C-Reactive Protein and Lymphocyte-to-Monocyte Ratio Predict Recurrence in Stage III Melanoma Patients with Microscopic Sentinel Lymph Node Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
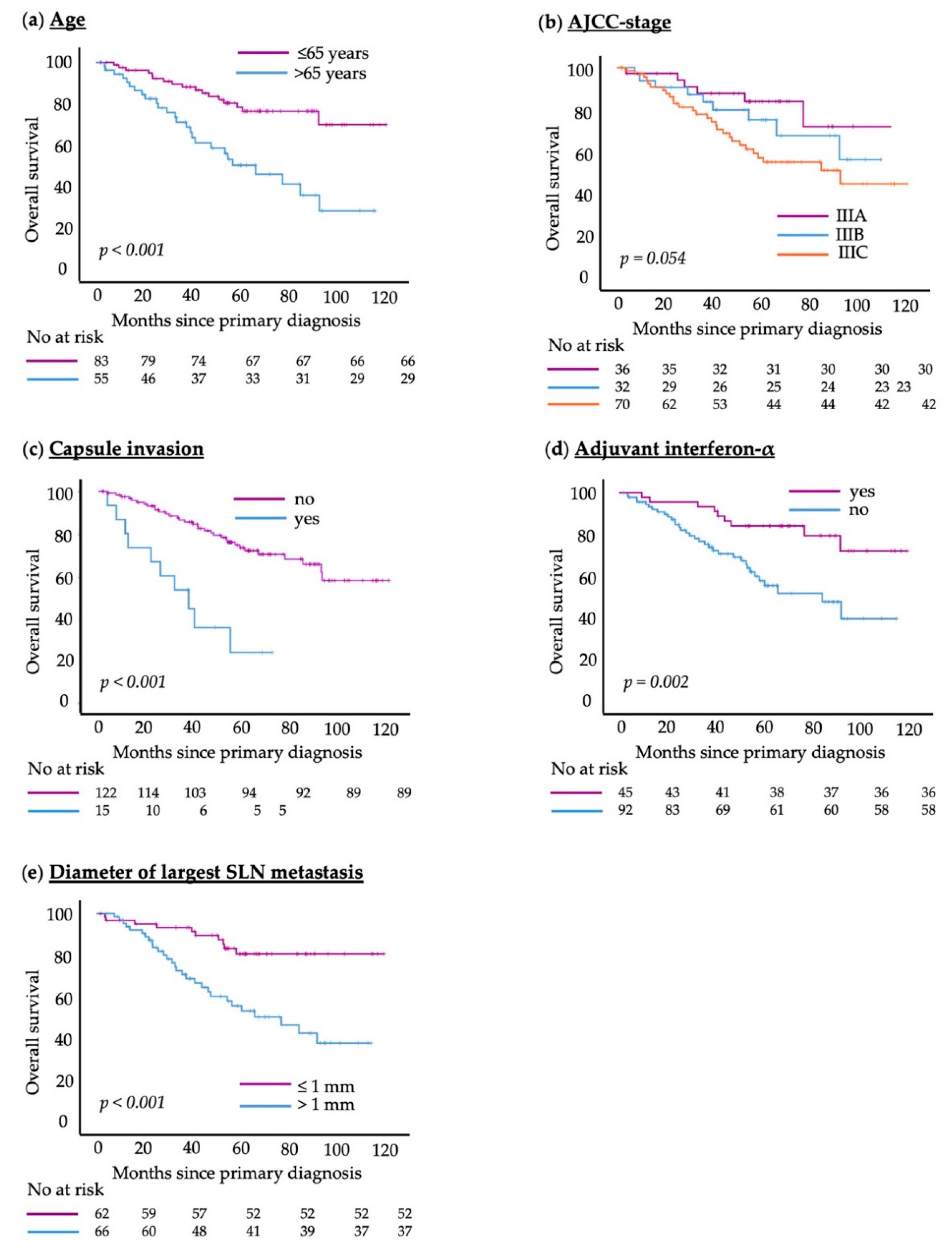
3.1. Patient and Disease Characteristics
3.2. Exploratory Analysis
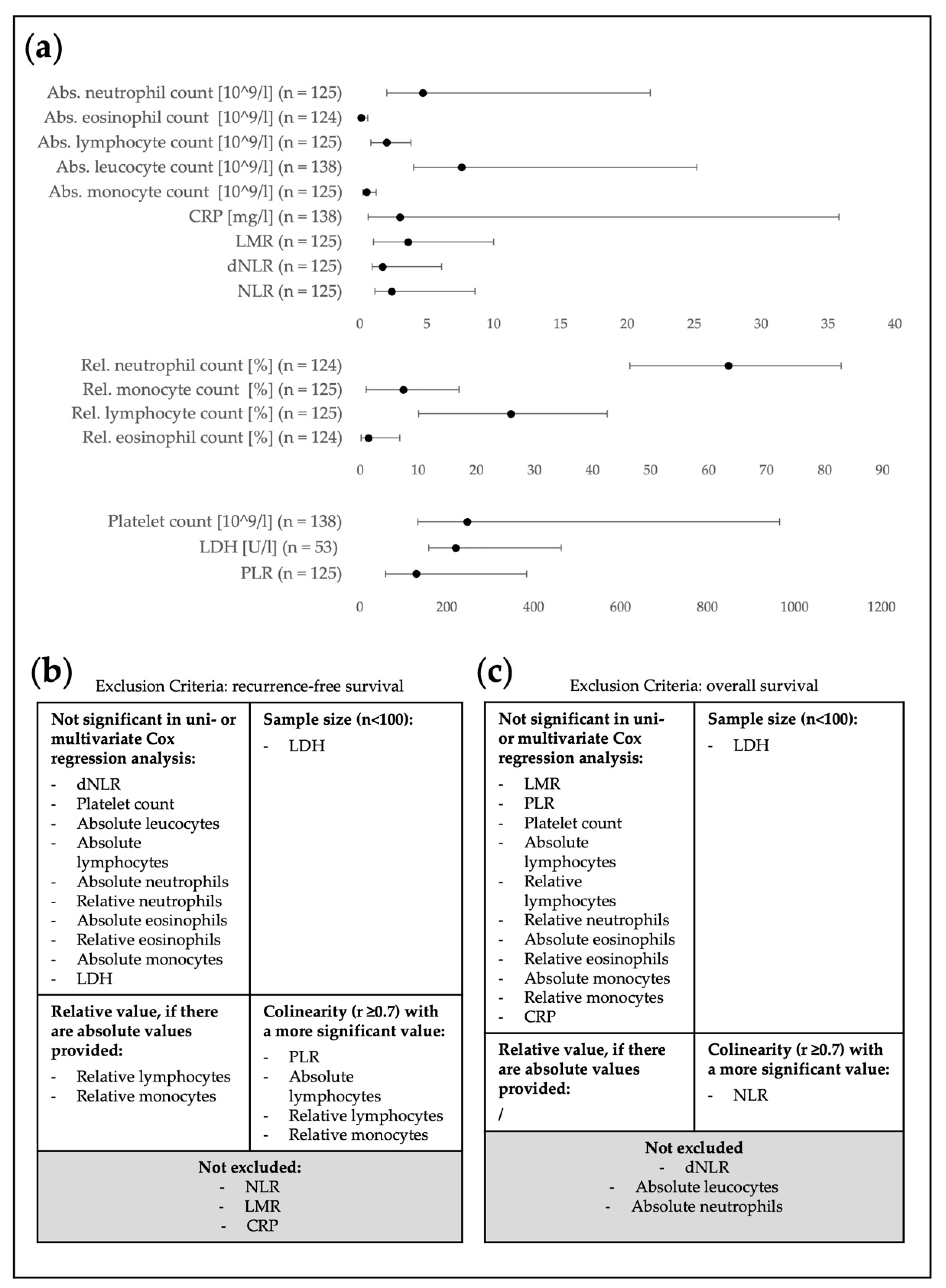
3.3. Pre-Selection of Relevant Blood Parameters
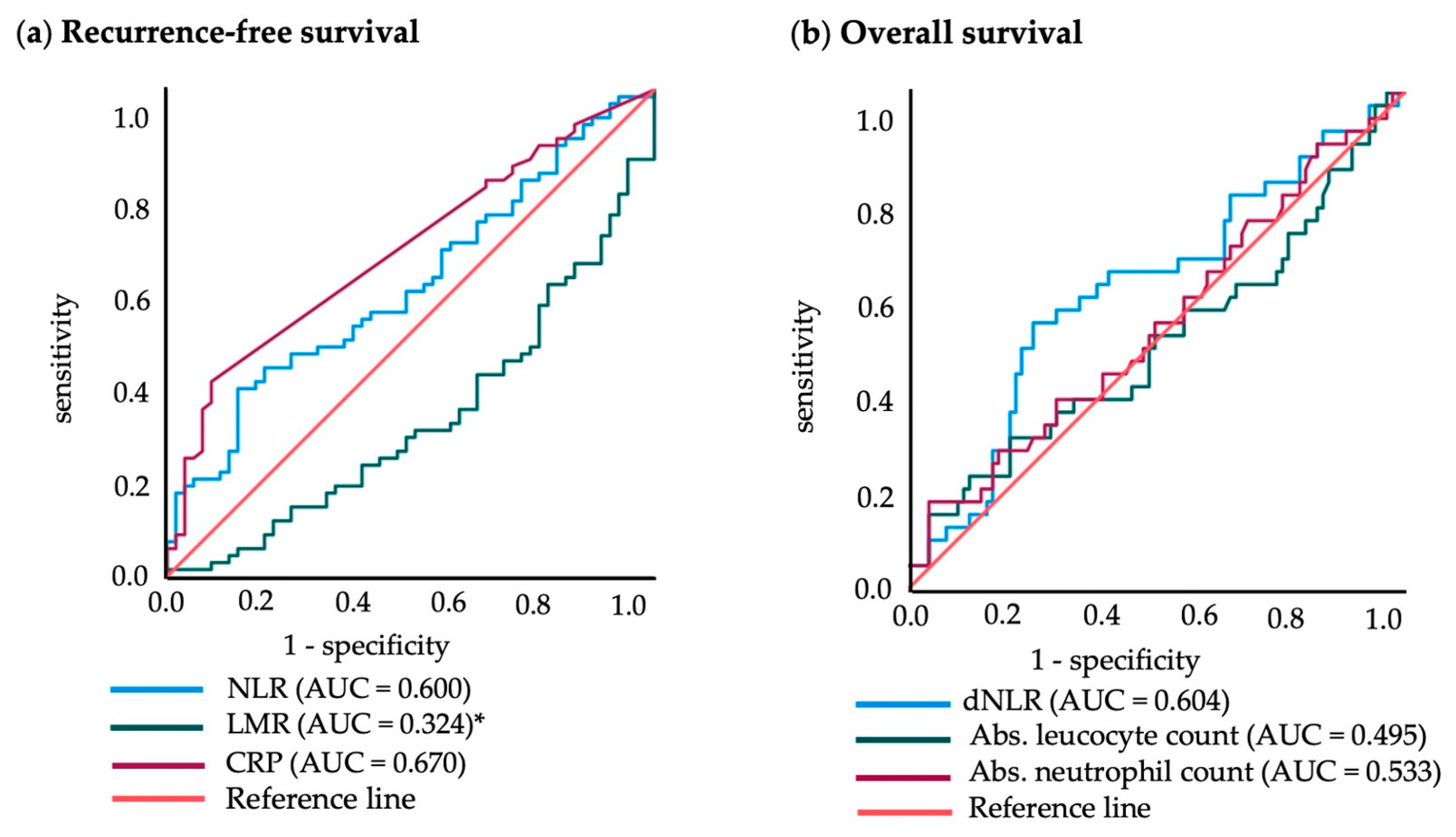
3.4. Cut-off Points for Blood Values
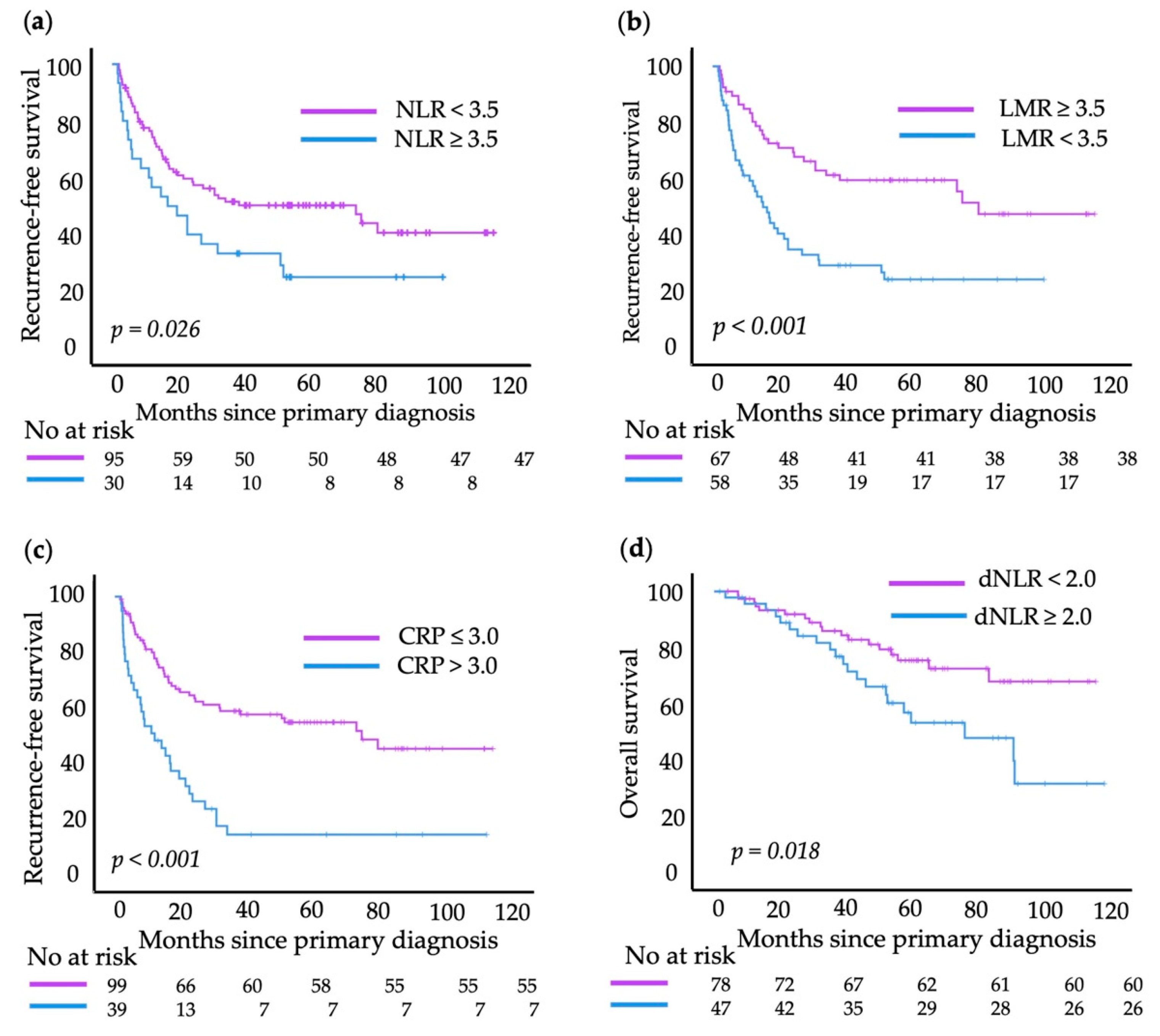
3.5. Hierarchical Multivariate Cox Regression Analysis
3.6. Hierarchical Multivariate Cox Regression Analysis
3.7. Combination of Dichotomized Blood Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Falk Delgado, A.; Zommorodi, S.; Falk Delgado, A. Sentinel Lymph Node Biopsy and Complete Lymph Node Dissection for Melanoma. Curr. Oncol. Rep. 2019, 21, 54. [Google Scholar] [CrossRef]
- Leiter, U.; Stadler, R.; Mauch, C.; Hohenberger, W.; Brockmeyer, N.H.; Berking, C.; Sunderkötter, C.; Kaatz, M.; Schatton, K.; Lehmann, P.; et al. Final Analysis of DeCOG-SLT Trial: No Survival Benefit for Complete Lymph Node Dissection in Patients with Melanoma with Positive Sentinel Node. J. Clin. Oncol. 2019, 37, 3000–3008. [Google Scholar] [CrossRef]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Chiarion-Sileni, V.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef]
- Liu, J.; Lin, P.C.; Zhou, B.P. Inflammation fuels tumor progress and metastasis. Curr. Pharm. Des. 2015, 21, 3032–3040. [Google Scholar] [CrossRef] [PubMed]
- Mirili, C.; Yilmaz, A.; Demirkan, S.; Bilici, M.; Basol Tekin, S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int. J. Clin. Oncol. 2019, 24, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, H.; Zhou, J.; Wang, B.; Chen, Y.; Kong, Y.; Xie, X.; Wang, X.; Fei, R.; Wei, L.; et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 141. [Google Scholar] [CrossRef]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef]
- Wagner, N.B.; Luttermann, F.; Gassenmaier, M.; Forschner, A.; Leiter, U.; Garbe, C.; Eigentler, T.K. Absolute and relative differential blood count predicts survival of AJCC stage I-II melanoma patients scheduled for sentinel lymph node biopsy. Australas J. Dermatol. 2020, 61, e310–e318. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.V.; Keeble, C.; Lo, M.C.I.; Thornton, O.; Peach, H.; Moncrieff, M.D.S.; Dewar, D.J.; Wade, R.G. The neutrophil-lymphocyte ratio and locoregional melanoma: A multicentre cohort study. Cancer Immunol. Immunother. 2020, 69, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Yang, Y.; Zhang, T.; Ma, X. Prognostic Value of Peripheral Inflammatory Markers in Preoperative Mucosal Melanoma: A Multicenter Retrospective Study. Front. Oncol. 2019, 9, 995. [Google Scholar] [CrossRef]
- Wade, R.G.; Robinson, A.V.; Lo, M.C.I.; Keeble, C.; Marples, M.; Dewar, D.J.; Moncrieff, M.D.S.; Peach, H. Baseline Neutrophil-Lymphocyte and Platelet-Lymphocyte Ratios as Biomarkers of Survival in Cutaneous Melanoma: A Multicenter Cohort Study. Ann. Surg. Oncol. 2018, 25, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Lino-Silva, L.S.; Salcedo-Hernandez, R.A.; Garcia-Perez, L.; Meneses-Garcia, A.; Zepeda-Najar, C. Basal neutrophil-to-lymphocyte ratio is associated with overall survival in melanoma. Melanoma Res. 2017, 27, 140–144. [Google Scholar] [CrossRef]
- Failing, J.J.; Yan, Y.; Porrata, L.F.; Markovic, S.N. Lymphocyte-to-monocyte ratio is associated with survival in pembrolizumab-treated metastatic melanoma patients. Melanoma Res. 2017, 27, 596–600. [Google Scholar] [CrossRef]
- Kopecky, J.; Kubecek, O.; Priester, P.; Vosmikova, H.; Cermakova, E.; Kyllarova, A. Prognostic value of blood cell count-derived ratios in BRAF-mutated metastatic melanoma. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub. 2022, 166, 393–404. [Google Scholar] [CrossRef]
- Zhang, F.; Gong, W. Prognostic Value of the Platelet-to-Lymphocyte Ratio in Patients with Melanoma: A Meta-Analysis. Front. Oncol. 2020, 10, 1116. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Ascierto, P.A.; Pigozzo, J.; Del Vecchio, M.; Maio, M.; Antonini Cappellini, G.C.; Guidoboni, M.; Queirolo, P.; Savoia, P.; Mandalà, M.; et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: Prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann. Oncol. 2018, 29, 524. [Google Scholar] [CrossRef]
- Capone, M.; Giannarelli, D.; Mallardo, D.; Madonna, G.; Festino, L.; Grimaldi, A.M.; Vanella, V.; Simeone, E.; Paone, M.; Palmieri, G.; et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J. Immunother. Cancer 2018, 6, 74. [Google Scholar] [CrossRef]
- Fang, S.; Wang, Y.; Sui, D.; Liu, H.; Ross, M.I.; Gershenwald, J.E.; Cormier, J.N.; Royal, R.E.; Lucci, A.; Schacherer, C.W.; et al. C-reactive protein as a marker of melanoma progression. J. Clin. Oncol. 2015, 33, 1389–1396. [Google Scholar] [CrossRef]
- Laino, A.S.; Woods, D.; Vassallo, M.; Qian, X.; Tang, H.; Wind-Rotolo, M.; Weber, J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 2020, 8, e000842. [Google Scholar] [CrossRef]
- Bartlett, E.K.; Flynn, J.R.; Panageas, K.S.; Ferraro, R.A.; Sta Cruz, J.M.; Postow, M.A.; Coit, D.G.; Ariyan, C.E. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer 2020, 126, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R.; Twedt, D.; Hellman, R. Absolute versus proportional differential leucocyte counts. Clin. Lab. Haematol. 1995, 17, 115–123. [Google Scholar] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Pylaeva, E.; Korschunow, G.; Spyra, I.; Bordbari, S.; Siakaeva, E.; Ozel, I.; Domnich, M.; Squire, A.; Hasenberg, A.; Thangavelu, K.; et al. During early stages of cancer, neutrophils initiate anti-tumor immune responses in tumor-draining lymph nodes. Cell Rep. 2022, 40, 111171. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Geuna, E.; Michalarea, V.; Guardascione, M.; Naumann, U.; Lorente, D.; Kaye, S.B.; de Bono, J.S. The neutrophil-lymphocyte ratio and its utilisation for the management of cancer patients in early clinical trials. Br. J. Cancer 2015, 112, 1157–1165. [Google Scholar] [CrossRef]
- Ma, J.; Kuzman, J.; Ray, A.; Lawson, B.O.; Khong, B.; Xuan, S.; Hahn, A.W.; Khong, H.T. Neutrophil-to-lymphocyte Ratio (NLR) as a predictor for recurrence in patients with stage III melanoma. Sci. Rep. 2018, 8, 4044. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.J.; McMillan, D.C.; Morrison, D.S.; Fletcher, C.D.; Horgan, P.G.; Clarke, S.J. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br. J. Cancer 2012, 107, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Schäfer, N.; Bode, C.; Borger, V.; Eichhorn, L.; Giordano, F.A.; Güresir, E.; Heimann, M.; Ko, Y.D.; Lehmann, F.; et al. Prognostic Value of Preoperative Inflammatory Markers in Melanoma Patients with Brain Metastases. J. Clin. Med. 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Iacono, D.; Basile, D.; Gerratana, L.; Vitale, M.G.; Pelizzari, G.; Cinausero, M.; Poletto, E.; Puglisi, F.; Fasola, G.; Minisini, A.M. Prognostic role of disease extent and lymphocyte-monocyte ratio in advanced melanoma. Melanoma Res. 2019, 29, 510–515. [Google Scholar] [CrossRef]
- Gandini, S.; Ferrucci, P.F.; Botteri, E.; Tosti, G.; Barberis, M.; Pala, L.; Battaglia, A.; Clerici, A.; Spadola, G.; Cocorocchio, E.; et al. Prognostic significance of hematological profiles in melanoma patients. Int. J. Cancer 2016, 139, 1618–1625. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics and Outcome | All Patients |
|---|---|
| n = 138 (100%) | |
| Age | |
| ≤65 years | 83 (60.1) |
| >65 years | 55 (39.9) |
| Median in years (IQR) * | 59 (45.0–72.0) |
| Gender | |
| Male | 71 (51,4) |
| Female | 67 (48.6) |
| Adjuvant interferon-α | |
| No | 92 (66.7) |
| Yes | 45 (32.6) |
| Unknown | 1 (0.7) |
| AJCC-stage | |
| IIIA | 36 (26.1) |
| IIIB | 32 (23.2) |
| IIIC | 70 (50.7) |
| IIID | / |
| Recurrence (locoregional and/or distant) | |
| No | 62 (44.9) |
| Yes | 76 (55.1) |
| -only locoregional | 15 (19.7) |
| -locoregional and distant | 61 (80.2) |
| Death | |
| No | 95 (68.8) |
| Yes | 43 (31.2) |
| Recurrence-free survival | |
| Median in months (IQR) | 23.3 (8.0–56.9) |
| Overall survival ** | |
| Median in months (IQR) | 53.4 (28.5–70.3) |
| Model 1: RFS | Model 2: RFS | Model 3: RFS | ||||
|---|---|---|---|---|---|---|
| Variable (reference bold) | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value |
| Age | ||||||
| (continuous) | 1.330 (0.974–1.817) | 0.073 | 1.286 (0.943–1.752) | 0.111 | 1.402 (1.054–1.865) | 0.020 |
| AJCC-stage | ||||||
| (A: n = 35) | ||||||
| (B: n = 28) | ||||||
| (C: n = 63) | ||||||
| IIIB vs. IIIA | 2.143 (0.885–5.188) | 0.091 | 2.151 (0.890–5.197) | 0.089 | 2.077 (0.888–4.857) | 0.092 |
| IIIC vs. IIIA | 3.461 (1.599–7.489) | 0.005 | 3.410 (1.575–7.384) | 0.002 | 3.707 (1.784–7.703) | <0.001 |
| IIID vs. IIIA | / | / | / | / | / | / |
| Capsule invasion | ||||||
| (n = 14; n = 112) | ||||||
| (no vs. yes) | 0.798 (0.398–1.356) | 0.568 | 0.808 (0.371–1.762) | 0.593 | 1.027 (0.485–2.176) | 0.944 |
| Adjuvant interferon-α | ||||||
| (n = 84; n = 42) | ||||||
| (yes vs. no) | 0.735 (0.398–1.356) | 0.324 | 0.763 (0.412–1.412) | 0.389 | 0.779 (0.434–1.399) | 0.403 |
| Size of largest SLN metastasis | ||||||
| (continuous) | 1.053 (0.898–1.234) | 0.524 | 1.028 (0.876–1.207) | 0.734 | 1.129 (0.974–1.307) | 0.107 |
| NLR | ||||||
| (n = 27; n = 87) | ||||||
| (≥3.5 vs. <3.5) | 1.512 (0.845–2.705) | 0.164 | ||||
| LMR | ||||||
| (n = 51; n = 63) | ||||||
| (<3.5 vs. ≥3.5) | 2.198 (1.301–3.715) | 0.003 | ||||
| CRP | ||||||
| (n = 92; n = 34) | ||||||
| (>3.0 vs. ≤3.0) | 3.355 (2.017–5.582) | <0.001 | ||||
| Model 4: OS | ||
|---|---|---|
| Variable (Reference bold) | HR (95%CI) | p-Value |
| Age | ||
| (continuous) | 1.438 (0.935–2.209) | 0.098 |
| AJCC-stage | ||
| (n = 35, n = 28, n = 63, n = 0) | ||
| IIIB vs. IIIA | 2.311 (0.699–7.647) | 0.170 |
| IIIC vs. IIIA | 2.199 (0.730–6.624) | 0.161 |
| IIID vs. IIIA | / | / |
| Capsule invasion | ||
| (n = 14; n = 112) | ||
| (no vs. yes) | 0.482 (0.196–1.1885) | 0.113 |
| Adjuvant interferon-α | ||
| (n = 84; n = 42) | ||
| (yes vs. no) | 0.404 (0.161–1.014) | 0.053 |
| Size of biggest SLN metastasis (continuous) | 1.272 (1.071–1.511) | 0.006 |
| dNLR | ||
| (n = 70; n = 44) | ||
| (≥2.0 vs. <2.0) | 2.428 (1.186–4.968) | 0.015 |
| Blood Values | Univariate Cox Regression Analysis n HR (95%CI) p-Value | Multivariate Cox Regression Analysis * n HR (95%CI) p-Value | ||
|---|---|---|---|---|
| Outcome: RFS | ||||
| Model 1: NLR | continuous (n = 125) | 1.236 (1.064–1.437) 0.006 | continuous (n = 114) | 1.340 (1.050–1.711) 0.019 |
| cut-off 3.5 (n = 30; n = 95) (≥3.5 vs. <3.5) | 1.761 (1.063–2.919) 0.028 | cut-off 3.5 (n = 27; n = 87) (≥3.5 vs. <3.5) | 1.512 (0.845–2.705) 0.164 | |
| Model 2: LMR | continuous (n = 125) | 0.689 (0.564–0.841) <0.001 | continuous (n = 114) | 0.608 (0.422–0.877) 0.008 |
| cut-off 3.5 (n = 58; n = 67) (<3.5 vs. ≥3.5) | 2.433 (1.505–3.934) <0.001 | cut-off 3.5 (n = 51; n = 63) (<3.5 vs. ≥3.5) | 2.198 (1.301–3.715) 0.003 | |
| Model 3: CRP | continuous (n = 138) | 1.065 (1.026–1.105) <0.001 | continuous (n = 126) | 1.457 (1.214–1.747) <0.001 |
| cut-off 3.0 (n = 39 vs. 99) (>3.0 vs. ≤3.0) | 2.841 (1.791–4.508) <0.001 | cut-off 3.0 (n = 34 vs. 92) (>3.0 vs. ≤3.0) | 3.355 (2.017–5.582) <0.001 | |
| Outcome: OS | ||||
| Model 4: dNLR | continuous (n = 124) | 1.287 (0.945–1.753) 0.109 | continuous (n = 114) | 1.410 (1.024–1.942) 0.035 |
| cut-off 2.0 (n = 47; n = 78) (≥2.0 vs. <2.0) | 2.102 (1.119–3.948) 0.021 | cut-off 2.0 (n = 44; n = 70) (≥2.0 vs. <2.0) | 2.428 (1.186–4.968) 0.015 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schildbach, V.A.S.; Horn, S.; Hidalgo-Gadea, G.; Johannis, W.; Mauch, C.; Franklin, C. C-Reactive Protein and Lymphocyte-to-Monocyte Ratio Predict Recurrence in Stage III Melanoma Patients with Microscopic Sentinel Lymph Node Metastasis. Cancers 2023, 15, 702. https://doi.org/10.3390/cancers15030702
Schildbach VAS, Horn S, Hidalgo-Gadea G, Johannis W, Mauch C, Franklin C. C-Reactive Protein and Lymphocyte-to-Monocyte Ratio Predict Recurrence in Stage III Melanoma Patients with Microscopic Sentinel Lymph Node Metastasis. Cancers. 2023; 15(3):702. https://doi.org/10.3390/cancers15030702
Chicago/Turabian StyleSchildbach, Viktoria Anna Sophie, Susanne Horn, Guillermo Hidalgo-Gadea, Wibke Johannis, Cornelia Mauch, and Cindy Franklin. 2023. "C-Reactive Protein and Lymphocyte-to-Monocyte Ratio Predict Recurrence in Stage III Melanoma Patients with Microscopic Sentinel Lymph Node Metastasis" Cancers 15, no. 3: 702. https://doi.org/10.3390/cancers15030702
APA StyleSchildbach, V. A. S., Horn, S., Hidalgo-Gadea, G., Johannis, W., Mauch, C., & Franklin, C. (2023). C-Reactive Protein and Lymphocyte-to-Monocyte Ratio Predict Recurrence in Stage III Melanoma Patients with Microscopic Sentinel Lymph Node Metastasis. Cancers, 15(3), 702. https://doi.org/10.3390/cancers15030702






