Is There a Role for Molecular Testing for Low-Risk Differentiated Thyroid Cancer? A Cost-Effectiveness Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
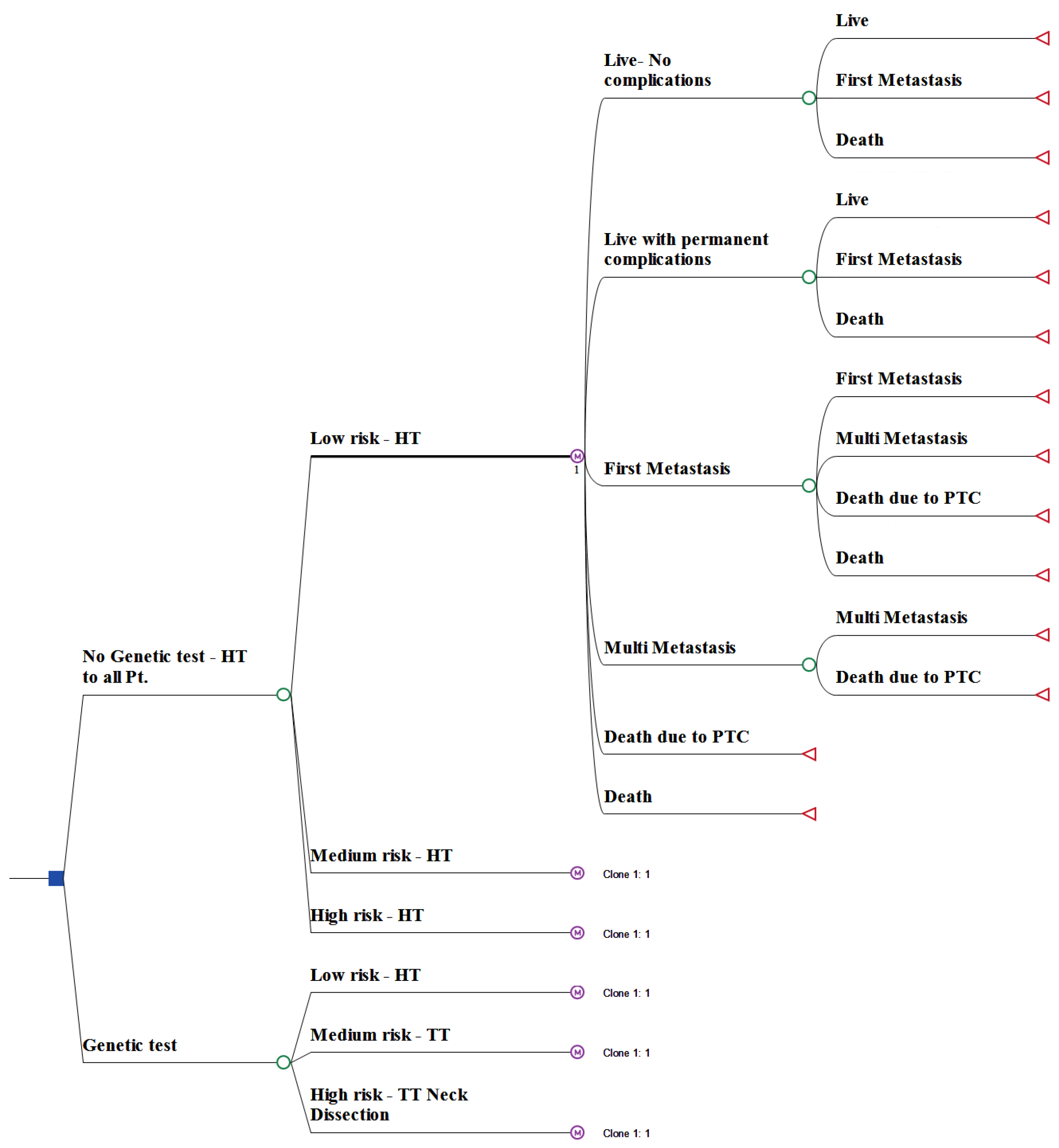
2.1. Model Structure
2.2. Clinical Data and Probabilities
2.3. Clinical Course
2.4. Costs
2.5. Sensitivity Analysis
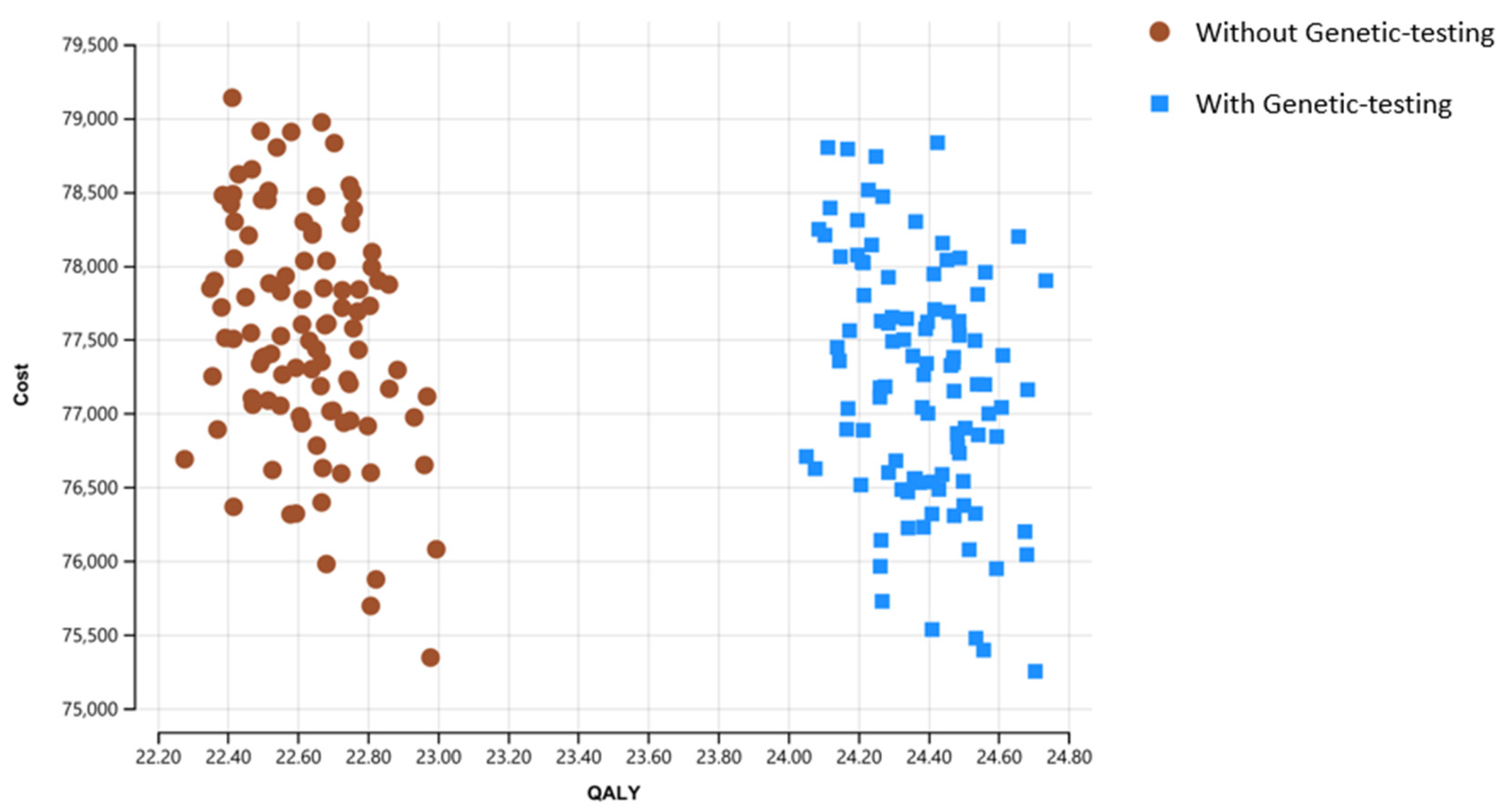
3. Results
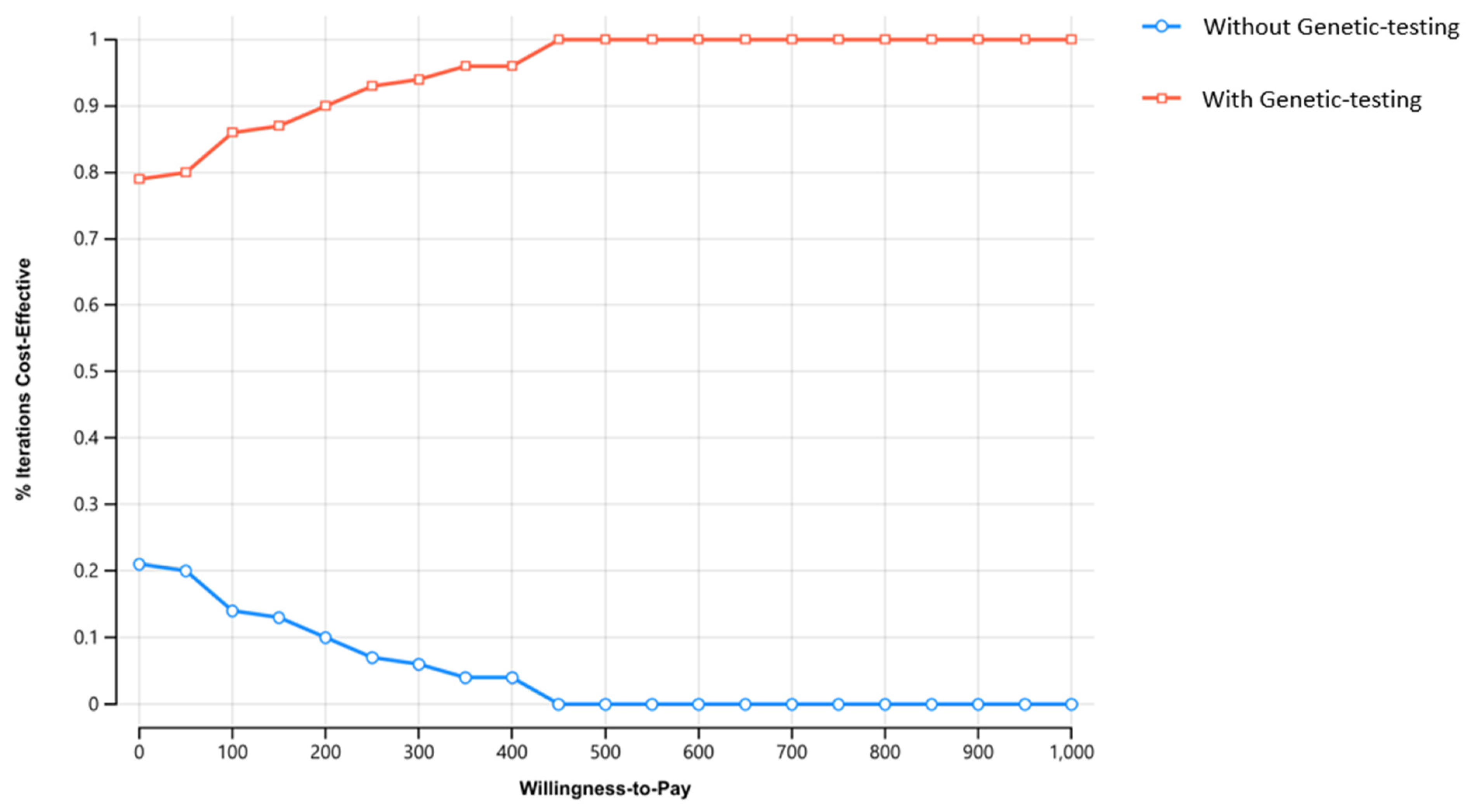
Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, M.; Williams, V.L.; Johnson, J.H.; Valderrabano, P. Thyroid Cancer Incidence Trends in the United States: Association with Changes in Professional Guideline Recommendations. Thyroid 2020, 30, 1132–1140. [Google Scholar] [CrossRef]
- Takano, T. Natural history of thyroid cancer [Review]. Endocr. J. 2017, 64, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer Statistics Review, 1975-2017—SEER Statistics. Available online: https://seer.cancer.gov/archive/csr/1975_2017/#citation (accessed on 21 October 2022).
- Wang, C.-C.C.; Friedman, L.; Kennedy, G.C.; Wang, H.; Kebebew, E.; Steward, D.L.; Zeiger, M.A.; Westra, W.H.; Wang, Y.; Khanafshar, E.; et al. A Large Multicenter Correlation Study of Thyroid Nodule Cytopathology and Histopathology. Thyroid 2011, 21, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Melo-Uribe, M.A.; Sanabria, Á.; Romero-Rojas, A.; Pérez, G.; Vargas, E.J.; Gutiérrez, V.; Abaúnza, M. The Bethesda system for reporting thyroid cytopathology in Colombia: Correlation with histopathological diagnoses in oncology and non-oncology institutions. J. Cytol. 2015, 32, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Blank, A.; Meiersdorf, S.; Hod, K.; Gabay, S.; Guindy, M.; Khafif, A. Impact of high-quality ultrasound following community ultrasound on surgical planning and active surveillance in patients with thyroid cancer. Clin. Endocrinol. 2021, 94, 990–997. [Google Scholar] [CrossRef]
- Vargas-Salas, S.; Martínez, J.R.; Urra, S.; Domínguez, J.M.; Mena, N.; Uslar, T.; Lagos, M.; Henríquez, M.; González, H.E. Genetic testing for indeterminate thyroid cytology: Review and meta-analysis. Endocr. Relat. Cancer 2018, 25, R163–R177. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association Between BRAF V600E Mutation and Mortality in Patients with Papillary Thyroid Cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef] [Green Version]
- Elisei, R.; Ugolini, C.; Viola, D.; Lupi, C.; Biagini, A.; Giannini, R.; Romei, C.; Miccoli, P.; Pinchera, A.; Basolo, F. BRAFV600E Mutation and Outcome of Patients with Papillary Thyroid Carcinoma: A 15-Year Median Follow-Up Study. J. Clin. Endocrinol. Metab. 2008, 93, 3943–3949. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.-L.; Wang, O.-C.; Zhang, X.-H.; Dai, X.-X.; Hu, X.-Q.; Qu, J.-M. The BRAF Mutation Is Predictive of Aggressive Clinicopathological Characteristics in Papillary Thyroid Microcarcinoma. Ann. Surg. Oncol. 2010, 17, 3294–3300. [Google Scholar] [CrossRef]
- Najafzadeh, M.; Marra, C.A.; Lynd, L.D.; Wiseman, S.M. Cost-Effectiveness of Using a Molecular Diagnostic Test to Improve Preoperative Diagnosis of Thyroid Cancer. Value Health 2012, 15, 1005–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.; How, J.; Tabah, R.J.; Mitmaker, E.J. Cost-Effectiveness of Molecular Testing for Thyroid Nodules with Atypia of Undetermined Significance Cytology. J. Clin. Endocrinol. Metab. 2014, 99, 2674–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.Y.; Roman, B.R.; Ma, J.C.M.; Ba, F.L.P.; Tuttle, R.M.; Shaha, A.R.; Shah, J.; Patel, S.G.; Ganly, I. Cost-effectiveness analysis of papillary thyroid cancer surveillance. Cancer 2015, 121, 4132–4140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balentine, C.J.; Vanness, D.J.; Schneider, D.F. Cost-effectiveness of lobectomy versus genetic testing (Afirma®) for indeterminate thyroid nodules: Considering the costs of surveillance. Surgery 2017, 163, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Rivas, A.M.; Nassar, A.; Zhang, J.; Casler, J.; Chindris, A.M.; Smallridge, R.; Bernet, V. Thyroseq®V2.0 Molecular Testing: A Cost-Effective Approach for the Evaluation of Indeterminate Thyroid Nodules. Endocr. Pract. 2018, 24, 780–788. [Google Scholar] [CrossRef]
- Prete, A.; de Souza, P.B.; Censi, S.; Muzza, M.; Nucci, N.; Sponziello, M. Update on Fundamental Mechanisms of Thyroid Cancer. Front. Endocrinol. 2020, 11, 102. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.Y.; Palmer, F.L.; Nixon, I.J.; Thomas, D.; Patel, S.G.; Shaha, A.R.; Shah, J.P.; Tuttle, R.M.; Ganly, I. Multi-Organ Distant Metastases Confer Worse Disease-Specific Survival in Differentiated Thyroid Cancer. Thyroid 2014, 24, 1594–1599. [Google Scholar] [CrossRef]
- Barczyński, M.; Konturek, A.; Stopa, M.; Nowak, W. Prophylactic central neck dissection for papillary thyroid cancer. Br. J. Surg. 2012, 100, 410–418. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Adam, M.A.; Pura, J.; Gu, L.; Dinan, M.A.; Tyler, D.S.; Reed, S.D.; Scheri, R.; Roman, S.A.; Sosa, J.A. Extent of Surgery for Papillary Thyroid Cancer Is Not Associated with Survival. Ann. Surg. 2014, 260, 601–607. [Google Scholar] [CrossRef]
- Liu, R.; Bishop, J.; Zhu, G.; Zhang, T.; Ladenson, P.W.; Xing, M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer. JAMA Oncol. 2017, 3, 202–208. [Google Scholar] [CrossRef]
- Xing, M. Genetic-guided Risk Assessment and Management of Thyroid Cancer. Endocrinol. Metab. Clin. North Am. 2019, 48, 109–124. [Google Scholar] [CrossRef]
- Pourrahmat, M.-M.; Kim, A.; Kansal, A.R.; Hux, M.; Pushkarna, D.; Fazeli, M.S.; Chung, K.C. Health state utility values by cancer stage: A systematic literature review. Eur. J. Health Econ. 2021, 22, 1275–1288. [Google Scholar] [CrossRef]
- Yip, L.; Gooding, W.E.; Nikitski, A.; Wald, A.I.; Carty, S.E.; Karslioglu-French, E.; Seethala, R.R.; Zandberg, D.P.; Ferris, R.L.; Nikiforova, M.N.; et al. Risk assessment for distant metastasis in differentiated thyroid cancer using molecular profiling: A matched case-control study. Cancer 2021, 127, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burman, K.D.; et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules with Indeterminate Cytology. JAMA Oncol. 2019, 5, 204–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzaferri, E.L. Management of Low-Risk Differentiated Thyroid Cancer. Endocr. Pract. 2007, 13, 498–512. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Park, S.Y.; Shin, J.H.; Oh, Y.L.; Choe, J.-H.; Kim, J.-H.; Kim, J.S.; Yim, H.S.; Kim, Y.-L.; Ki, C.-S.; et al. Highly Sensitive and Specific Molecular Test for Mutations in the Diagnosis of Thyroid Nodules: A Prospective Study of BRAF-Prevalent Population. Int. J. Mol. Sci. 2020, 21, 5629. [Google Scholar] [CrossRef] [PubMed]
- Hier, J.; Avior, G.; Pusztaszeri, M.; Krasner, J.R.; Alyouha, N.; Forest, V.-I.; Hier, M.P.; Mlynarek, A.; Richardson, K.; Sadeghi, N.; et al. Molecular testing for cytologically suspicious and malignant (Bethesda V and VI) thyroid nodules to optimize the extent of surgical intervention: A retrospective chart review. J. Otolaryngol.-Head Neck Surg. 2021, 50, 1–7. [Google Scholar] [CrossRef]
- Tessler, I.; Shochat, I.; Cohen, O.; Meir, A.; Avior, G. Positive Correlation of Thyroid Nodule Cytology with Molecular Profiling—A Single-Center Experience. Endocr. Pathol. 2021, 32, 480–488. [Google Scholar] [CrossRef]
- Ronen, O.; Oichman, M. National differences in cost analysis of Afirma Genomic sequencing classifier. Clin. Endocrinol. 2020, 94, 717–724. [Google Scholar] [CrossRef]
- Nicholson, K.; Roberts, M.S.; McCoy, K.L.; Carty, S.E.; Yip, L. Molecular Testing Versus Diagnostic Lobectomy in Bethesda III/IV Thyroid Nodules: A Cost-Effectiveness Analysis. Thyroid 2019, 29, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Aydemirli, M.D.; Snel, M.; van Wezel, T.; Ruano, D.; Obbink, C.M.H.; Hout, W.B.V.D.; Schepers, A.; Morreau, H. Yield and costs of molecular diagnostics on thyroid cytology slides in the Netherlands, adapting the Bethesda classification. Endocrinol. Diabetes Metab. 2021, 4, e00293. [Google Scholar] [CrossRef] [PubMed]
- Sancho, J.J.; Lennard, T.W.J.; Paunovic, I.; Triponez, F.; Sitges-Serra, A. Prophylactic central neck disection in papillary thyroid cancer: A consensus report of the European Society of Endocrine Surgeons (ESES). Langenbeck’s Arch. Surg. 2013, 399, 155–163. [Google Scholar] [CrossRef]
- Chan, W.W.L.; Kwong, D.L.W. Radioactive Iodine for Papillary Thyroid Carcinoma. Methods Mol Biol. 2022, 2534, 225–241. [Google Scholar] [CrossRef] [PubMed]

| Variable | Base | Low | High | Refs |
|---|---|---|---|---|
| Age | 45 | 40 | 50 | |
| Cost of treatment in distance metastases | 22,008 | 17,500 | 26,500 | [15] |
| Cost of thyroid lobectomy surgery | 3538 | 2000 | 5000 | [16] |
| Cost of neck dissection surgery | 7731 | 1000 | 4000 | [16] |
| Cost of genetic testing panel | 4056 | 2000 | 6000 | [16] |
| Cost of total thyroidectomy surgery | 4102 | 2500 | 5500 | [16] |
| Rate of female gender | 0.8 | 0.7 | 0.9 | |
| Rate of first metastasis in high-risk genetic group | 0.193 | 0.1 | 0.3 | |
| Rate of first metastasis in intermediate-risk genetic group | 0.047 | 0.01 | 0.1 | |
| Rate of first metastasis in low-risk genetic group | 0.002 | 0.001 | 0.01 | |
| Prevalence of high-risk genetic group | 0.15 | 0.01 | 0.3 | [17] |
| prevalence of low-risk genetic group | 0.23 | 0.2 | 0.4 | |
| Rate of death in first distant metastasis | 0.05 | 0.02 | 0.1 | [18] |
| Rate of transition from first metastasis to multi-distant metastasis | 0.566 | 0.4 | 0.7 | [18] |
| Rate of death in multi-distant metastasis | 0.35 | 0.3 | 0.4 | [18] |
| Relative risk for the rate for first metastasis of TT with ND vs. HT in high-risk genetic group | 0.6 | 0.5 | 0.95 | [19,20,21,22,23] |
| Relative risk for the rate for first metastasis of TT vs. HT in intermediate-risk genetic group | 0.8 | 0.5 | 0.95 | [19,20,21,22,23] |
| Time horizon (Years) | 100 | 90 | 110 | Assumption |
| Utility of Metastasis | 0.8 | 0.5 | 0.9 | [24] |
| Strategy | Cost | Incremental Cost | Effectiveness | Incremental Effectiveness | ICER |
|---|---|---|---|---|---|
| Without genetic testing | 75,103 | 22.256 | |||
| With genetic testing | 75,430 | 327 | 23.972 | 1.716 | 190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessler, I.; Leshno, M.; Feinmesser, G.; Alon, E.E.; Avior, G. Is There a Role for Molecular Testing for Low-Risk Differentiated Thyroid Cancer? A Cost-Effectiveness Analysis. Cancers 2023, 15, 786. https://doi.org/10.3390/cancers15030786
Tessler I, Leshno M, Feinmesser G, Alon EE, Avior G. Is There a Role for Molecular Testing for Low-Risk Differentiated Thyroid Cancer? A Cost-Effectiveness Analysis. Cancers. 2023; 15(3):786. https://doi.org/10.3390/cancers15030786
Chicago/Turabian StyleTessler, Idit, Moshe Leshno, Gilad Feinmesser, Eran E. Alon, and Galit Avior. 2023. "Is There a Role for Molecular Testing for Low-Risk Differentiated Thyroid Cancer? A Cost-Effectiveness Analysis" Cancers 15, no. 3: 786. https://doi.org/10.3390/cancers15030786
APA StyleTessler, I., Leshno, M., Feinmesser, G., Alon, E. E., & Avior, G. (2023). Is There a Role for Molecular Testing for Low-Risk Differentiated Thyroid Cancer? A Cost-Effectiveness Analysis. Cancers, 15(3), 786. https://doi.org/10.3390/cancers15030786





