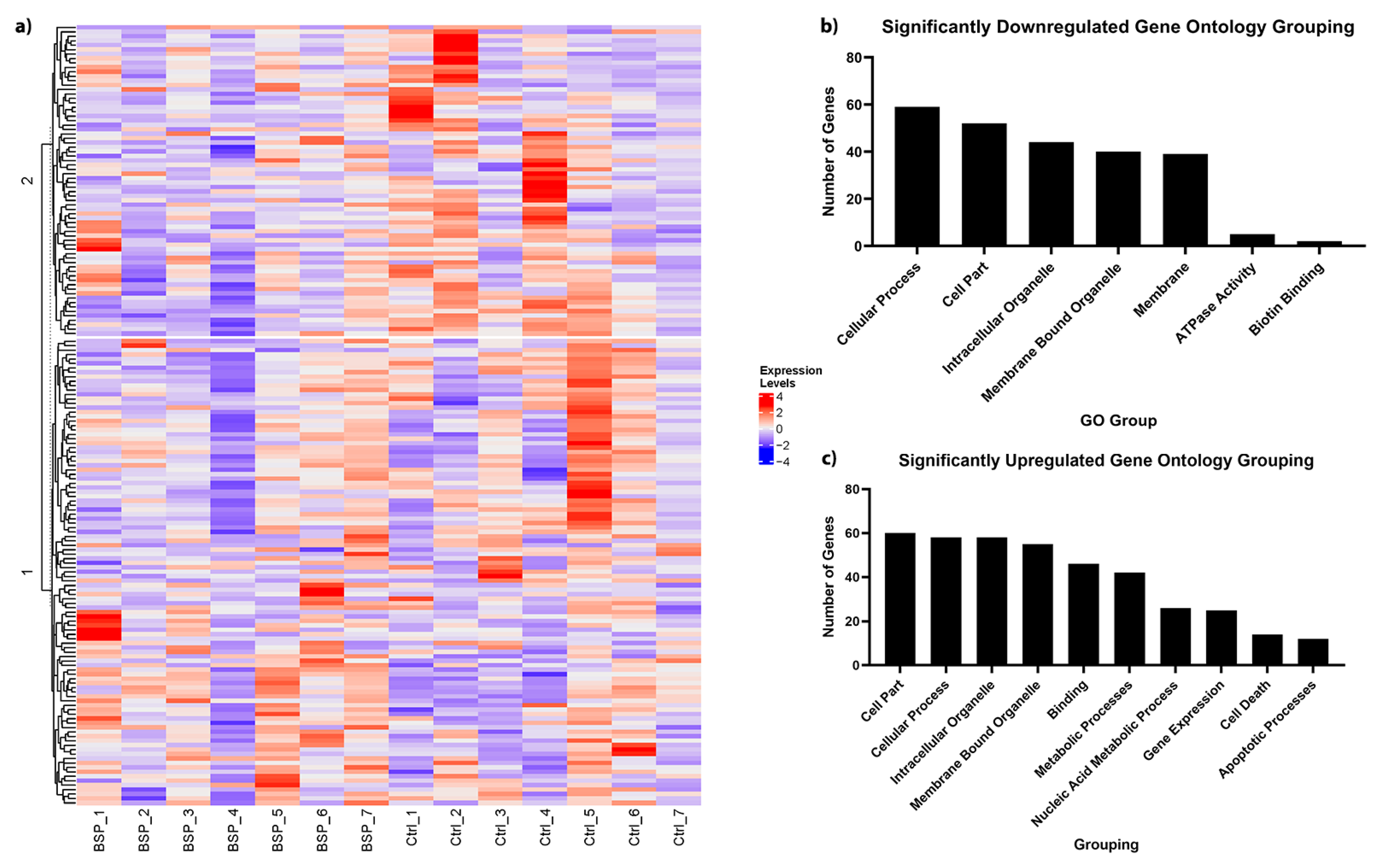
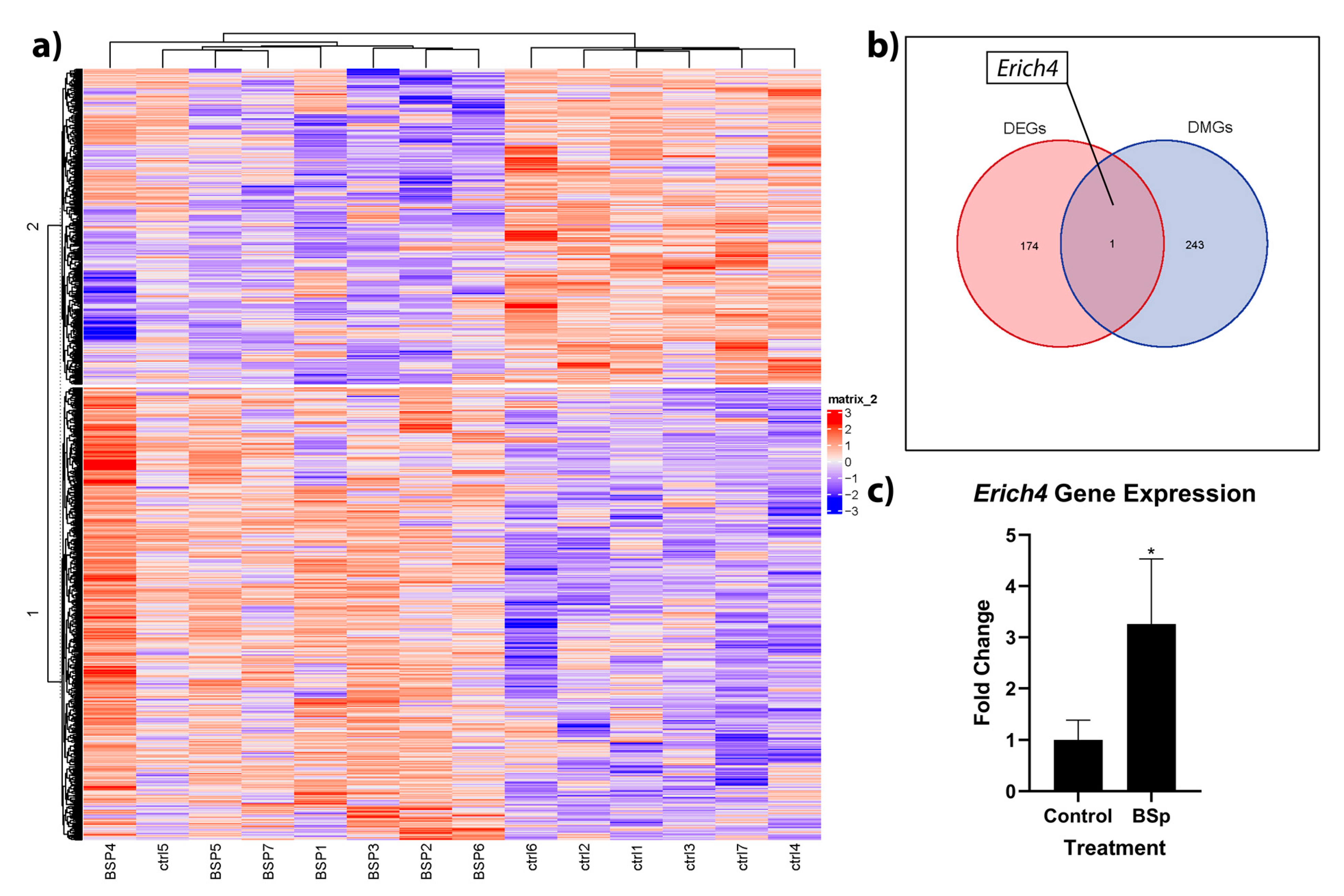
| Gene ID | Name | Log FC | Avg. Exp. | adj. p Value |
|---|---|---|---|---|
| Pcdhb1 | Protocadherin Beta 1 | −3.39609 | −4.2328285 | 0.0008754 |
| Vmn2r29 | Vomeronasal 2, receptor 29 | −2.91192 | −4.2173375 | 0.04718332 |
| Gm21962 | Predicted gene, 21962 | −2.86629 | −3.8732011 | 0.02353836 |
| Gm28778 | Predicted gene 28778 | −2.681 | −4.5447846 | 0.0008754 |
| Mroh8 | Maestro Heat Like Repeat Family Member 8 | −2.55521 | −4.6539474 | 0.00107869 |
| Olfr798 | Olfactory receptor 798 | −2.33029 | −4.2038604 | 0.00553995 |
| Prss39 | Protease, serine 39 | −2.30801 | −4.0433775 | 0.04061123 |
| Olfr837 | Olfactory receptor 837 | −2.27784 | −4.3915511 | 0.00413192 |
| Olfr592 | Olfactory receptor 592 | −2.21951 | −4.1896308 | 0.00413192 |
| Olfr1181 | Olfactory receptor 1181 | −2.21231 | −4.669899 | 0.04061123 |
| Neurod6 | Neuronal Differentiation 6 | −2.12643 | −4.5476479 | 0.00802338 |
| Gm2897 | Predicted gene 2897 | −2.11836 | −4.4376761 | 0.04726518 |
| Slc51b | Solute Carrier Family 51 Subunit Beta | −2.11418 | −4.6957968 | 0.02815029 |
| Vmn1r210 | Vomeronasal 1 receptor 210 | −2.11002 | −4.4579116 | 0.02274392 |
| Olfr1228 | Olfactory receptor 1228 | −2.06907 | −4.8911034 | 0.00490394 |
| Pcsk1 | Proprotein convertase subtilisin/kexin type 1 | −1.95999 | −2.733677 | 0.00720816 |
| Srarp | Steroid Receptor Associated And Regulated Protein | −1.9012 | −4.7484372 | 0.04726518 |
| Hoxc10 | Homeobox C10 | −1.89364 | −4.2726565 | 0.01709571 |
| Chrdl2 | Chordin Like 2 | −1.799 | −4.4780566 | 0.04187643 |
| Slc6a21 | Solute carrier family 6 member 21 | −1.77935 | −5.1522347 | 0.02831963 |
| Gene ID | Name | Log FC | Avg. Exp. | adj. p value |
|---|---|---|---|---|
| Gm2237 | Predicted gene 2237 | 3.158839 | −4.602845 | 0.0008754 |
| Lman1l | Lectin, Mannose Binding 1 Like | 2.951392 | −4.1578837 | 0.00107869 |
| Clec4e | C-Type Lectin Domain Family 4 Member E | 2.886333 | −3.9958048 | 0.01925175 |
| Kif19b | Kinesin Family Member 19 | 2.583289 | −4.5418484 | 0.0008754 |
| Armc12 | Armadillo Repeat Containing 12 | 2.483762 | −4.2946274 | 0.00113373 |
| Tmem200c | Transmembrane Protein 200C | 2.018997 | −4.6153797 | 0.02619552 |
| Olfr361 | Olfactory receptor 361 | 1.983424 | −4.2906929 | 0.04288585 |
| Sncb | Synuclein Beta | 1.923413 | −4.9915667 | 0.01746493 |
| Tmem59l | Transmembrane Protein 59 Like | 1.858611 | −4.7316851 | 0.02557444 |
| Gm11168 | Predicted gene 11168 | 1.802597 | −4.6737187 | 0.03114816 |
| Erich4 | Glutamate Rich 4 | 1.791582 | −4.6379376 | 0.04726518 |
| Treml1 | Triggering Receptor Expressed On Myeloid Cells Like 1 | 1.710026 | −4.8032762 | 0.01326325 |
| Olfr922 | Olfactory receptor 922 | 1.645667 | −4.8070893 | 0.01625371 |
| Mmp13 | Matrix metallopeptidase 13 | 1.119203 | 1.333663 | 0.0463051 |
| Prmt6 | Protein Arginine Methyltransferase 6 | 1.092539 | 3.0074275 | 0.00815202 |
| Tspan4 | Tetraspanin 4 | 0.904403 | 5.6742821 | 0.04244361 |
| Sphk1 | Sphingosine Kinase 1 | 0.898623 | 4.7451435 | 0.01787144 |
| Parp6 | Poly(ADP-Ribose) Polymerase Family Member 6 | 0.806976 | 2.9441413 | 0.0113267 |
| Adam8 | ADAM metallopeptidase domain 8 | 0.779123 | 3.0242021 | 0.04726518 |
| Coq10b | Coenzyme Q10B | 0.622019 | 4.0602847 | 0.02931014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brane, A.; Arora, I.; Tollefsbol, T.O. Peripubertal Nutritional Prevention of Cancer-Associated Gene Expression and Phenotypes. Cancers 2023, 15, 674. https://doi.org/10.3390/cancers15030674
Brane A, Arora I, Tollefsbol TO. Peripubertal Nutritional Prevention of Cancer-Associated Gene Expression and Phenotypes. Cancers. 2023; 15(3):674. https://doi.org/10.3390/cancers15030674
Chicago/Turabian StyleBrane, Andrew, Itika Arora, and Trygve O. Tollefsbol. 2023. "Peripubertal Nutritional Prevention of Cancer-Associated Gene Expression and Phenotypes" Cancers 15, no. 3: 674. https://doi.org/10.3390/cancers15030674
APA StyleBrane, A., Arora, I., & Tollefsbol, T. O. (2023). Peripubertal Nutritional Prevention of Cancer-Associated Gene Expression and Phenotypes. Cancers, 15(3), 674. https://doi.org/10.3390/cancers15030674






