Simple Summary
Systemic therapy, including immunotherapy for unresectable hepatocellular carcinoma, has rapidly progressed worldwide. The aim of the study is to investigate the clinical usefulness of comprehensive genomic profiling in patients with unresectable hepatocellular carcinoma who received multiple molecular-targeted agents. Previous studies about the genomic status of hepatocellular carcinoma were limited to patients treated with resection, ablation, or a few agents. This is the first study to reveal gene mutations in patients who received multiple molecular-targeted therapies in real-world practice. The number of patients in this study was small. However, the results of this study would have an impact on future research into novel treatments for unresectable hepatocellular carcinoma. The best timing for performing comprehensive genomic profiling should be discussed, and further research is needed to develop personalized treatment and novel therapeutic agents for unresectable hepatocellular carcinoma.
Abstract
The molecular mechanism of hepatocellular carcinoma (HCC) is partially demonstrated. Moreover, in the patients receiving multiple molecular-targeted therapies, the gene alternations are still unknown. Six molecular-targeted therapies of unresectable HCC (uHCC) and comprehensive genomic profiling (CGP) have been approved in clinical practice. Hence, the utility of CGP in patients with uHCC treated with multiple molecular-targeted agents is investigated. The data of the patients with uHCC who received CGP tests were collected, retrospectively, between February 2021 and May 2022. Gene alterations detected by foundation testing, excluding variants of unknown significance, were reported in all nine patients. The samples for CGP were derived from liver tumor biopsy (n = 2), surgical specimens of bone metastases (n = 2), and blood (n = 5). The median number of systemic therapies was four. Seven patients were candidates eligible for clinical trials. One patient with a high tumor mutation burden (TMB) could receive pembrolizumab after CGP. This study presented genomic alternations after receiving multiple molecular-targeted therapies. However, further investigation needs to be conducted to develop personalized therapies and invent newer agents for treating HCC.
1. Introduction
Liver cancer is a global health problem and has become the second and sixth most common cause of cancer-associated deaths in men and women, respectively, in 2020 [1]. Hepatocellular carcinoma (HCC) accounts for 75–85% of liver cancer cases. The life expectancy of patients with HCC has improved considerably with rapid advancement in systemic therapy, including immunotherapy. However, based on recent clinical trials, the median overall survival of advanced HCC is 15–19 months [2,3,4]. Moreover, a few biomarkers were used for decision-making. The features of the molecular pathogenesis and drivers of HCC have already been reported partially [5,6,7]. However, most previous studies included only patients who had undergone resection or received a few systemic therapies. In patients with unresectable HCC (uHCC), six systemic therapies, including sorafenib [8,9], regorafenib [10], Lenvatinib [2], ramucirumab (only AFP ≥ 400 ng/mL) [11], cabozantinib [12], and atezolizumab plus bevacizumab [3], have been reimbursed by national health insurance in Japan. Moreover, clinical sequencing in tissue specimens has been covered by national health insurance since June 2019. Subsequently, a liquid biopsy was also approved in August 2021 [13,14]. This has led to a rapidly expanding number of individualized therapies that specifically target comprehensive genomic profiling in a patient’s tumor. Personalized cancer therapy could be achieved by regulating the mutation status of specific molecular drivers in critical signaling pathways. However, its clinical utility in uHCC remains unknown. In this study, the clinical utility of comprehensive genomic profiling with Foundation One® CDx (F1CDx) and Foundation One® Liquid CDx (F1LCDx) in real-world practice is investigated. These systems are used to identify potentially actionable genetic alterations and perform precision individualized therapies. Furthermore, the genomic status in patients with HCC after receiving multiple systemic therapies in real-world practice would be revealed in the future to develop newer molecular-targeted agents.
2. Materials and Methods
2.1. Study Protocol
The patients who had uHCC and underwent F1CDx or F1LCDx at our hospital between February 2021 and May 2022 were, retrospectively, investigated. All the patients either progressed or were finishing the standard systemic therapy. Before enrolling for the study, written informed consent was obtained from each patient. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki, and the study was approved by the institutional ethics review committee (approval number: 2102). The sequencing test, data analysis, and annotation were conducted by Foundation Medicine Inc. The final report on F1CDx or F1LCDx included any detected genomic findings and FDA-approved therapeutic options.
2.2. Clinical and Laboratory Data
The clinical data, including the age, sex, performance status, liver function, renal function, nutritional status, tumor markers (AFP and PIVKA-II), and imaging findings from the initial systemic treatment for HCC to the last visit after the comprehensive genomic profiling test were collected. Each systemic treatment was performed as per the manufacturer’s guidelines. Dynamic computed tomography (CT) was performed at baseline and, thereafter, every 6–12 weeks. Dynamic magnetic resonance imaging (MRI) was conducted for patients allergic to the contrast agents of the CT scan. The treatment response was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST ver1.1) or Modified Response Evaluation Criteria in Solid Tumors (mRECIST). Adverse events (AEs) were reported as per the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
2.3. F1CDx and F1LCDx
F1CDx and F1LCDx are qualitative next-generation sequencing that use targeted high-throughput hybridization-based capture technology for the detection of substitutions, insertion and deletion alterations, and copy number alterations (CNAs) in 324 genes and select gene rearrangements, using DNA isolated from formalin-fixed, paraffin-embedded (FFPE) tumor tissue specimens or blood. These tests are intended to identify patients who may benefit from treatment with therapies in accordance with approved therapeutic product labeling. Foundation One® Liquid CDx is an FDA-approved companion diagnostic that analyzes guideline-recommended genes from a simple blood draw. It is the only FDA-approved blood-based test to analyze over 300 genes—making it the most comprehensive FDA-approved liquid biopsy on the market. To detect base substitution, reads with low mapping (mapping quality < 25) or base calling quality (base calls with quality ≤ 2) were discarded. Final calls were made at mutant allele frequency (MAF) ≥ 5% (MAF ≥ 1% at hotspots). Variants were classified as variants of uncertain (VUS) when the significance and impact upon cancer progression were unknown due to a lack of reported evidence and conclusive change in function based on the previous reports [15,16]. Genomic DNA control samples were analyzed at a central laboratory testing service.
2.4. Expert Panel Discussion
After F1CDx or F1LCDx, each case was reviewed at the expert panel discussion with specialists, including genetic counselors, pathologists, medical oncologists, bioinformaticians, clinical research coordinators, and primary physicians. Based on the patient’s medical treatment or family history, the genetic results of actionable genomic alterations and treatment options were carefully evaluated by these specialists.
2.5. Treatment after F1CDX or F1LCDX
After the expert panel discussion, each doctor explained the results to the patients. Additionally, if relevant clinical trials were available, the patients were introduced to the National Cancer Center. If relevant clinical trials were not available, the therapeutic strategies were decided based on the discussion with the tumor board.
3. Results
3.1. Patient Characteristics
During the study period, nine patients received F1CDX or F1LCDx. The characteristics of the patients with F1CDX or F1LCDx are listed in Table 1. The patients defined as others were without hepatitis B virus (HBV), hepatitis C virus (HCV), or alcohol consumption. The mean age of patients defined as others was 30 years old and younger than those with HBV, HCV, or alcohol consumption. Four patients had a smoking history (current or former smokers). One patient with HBV was treated for esophageal varices by endoscopic variceal ligation (EVL) before CGP. Three patients (two patients with HBV and one patient with HCV) had low platelets (<10⁵/µL) at the CGP test. All patients had no cancer other than hepatocellular carcinoma.

Table 1.
Baseline characteristics of the patients at comprehensive genomic profiling test.
Extrahepatic metastasis was observed in seven patients; peritoneal dissemination (n = 1), lung only (n = 3), and lung and bone (n = 3). The previous systemic therapies in each patient are shown in Table 2. The number of systemic therapies received was six in one patient, five in three patients, and four in five patients. The samples for F1CDX or F1LCDx were from liver tumor biopsy (n = 2), surgical specimens of bone metastases (n = 2), and blood (n = 5). All patients who received F1LCDx had extrahepatic metastasis, while two of four patients evaluated by F1CDx had only intrahepatic lesions, including major vascular invasion. Liver function was maintained as Child–Pugh A in three of four patients with F1CDx, while two of five patients were diagnosed as Child–Pugh B at the CGP test.

Table 2.
The results of comprehensive genomic profiling test.
3.2. Common Alterations in Patients with HCC
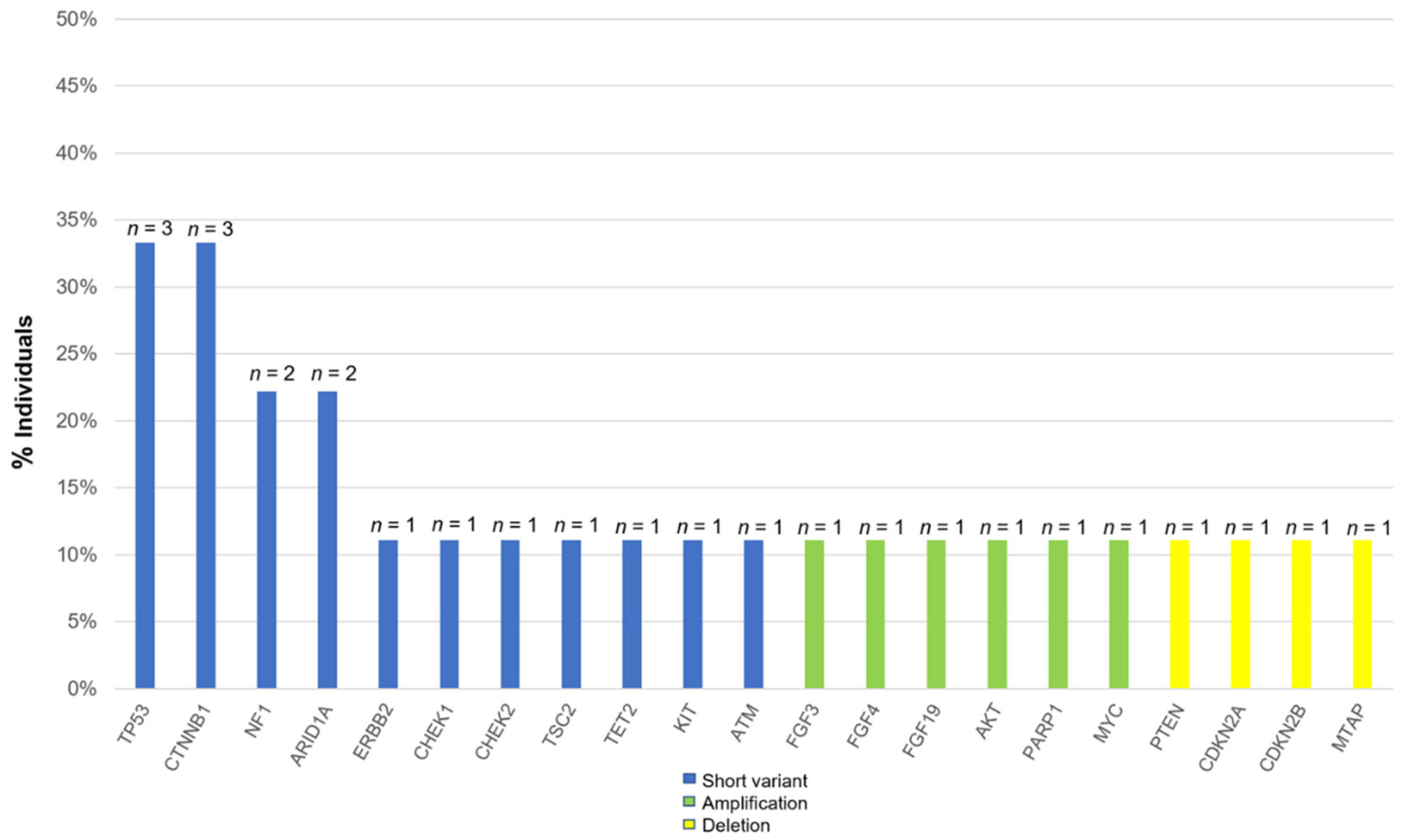
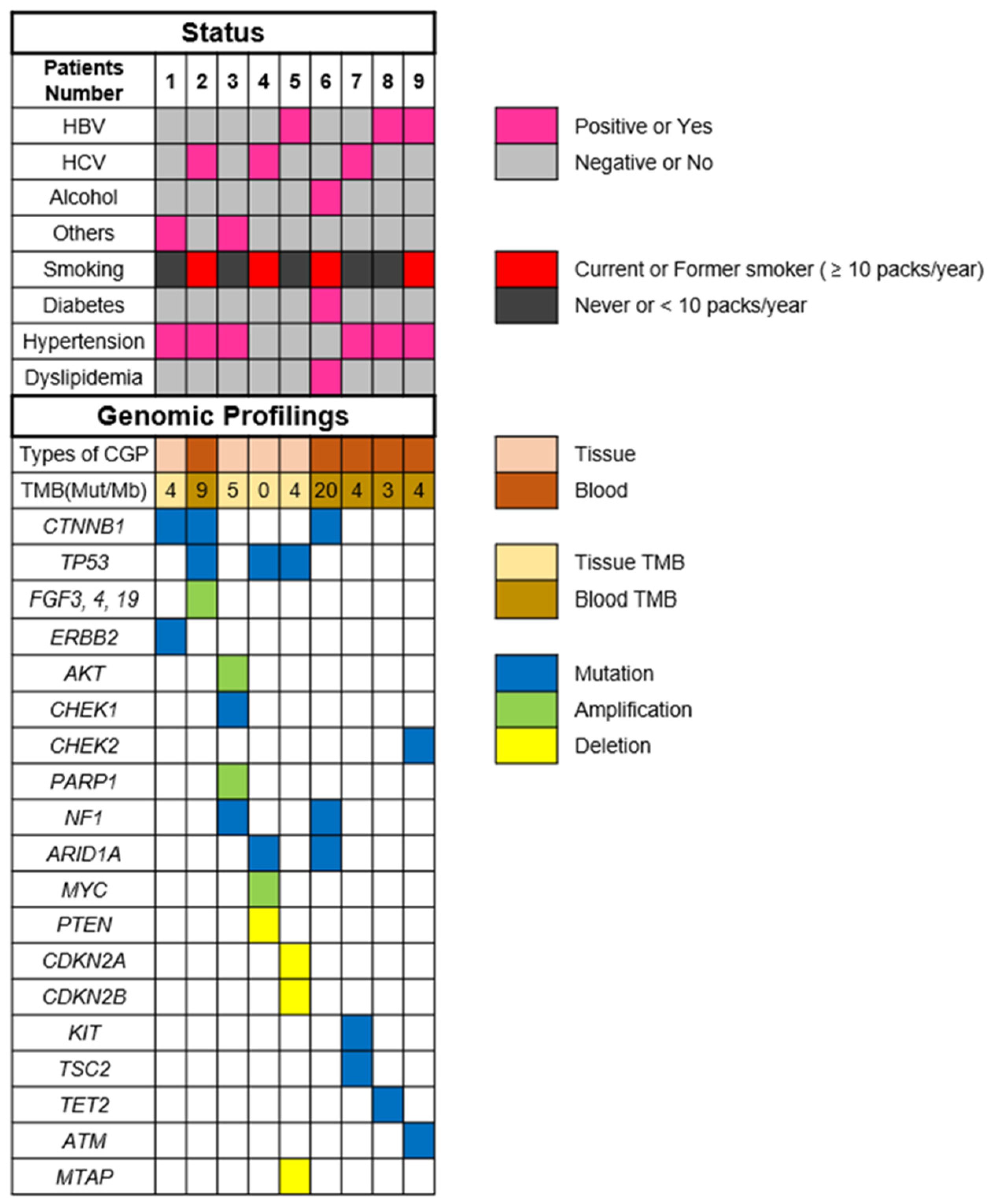
The most commonly altered genes, excluding VUS in HCC, are shown in Figure 1. There are alterations in all patients with HCC. The two most frequent alterations observed were TP53 (n = 3, 33.3%) and CTNNB1 (n = 3, 33.3%). Four patients were investigated by using F1CDx, and the other five patients were investigated by using F1LCDx (Table 1). Five patients performed F1LCDx, and detectable alterations were seen in all patients. Based on the results of comprehensive genomic profiling, four out of five patients were candidates for the clinical trials. In Table S1, Table 2 and Table S2, the genomic results of nine patients are shown. All patients had detectable alterations, and the median number of alterations (except VUS) per patient was four (2–5). The results of tumor mutation burden (TMB) and microsatellite instability (MSI) were obtained. The median number of TMB was four (range 0–20). Only one patient had TMB-high (TMB ≥ 10 mutations/megabase), whereas none of the patients had MSI-high (MSI sensor scores ≥ 10). An alternation suspected of a hereditary tumor was not seen in any patient. The results of comprehensive genomic profiling in patients with uHCC are illustrated in Figure 2. Five patients performed F1LCDx, and detectable alterations were seen in all patients. All patients evaluated with F1LCDx had extrahepatic metastasis. One patient received six regimens, and four of five patients experienced four regimens before CGP. Based on the results of comprehensive genomic profiling, four out of five patients were candidates for the clinical trials.
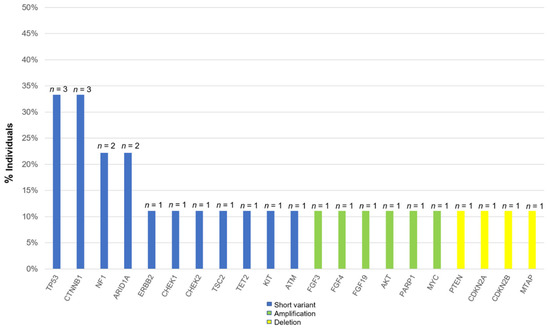
Figure 1.
Percentage of all alterations in patients with unresectable hepatocellular carcinoma.
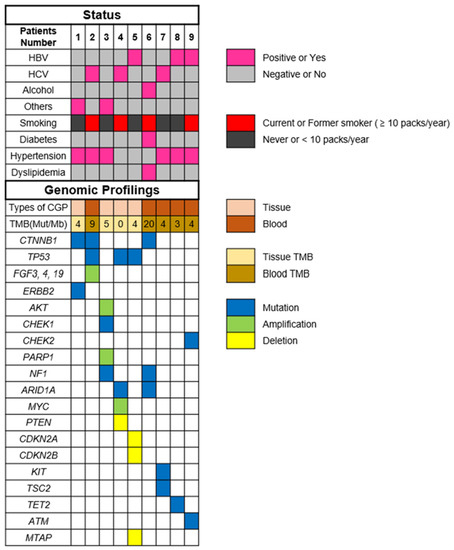
Figure 2.
Results of comprehensive genomic profiling in patients with unresectable hepatocellular carcinoma. Abbreviations: TMB—tumor mutation burden; HBV—hepatitis B virus; HCV—hepatitis C virus; and others mean non-HBV, non-HCV, and non-alcohol.
3.3. Treatment after The Comprehensive Genomic Profiling
The clinical outcome after CGP is described in Table 3. Based on the results of the comprehensive genomic profiling, the patient with TMB-high was treated with pembrolizumab, whereas the other patient was treated with regorafenib. This decision was taken based on the profiling outcome by the expert panel, which revealed that regorafenib would be a more effective option. This patient was retreated with regorafenib and survived for five months after the retreatment. The OS from the initial liver resection was 12.5 years, and survival from the initiation of sorafenib treatment for lung metastases was 4.5 years. One patient with a CTNNB1 mutation received cabozantinib as the fifth line of treatment and maintained SD for 11 months. Based on the result of gene profiling by multigene panel test, the other patient with TSC2 planned to participate in everolimus’s prospective trial of patient-proposed healthcare services with multiple targeted agents. Of these nine patients, seven (77.8%) were eligible for participating in ≥1 clinical trial option. However, none could enter the clinical trials. The reasons for the inability of patients to participate in the trials are listed in Table 3. Of the nine patients, three could not participate due to renal dysfunction or low platelet counts, and one due to HBV infection. The median survival duration from the comprehensive genomic profiling was 4 (2–18) months.

Table 3.
Clinical outcome after comprehensive genomic profiling test.
4. Discussion
Based on our knowledge, this is the first report to reveal the clinical utility of comprehensive genomic profiling in patients with uHCC after receiving multiple molecular-targeted therapies. Llovet et al. [5] reviewed the molecular pathogenesis of HCC and revealed that most molecular alternations were undruggable, with only 20–25% of tumors having an actionable driver mutation. However, all nine patients in this study had alterations, and the median number of alterations per patient was four. Of the nine patients, seven (77.8%) were eligible for participating in the ≥1 clinical trial, which was conducted as per the comprehensive genomic profiling in malignant tumors. The results of this study differed from past studies because of the different backgrounds of the patients. In this study, all patients received atezolizumab plus bevacizumab and received more than three molecular-targeted therapies. The patients who received sequential therapies for HCC had actionable driver mutations more frequently compared to those registered in previous studies. In recent studies [5,6,7], CTNNB1 mutations were observed in 33% of patients. CTTNB1 mutation is associated with activation of Wnt/β-catenin signaling and enriched in non-T-cell inflamed HCCs, which demonstrated a poor clinical response to an immune checkpoint inhibitor. Ogawa K et al. [17] reported that in patients with HCC, the treatment effect of atezolizumab plus bevacizumab with mutant CTNNB1 was comparable to the patients with wild-type CTNNB1. In this study, of the nine patients, three had a CTNNB1 mutation. The best responses during atezolizumab plus bevacizumab were stable disease (SD) in two patients and progressive disease (PD) in one patient, as per RECIST ver1.1.
One of the most important findings of this study was revealing the utility of liquid biopsy in patients with uHCC. Of the five patients performing F1LCDx, detectable alterations were seen in all patients. Based on the results of comprehensive genomic profiling, four out of five patients were candidates for the clinical trials. Translating molecular knowledge into precision oncology has been perceived as difficult in HCC because of the existence of intratumoral heterogeneity and multifocal tumors. Under such circumstances, a liquid biopsy, which is a noninvasive technique with the demonstration of the genetic information representative of the tumor genome, could be highly valuable.
Although this study included a small number of patients, some patients had significant mutations associated with β-catenin inhibitors and mTOR inhibitors. In this study, of the nine patients, three had CTNNB1 mutation, which is associated with β-catenin degradation. Subsequently, CTNNB1 mutation leads to the constitutive activation of Wnt/β-catenin signaling. An orally active selective inhibitor of the interaction between β-catenin and CREB binding protein, E7386 [18], demonstrated antitumor activity through the modulation of the Wnt/β-catenin signaling pathway. In Japan, the clinical trial of combination therapy with E7386 and PD-1 antibody (ClinicalTrials.gov Identifier: NCT05091346) is ongoing. The other anticipated agent is the mTOR inhibitor. In HCC, the PI3K/AKT/mTOR signaling pathway was reported, and its activation was frequently detected [19,20]. Chen J.S. et al. [21] reported that the PI3K/PTEN/AKT/mTOR pathway was involved in invasion and metastasis in HCC. Ocana A. et al. [22] revealed that the activation of the PI3K/mTOR/AKT pathway was associated with significantly worse 5-year survival in solid tumors. In this study, molecular aberrations led to the putative activation of the mTOR pathway and were detected in five of the nine patients. In advanced HCC, a preliminary antitumor effect was reported in a phase I/II study of mTOR inhibition using the rapamycin derivate RAD001 (everolimus) [23]. However, in a subsequent phase III trial (EVOLVE-1), everolimus did not meet the primary endpoint [24]. The patients who participated in this study did not receive sequential therapies and were treated only with sorafenib.
All nine patients in this study had gene alternations, and one patient received pembrolizumab based on the results of CGP. The other patient participated in the clinical trial for unresectable HCC instead of the everolimus’s prospective trial of patient-proposed healthcare services with multiple targeted agents. Three patients visited the National Cancer Center Hospital to participate in the clinical trials based on CGP. However, they were excluded because of renal dysfunction, low platelet count, or HBV infection. In Japan, comprehensive genomic profiling tests are approved only in patients who have finished the standard therapies or have received the last recommended treatments. Furthermore, the timing of the comprehensive genomic profiling tests should be discussed at a multidisciplinary tumor board.
This study has a few limitations. First, it was a single-centric study. Hence, the sample size was very small. We could not perform any statistical analysis. Second, all the participants of this study were Japanese. Hence, ethnicity could not be discussed. In the future, further research should be conducted with international multicentric investigations, including a large sample size to confirm the results of this study. Third, this study was conducted in Japan. However, the reimbursement laws differ in each country. Further studies, including a large number of patients in multi centers, are necessary.
This study is the first to reveal the usefulness of comprehensive genomic profiling in patients with uHCC receiving multiple molecular-targeted therapies. Although no patient could participate in the clinical trials based on CGP, the reasons were associated with organ function or viral infection and not gene alternations. The results of this study contribute to a better understanding of the genomic status and development of newer molecular-targeted agents for HCC.
5. Conclusions
Comprehensive genomic profiling with tumor tissue or patient’s blood might be useful in patients with HCC receiving sequential molecular-targeted therapies. In the future, the best timing for comprehensive genomic profiling should be discussed to provide more personalized treatment to the patient.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15030719/s1, Table S1: Genomic results and clinical outcome in 9 patients, Table S2: The information on the clinical trial options and investigational candidate drugs.
Author Contributions
Conceptualization, S.I. and K.T. (Kaoru Tsuchiya); methodology, S.I. and K.T. (Kaoru Tsuchiya); software, S.I.; validation, S.I. and K.T. (Kaoru Tsuchiya); formal analysis, S.I.; investigation, S.I. and K.T. (Kaoru Tsuchiya); resources, N.U., K.S., Y.T. (Yuki Tanaka), H.M. (Haruka Miyamoto), M.Y., S.I, H.M. (Hiroaki Matsumoto), T.N., T.K., K.T. (Kenta Takaura), S.T., C.M., N.T., Y.Y., Y.T. (Yuka Takahashi), H.N. and U.S.; data curation, S.I. and K.T. (Kaoru Tsuchiya); writing—original draft preparation, S.I., K.T. (Kaoru Tsuchiya) and Y.K.; writing—review and editing, K.T. (Kenta Takaura), Y.Y., H.N., Y.A., R.O., M.K. and N.I.; visualization, S.I. and K.T. (Kaoru Tsuchiya); supervision, Y.A., R.O. and N.I.; project administration, S.I. and K.T. (Kaoru Tsuchiya); funding acquisition, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
Masayuki Kurosaki received funding support from the Japan Agency for Medical Research and Development (JP20fk0210067h0001).
Institutional Review Board Statement
The study was conducted as per the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Musashino Red Cross Hospital (ethics code: 2102; approval date 28 July 2021).
Informed Consent Statement
Written and informed consent was obtained from all participants involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to Shinobu Nagai, Nobuko Okamura, and Yaeko Kobayashi for their assistance.
Conflicts of Interest
Kaoru Tsuchiya, Masayuki Kurosaki, and Namiki Izumi received advisory board fees and honoraria for the speakers’ bureau from Eli Lilly Japan, Chugai Pharmaceutical Company, Takeda, and Eisai. The Japanese Ministry of Health, Labour, and Welfare had no role in the study design, data collection, data analysis, data interpretation, manuscript writing, or the publication of the results.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus Sorafenib in First-Line Treatment of Patients with Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus Atezolizumab versus Sorafenib for Advanced Hepatocellular Carcinoma (COSMIC-312): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef]
- Llovet, J.M.; Pinyol, R.; Kelley, R.K.; El-Khoueiry, A.; Reeves, H.L.; Wang, X.W.; Gores, G.J.; Villanueva, A. Molecular Pathogenesis and Systemic Therapies for Hepatocellular Carcinoma. Nat. Cancer 2022, 3, 386–401. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular Therapies and Precision Medicine for Hepatocellular Carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef]
- Ding, X.; He, M.; Chan, A.W.H.; Song, Q.X.; Sze, S.C.; Chen, H.; Man, M.K.H.; Man, K.; Chan, S.L.; Lai, P.B.S.; et al. Genomic and Epigenomic Features of Primary and Recurrent Hepatocellular Carcinomas. Gastroenterology 2019, 157, 1630–1645.e6. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after Sorafenib in Patients with Advanced Hepatocellular Carcinoma and Increased α-Fetoprotein Concentrations (REACH-2): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, Y.; Kano, Y.; Tohyama, K.; Matsudera, S.; Kumaki, Y.; Takahashi, K.; Mitsumura, T.; Harada, Y.; Sato, A.; Nakamura, H.; et al. Clinical Utility of Comprehensive Genomic Profiling in Japan: Result of PROFILE-F Study. PLoS ONE 2022, 17, e0266112. [Google Scholar] [CrossRef] [PubMed]
- Noji, R.; Tohyama, K.; Kugimoto, T.; Kuroshima, T.; Hirai, H.; Tomioka, H.; Michi, Y.; Tasaki, A.; Ohno, K.; Ariizumi, Y.; et al. Comprehensive Genomic Profiling Reveals Clinical Associations in Response to Immune Therapy in Head and Neck Cancer. Cancers 2022, 14, 3476. [Google Scholar] [CrossRef] [PubMed]
- Frampton, G.M.; Fichtenholtz, A.; Otto, G.A.; Wang, K.; Downing, S.R.; He, J.; Schnall-Levin, M.; White, J.; Sanford, E.M.; An, P.; et al. Development and Validation of a Clinical Cancer Genomic Profiling Test Based on Massively Parallel DNA Sequencing. Nat. Biotechnol. 2013, 31, 1023–1031. [Google Scholar] [CrossRef]
- Woodhouse, R.; Li, M.; Hughes, J.; Delfosse, D.; Skoletsky, J.; Ma, P.; Meng, W.; Dewal, N.; Milbury, C.; Clark, T.; et al. Clinical and Analytical Validation of FoundationOne Liquid CDx, a Novel 324-Gene CfDNA-Based Comprehensive Genomic Profiling Assay for Cancers of Solid Tumor Origin. PLoS ONE 2020, 15, e0237802. [Google Scholar] [CrossRef]
- Ogawa, K.; Kanzaki, H.; Chiba, T.; Ao, J.; Qiang, N.; Ma, Y.; Zhang, J.; Yumita, S.; Ishino, T.; Unozawa, H.; et al. Effect of Atezolizumab plus Bevacizumab in Patients with Hepatocellular Carcinoma Harboring CTNNB1 Mutation in Early Clinical Experience. J. Cancer 2022, 13, 2656–2661. [Google Scholar] [CrossRef]
- Yamada, K.; Hori, Y.; Inoue, S.; Yamamoto, Y.; Iso, K.; Kamiyama, H.; Yamaguchi, A.; Kimura, T.; Uesugi, M.; Ito, J.; et al. E7386, a Selective Inhibitor of the Interaction between β-Catenin and CBP, Exerts Antitumor Activity in Tumor Models with Activated Canonical Wnt Signaling. Cancer Res. 2021, 81, 1052–1062. [Google Scholar] [CrossRef]
- Sun, E.J.; Wankell, M.; Palamuthusingam, P.; McFarlane, C.; Hebbard, L. Targeting the PI3K/Akt/MTOR Pathway in Hepatocellular Carcinoma. Biomedicines 2021, 9, 1639. [Google Scholar] [CrossRef]
- Matter, M.S.; Decaens, T.; Andersen, J.B.; Thorgeirsson, S.S. Targeting the MTOR Pathway in Hepatocellular Carcinoma: Current State and Future Trends. J. Hepatol. 2014, 60, 855–865. [Google Scholar] [CrossRef]
- Chen, J.-S.; Wang, Q.; Fu, X.-H.; Huang, X.-H.; Chen, X.-L.; Cao, L.-Q.; Chen, L.-Z.; Tan, H.-X.; Li, W.; Bi, J.; et al. Involvement of PI3K/PTEN/AKT/MTOR Pathway in Invasion and Metastasis in Hepatocellular Carcinoma: Association with MMP-9. Hepatol. Res. 2009, 39, 177–186. [Google Scholar] [CrossRef]
- Ocana, A.; Vera-Badillo, F.; Al-Mubarak, M.; Templeton, A.J.; Corrales-Sanchez, V.; Diez-Gonzalez, L.; Cuenca-Lopez, M.D.; Seruga, B.; Pandiella, A.; Amir, E. Activation of the PI3K/MTOR/AKT Pathway and Survival in Solid Tumors: Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e95219. [Google Scholar] [CrossRef]
- Zhu, A.X.; Abrams, T.A.; Miksad, R.; Blaszkowsky, L.S.; Meyerhardt, J.A.; Zheng, H.; Muzikansky, A.; Clark, J.W.; Kwak, E.L.; Schrag, D.; et al. Phase 1/2 Study of Everolimus in Advanced Hepatocellular Carcinoma. Cancer 2011, 117, 5094–5102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Kudo, M.; Assenat, E.; Cattan, S.; Kang, Y.-K.; Lim, H.Y.; Poon, R.T.P.; Blanc, J.-F.; Vogel, A.; Chen, C.-L.; et al. Effect of Everolimus on Survival in Advanced Hepatocellular Carcinoma after Failure of Sorafenib: The EVOLVE-1 Randomized Clinical Trial. JAMA 2014, 312, 57–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).