Associations between Lifestyle Factors and Neurocognitive Impairment among Chinese Adolescent and Young Adult (AYA) Survivors of Sarcoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Treatment Regimens
2.4. Study Outcomes
2.5. Lifestyle Factors
2.6. Covariates
2.7. Statistical Analysis
3. Results
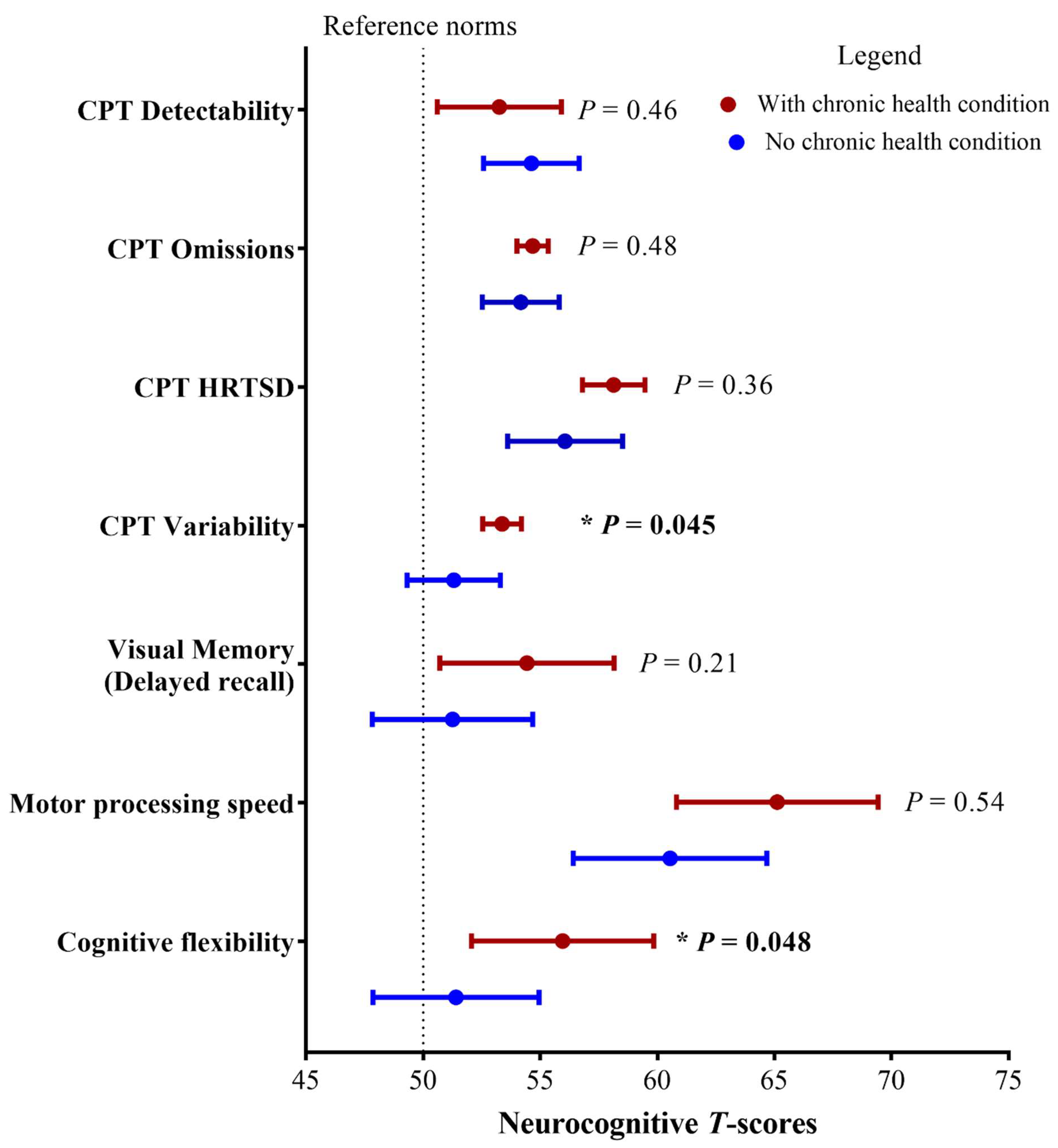
3.1. Associations between Clinical/Treatment Factors and Neurocognitive Outcomes
3.2. Lifestyle Factors
3.3. Associations between Lifestyle Factors and Neurocognitive Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Close, A.G.; Dreyzin, A.; Miller, K.D.; Seynnaeve, B.K.N.; Rapkin, L.B. Adolescent and young adult oncology-past, present, and future. CA Cancer. J. Clin. 2019, 69, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Hong Kong Cancer Registry, Hospital Authority. Available online: https://www3.ha.org.hk/cancereg/ (accessed on 1 December 2022).
- Edelmann, M.N.; Daryani, V.M.; Bishop, M.W.; Liu, W.; Brinkman, T.M.; Stewart, C.F.; Mulrooney, D.A.; Kimberg, C.; Ness, K.K.; Cheung, Y.T.; et al. Neurocognitive and Patient-Reported Outcomes in Adult Survivors of Childhood Osteosarcoma. JAMA Oncol. 2016, 2, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Norsker, F.N.; Boschini, C.; Rechnitzer, C.; Holmqvist, A.S.; Tryggvadottir, L.; Madanat-Harjuoja, L.-M.; Schrøder, H.; Scheike, T.H.; Hasle, H.; Winther, J.F.; et al. Risk of late health effects after soft-tissue sarcomas in childhood—A population-based cohort study within the Adult Life after Childhood Cancer in Scandinavia research programme. Acta Oncol. 2020, 59, 1246–1256. [Google Scholar] [CrossRef]
- Bishop, M.W.; Ness, K.K.; Li, C.; Liu, W.; Srivastava, D.K.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Pappo, A.S.; Robison, L.L.; et al. Cumulative Burden of Chronic Health Conditions in Adult Survivors of Osteosarcoma and Ewing Sarcoma: A Report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.; Zheng, D.J.; Netson-Amore, K.L.; Kadan-Lottick, N.S. Cognitive Impairment in Survivors of Pediatric Extracranial Solid Tumors and Lymphomas. J. Clin. Oncol. 2021, 39, 1727–1740. [Google Scholar] [CrossRef]
- Tonning Olsson, I.; Brinkman, T.M.; Wang, M.; Ehrhardt, M.J.; Banerjee, P.; Mulrooney, D.A.; Huang, I.C.; Ness, K.K.; Bishop, M.W.; Srivastava, D.; et al. Neurocognitive and psychosocial outcomes in adult survivors of childhood soft-tissue sarcoma: A report from the St. Jude Lifetime Cohort. Cancer 2020, 126, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Kadan-Lottick, N.S.; Zheng, D.J.; Wang, M.; Bishop, M.W.; Srivastava, D.K.; Ross, W.L.; Rodwin, R.L.; Ness, K.K.; Gibson, T.M.; Spunt, S.L.; et al. Patient-reported neurocognitive function in adult survivors of childhood and adolescent osteosarcoma and Ewing sarcoma. J. Cancer Surviv. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Lim, E.; Ng, T.; Shih, V.; Quek, R.; Cheung, Y.T. Symptom burden and medication use in adult sarcoma patients. Support Care Cancer 2015, 23, 1709–1717. [Google Scholar] [CrossRef]
- Plas, E.V.D.; Modi, A.J.; Li, C.K.; Krull, K.R.; Cheung, Y.T. Cognitive Impairment in Survivors of Pediatric Acute Lymphoblastic Leukemia Treated with Chemotherapy Only. J. Clin. Oncol. 2021, 39, 1705–1717. [Google Scholar] [CrossRef]
- Janeway, K.A.; Barkauskas, D.A.; Krailo, M.D.; Meyers, P.A.; Schwartz, C.L.; Ebb, D.H.; Seibel, N.L.; Grier, H.E.; Gorlick, R.; Marina, N. Outcome for adolescent and young adult patients with osteosarcoma: A report from the Children’s Oncology Group. Cancer 2012, 118, 4597–4605. [Google Scholar] [CrossRef]
- Whelan, J.S.; Davis, L.E. Osteosarcoma, Chondrosarcoma, and Chordoma. J. Clin. Oncol. 2018, 36, 188–193. [Google Scholar] [CrossRef]
- Krull, K.R.; Sabin, N.D.; Reddick, W.E.; Zhu, L.; Armstrong, G.T.; Green, D.M.; Arevalo, A.R.; Krasin, M.J.; Srivastava, D.K.; Robison, L.L.; et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3618–3624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunin-Batson, A.S.; Klosky, J.L.; Carlson-Green, B.; Brinkman, T.M. Health Behaviors and Neurocognitive Function in Survivors of Childhood Cancer. J. Clin. Oncol. 2021, 39, 1786–1794. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Yang, L.S.; Ma, J.C.T.; Woo, P.H.K.; Luk, S.M.S.; Chan, T.C.H.; Lee, V.W.-Y.; Yeung, N.C.-Y.; Li, C.-K.L. A Survey of Health Behaviour Practices of Childhood Cancer Survivors in Hong Kong and Their Expectations for a Local Cancer Survivorship Program. Hong Kong Med. J. 2021, 28, 33–44. [Google Scholar]
- Phillips, N.S.; Howell, C.R.; Lanctot, J.Q.; Partin, R.E.; Pui, C.H.; Hudson, M.M.; Robison, L.L.; Krull, K.R.; Ness, K.K. Physical fitness and neurocognitive outcomes in adult survivors of childhood acute lymphoblastic leukemia: A report from the St. Jude Lifetime cohort. Cancer 2020, 126, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, T.M.; Lown, E.A.; Li, C.; Tonning Olsson, I.; Marchak, J.G.; Stuber, M.L.; Vuotto, S.; Srivastava, D.; Nathan, P.C.; Leisenring, W.M.; et al. Alcohol consumption behaviors and neurocognitive dysfunction and emotional distress in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Addiction 2019, 114, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasalkar, D.D.; Chu, W.C.; Lee, V.; Paunipagar, B.K.; Cheng, F.W.; Li, C.K. Pulmonary metastases in children with osteosarcoma: Characteristics and impact on patient survival. Pediatr. Radiol. 2011, 41, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.P.Y.; Hui, W.F.; Wong, K.C.; Fong, B.S.T.; Luk, C.W.; Li, C.K. Paediatric high-grade osteosarcoma and its prognostic factors: 10-year retrospective study. Hong Kong Med. J. 2022, 28, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yam, P.P.-Y.; Yang, L.S.; Sato, S.; Li, C.K.; Cheung, Y.T. Neurocognitive impairment in Asian childhood cancer survivors: A systematic review. Cancer Metastasis Rev. 2020, 39, 27–41. [Google Scholar] [CrossRef]
- Conners, C.K.; Sitarenios, G. Conners’ Continuous Performance Test (CPT). Encycl. Clin. Neuropsychol. 2011, 681–683. [Google Scholar] [CrossRef]
- Casarotti, A.; Papagno, C.; Zarino, B. Modified Taylor Complex Figure: Normative data from 290 adults. J. Neuropsychol. 2014, 8, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.; Sherman, E.M.S.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed.; Oxford University Press: Oxford, UK, 2006; pp. 401–562. [Google Scholar]
- Lee, T.M.; Yuen, K.S.; Chan, C.C.H. Normative Data for Neuropsychological Measures of Fluency, Attention, and Memory Measures for Hong Kong Chinese. J. Clin. Exp. Neuropsychol. 2002, 24, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Kang, L.; Yao, S.; Ma, Y.; Li, T.; Liang, Y.; Cheng, Z.; Xu, Y.; Shi, J.; Xu, X.; et al. The MATRICS Consensus Cognitive Battery (MCCB): Co-norming and standardization in China. Schizophr. Res. 2015, 169, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Grooved Pegboard Test User’s Manual. Available online: https://www.advys.be/docs/GroovedPegboardTestManual.pdf (accessed on 1 June 2022).
- Barlow-Krelina, E.; Chen, Y.; Yasui, Y.; Till, C.; Gibson, T.M.; Ness, K.K.; Leisenring, W.M.; Howell, R.M.; Nathan, P.C.; Oeffinger, K.C.; et al. Consistent Physical Activity and Future Neurocognitive Problems in Adult Survivors of Childhood Cancers: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2020, 38, 2041–2052. [Google Scholar] [CrossRef]
- Koevoets, E.W.; Schagen, S.B.; de Ruiter, M.B.; Geerlings, M.I.; Witlox, L.; van der Wall, E.; Stuiver, M.M.; Sonke, G.S.; Velthuis, M.J.; Jobsen, J.J.; et al. Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: A randomized controlled trial (PAM study). Breast Cancer Res. 2022, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.L.; Medina, K.L.; Padula, C.B.; Tapert, S.F.; Brown, S.A. Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J. Child. Adolesc. Subst. Abuse 2011, 20, 135–154. [Google Scholar] [CrossRef] [Green Version]
- Lown, E.A.; Hijiya, N.; Zhang, N.; Srivastava, D.K.; Leisenring, W.M.; Nathan, P.C.; Castellino, S.M.; Devine, K.A.; Dilley, K.; Krull, K.R.; et al. Patterns and predictors of clustered risky health behaviors among adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer 2016, 122, 2747–2756. [Google Scholar] [CrossRef] [Green Version]
- Parsons, H.M.; Jewett, P.I.; Sadak, K.; Turcotte, L.M.; Vogel, R.I.; Blaes, A.H. e-Cigarette Use Among Young Adult Cancer Survivors Relative to the US Population. JAMA Oncol. 2020, 6, 923–926. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Brinkman, T.M.; Mulrooney, D.A.; Mzayek, Y.; Liu, W.; Banerjee, P.; Panoskaltsis-Mortari, A.; Srivastava, D.; Pui, C.H.; Robison, L.L.; et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer 2017, 123, 3410–3419. [Google Scholar] [CrossRef] [Green Version]
- Tonning Olsson, I.; Lubas, M.M.; Li, C.; Mandrell, B.N.; Banerjee, P.; Howell, C.R.; Ness, K.K.; Srivastava, D.; Robison, L.L.; Hudson, M.M.; et al. Insomnia and Neurocognitive Functioning in Adult Survivors of Childhood Cancer. JNCI Cancer Spectr. 2020, 4, pkaa008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Yang, L.S.; Yam, P.; Lam, C.S.; Chan, A.S.; Li, C.K.; Cheung, Y.T. Neurocognitive and Behavioral Outcomes of Chinese Survivors of Childhood Lymphoblastic Leukemia. Front. Oncol. 2021, 11, 655669. [Google Scholar] [CrossRef] [PubMed]
- Bannai, A.; Tamakoshi, A. The association between long working hours and health: A systematic review of epidemiological evidence. Scand. J. Work Environ. Health 2014, 40, 5–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.C.; Lopez, V.; Joyce Chung, O.K.; Ho, K.Y.; Chiu, S.Y. The impact of cancer on the physical, psychological and social well-being of childhood cancer survivors. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2013, 17, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.P.; Choi, K.C.; Li, A.M.; Hui, S.S.; Chan, M.H.; Wing, Y.K.; Ma, R.C.; Lam, C.W.; Lau, J.T.; So, W.Y.; et al. Association between physical activity and cardiovascular risk in Chinese youth independent of age and pubertal stage. BMC Public Health 2010, 10, 303. [Google Scholar] [CrossRef] [Green Version]
- Achenbach, T.M.; Ivanova, M.Y.; Rescorla, L.A. Empirically based assessment and taxonomy of psychopathology for ages 1½-90+ years: Developmental, multi-informant, and multicultural findings. Compr. Psychiatry 2017, 79, 4–18. [Google Scholar] [CrossRef]
- National Institute on Alcohol Abuse and Alcoholism, National Institute of Health, US Government. Drinking Levels Defined. Available online: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking (accessed on 25 October 2022).
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Centre for Health Protection. The Government of the Hong Kong Special Administrative Region. “Sleep”. Available online: https://www.chp.gov.hk/en/statistics/data/10/757/5525.html (accessed on 25 October 2022).
- Varni, J.W.; Burwinkle, T.M.; Katz, E.R.; Meeske, K.; Dickinson, P. The PedsQL in pediatric cancer: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer 2002, 94, 2090–2106. [Google Scholar] [CrossRef]
- Varni, J.W.; Limbers, C.A. The PedsQL™ Multidimensional Fatigue Scale in young adults: Feasibility, reliability and validity in a University student population. Qual. Life Res. 2007, 17, 105–114. [Google Scholar] [CrossRef]
- Li, J.; Brisson, C.; Clays, E.; Ferrario, M.M.; Ivanov, I.D.; Landsbergis, P.; Leppink, N.; Pega, F.; Pikhart, H.; Prüss-Üstün, A.; et al. WHO/ILO work-related burden of disease and injury: Protocol for systematic reviews of exposure to long working hours and of the effect of exposure to long working hours on ischaemic heart disease. Environ. Int. 2018, 119, 558–569. [Google Scholar] [CrossRef]
- Ewig, C.L.Y.; Cheng, Y.M.; Li, H.S.; Wong, J.C.L.; Cho, A.H.Y.; Poon, F.M.H.; Li, C.K.; Cheung, Y.T. Use of Chronic Prescription Medications and Prevalence of Polypharmacy in Survivors of Childhood Cancer. Front. Oncol. 2021, 11, 642544. [Google Scholar] [CrossRef] [PubMed]
- Ewig, C.L.-Y.; Hui, K.H.; Lee, S.L.K.; Leung, A.W.K.; Wong, G.L.-H.; Li, C.K.; Cheung, Y.T. Medication Burden Among Pediatric Cancer Survivors: Analysis of a Population-Wide Electronic Database in Hong Kong. JNCI Cancer Spectr. 2022, 6, pkac059. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Jiang, J.Y.; Tang, J.L.; Lam, A.; Fung, H.; Mercer, S.W. Health services research in the public healthcare system in Hong Kong: An analysis of over 1 million antihypertensive prescriptions between 2004–2007 as an example of the potential and pitfalls of using routinely collected electronic patient data. BMC Health Serv. Res. 2008, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.M.; Krull, K.R.; Howell, C.R.; Banerjee, P.; Brinkman, T.M.; Kaste, S.C.; Partin, R.E.; Srivastava, D.; Yasui, Y.; Armstrong, G.T.; et al. Physiologic Frailty and Neurocognitive Decline Among Young-Adult Childhood Cancer Survivors: A Prospective Study from the St Jude Lifetime Cohort. J. Clin. Oncol. 2021, 39, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Khan, R.B.; Liu, W.; Brinkman, T.M.; Edelmann, M.N.; Reddick, W.E.; Pei, D.; Panoskaltsis-Mortari, A.; Srivastava, D.; Cheng, C.; et al. Association of Cerebrospinal Fluid Biomarkers of Central Nervous System Injury with Neurocognitive and Brain Imaging Outcomes in Children Receiving Chemotherapy for Acute Lymphoblastic Leukemia. JAMA Oncol. 2018, 4, e180089. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Society. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Mayo, S.J.; Lustberg, M.; Dhillon, M.H.; Nakamura, Z.M.; Allen, D.H.; Von Ah, D.; CJanelsins, M.; Chan, A.; Olson, K.; Tan, C.J.; et al. Cancer-related cognitive impairment in patients with non-central nervous system malignancies: An overview for oncology providers from the MASCC Neurological Complications Study Group. Support Care Cancer 2021, 29, 2821–2840. [Google Scholar] [CrossRef]
- Shahid, M.; Kim, J. Exercise May Affect Metabolism in Cancer-Related Cognitive Impairment. Metabolites 2020, 10, 377. [Google Scholar] [CrossRef]
- Tari, B.; Vanhie, J.J.; Belfry, G.R.; Shoemaker, J.K.; Heath, M. Increased cerebral blood flow supports a single-bout postexercise benefit to executive function: Evidence from hypercapnia. J. Neurophysiol. 2020, 124, 930–940. [Google Scholar] [CrossRef]
- Cai, H.; Li, G.; Hua, S.; Liu, Y.; Chen, L. Effect of exercise on cognitive function in chronic disease patients: A meta-analysis and systematic review of randomized controlled trials. Clin. Interv. Aging 2017, 12, 773–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsey, I.; Chan, A.; Charalambous, A.; Cheung, Y.T.; Darling, H.S.; Eng, L.; Grech, L.; Hart, N.H.; Kirk, D.; Mitchell, S.A.; et al. Exercise counselling and referral in cancer care: An international scoping survey of health care practitioners’ knowledge, practices, barriers, and facilitators. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2022, 30, 9379–9391. [Google Scholar] [CrossRef]
- Ezenwankwo, E.F.; Nnate, D.A.; Usoro, G.D.; Onyeso, C.P.; Anieto, I.B.; Ibeneme, S.C.; Albertus, Y.; Lambert, V.E.; Ezeukwu, A.O.; Abaraogu, U.O.; et al. A scoping review examining the integration of exercise services in clinical oncology settings. BMC Health Serv. Res. 2022, 22, 236. [Google Scholar] [CrossRef]
- The Centre for Health Protection. The Government of Hong Kong Special Administrative Region. “Alcohol Consumption”. Available online: https://www.chp.gov.hk/en/healthtopics/content/25/8798.html (accessed on 25 October 2022).
- The Government of the Hong Kong Special Administrative Region. Press Release “FHB Announces the First Incidence for Latest Smoking Prevalence Rate in Hong Kong to Attain Single Digit”. 2022. Available online: https://www.info.gov.hk/gia/general/202205/26/P2022052600683.htm#:~:text=The%20Secretary%20for%20Food%20and,9.5%20per%20cent%20in%202021 (accessed on 25 October 2022).
- Symmes, A.; Winters, K.C.; Fahnhorst, T.; Botzet, A.; Lee, S.; August, G.; Realmuto, G. The Association Between Attention-Deficit Hyperactivity Disorder and Nicotine Use Among Adolescents and Young Adults. J. Child Adolesc. Subst. Abuse 2015, 24, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Anstey, K.J.; Chen, R. Invited Commentary: Secondhand Smoke-an Underrecognized Risk Factor for Cognitive Decline. Am. J. Epidemiol. 2018, 187, 919–921. [Google Scholar] [CrossRef]
- Xie, Z.; Ossip, D.J.; Rahman, I.; O’Connor, R.J.; Li, D. Electronic cigarette use and subjective cognitive complaints in adults. PLoS ONE 2020, 15, e0241599. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.E.; Hatton, S.N.; Lyons, M.J.; Puckett, O.K.; Whitsell, N.; Kremen, W.S. Young adult cognitive ability moderates effects of lifestyle behaviors on brain aging in late midlife: A longitudinal study of cognitive reserve. Alzheimer’s Dement. 2020, 16, e046011. [Google Scholar] [CrossRef]
- Cadar, D.; Pikhart, H.; Mishra, G.; Stephen, A.; Kuh, D.; Richards, M. The role of lifestyle behaviors on 20-year cognitive decline. J. Aging Res. 2012, 2012, 304014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Ecker, B.L.; Tang, R.; Maggino, L.; Roses, R.E.; DeMatteo, R.P.; Fraker, D.L.; Karakousis, G.C. Trends in practice patterns and outcomes: A decade of sarcoma care in the United States. Surg. Oncol. 2019, 29, 168–177. [Google Scholar] [CrossRef]

| Characteristics | All Survivors N = 116 | Osteosarcoma N = 57 | Soft-Tissue Sarcoma N = 59 |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Sex | |||
| Male | 60 (51.7) | 31 (54.4) | 29 (49.2) |
| Female | 56 (48.3) | 26 (45.6) | 30 (50.8) |
| Age at interview (years) * | 28.2 (8.2) | 29.3 (8.2) | 27.2 (8.1) |
| >15–18 years | 15 (12.9) | 6 (10.5) | 9 (15.3) |
| ≥18–30 years | 62 (53.4) | 29 (50.9) | 33 (55.9) |
| >30 years | 39 (33.7) | 22 (38.6) | 17 (28.8) |
| Age of Diagnosis (years) * | 13.3 (7.2) | 13.0 (5.2) | 13.5 (8.8) |
| <15 years (diagnosed at childhood) | 82 (70.7) | 44 (77.2) | 38 (64.4) |
| ≥15–18 years (diagnosed at adolescence) | 19 (16.4) | 8 (14.0) | 11 (18.6) |
| ≥18–39 years (diagnosed at young adulthood) | 15 (12.9) | 5 (8.8) | 10 (17.0) |
| Years from diagnosis * | 14.9 (7.6) | 16.2 (7.5) | 13.7 (7.5) |
| ≤5 years | 16 (13.8) | 3 (5.3) | 13 (22.0) |
| >5–10 years | 21 (18.1) | 11 (19.3) | 10 (16.9) |
| >10–20 years | 52 (44.8) | 27 (47.4) | 25 (42.3) |
| >20 years | 27 (23.2) | 16 (28.0) | 11 (18.6) |
| Education level | |||
| Secondary school or below | 42 (36.2) | 22 (38.6) | 20 (33.9) |
| Post-secondary school or above | 70 (60.3) | 33 (57.9) | 37 (62.7) |
| Employment status | |||
| Employed | 81 (69.8) | 43 (75.4) | 38 (64.4) |
| Not employed | 20 (17.2) | 8 (14.0) | 12 (20.3) |
| Students | 15 (12.9) | 6 (10.5) | 9 (15.3) |
| Clinical characteristics | |||
| Tumor site (for osteosarcoma) | |||
| Femur | - | 32 (56.1) | |
| Tibia | - | 14 (24.7) | |
| Humerus | - | 5 (8.8) | |
| Fibula | - | 3 (5.2) | |
| Others (hip, neck) | - | 3 (5.2) | |
| Tumor site (for soft-tissue sarcoma) | |||
| Lower extremity | - | - | 18 (30.5) |
| Upper extremity | - | - | 6 (10.2) |
| Abdomen/pelvis | - | - | 17 (28.8) |
| Head and neck | - | - | 10 (16.9) |
| Chest | - | - | 8 (13.6) |
| Subtypes (for soft-tissue sarcoma) | |||
| Rhabdomyosarcoma | - | - | 15 (25.4) |
| Ewing sarcoma | - | - | 11 (18.6) |
| Liposarcoma | - | - | 6 (10.2) |
| Synovial Sarcoma | - | - | 5 (8.5) |
| Clear Cell Sarcoma | - | - | 4 (6.8) |
| PNET | - | - | 3 (5.1) |
| Others | - | - | 15 (25.4) |
| Relapse | |||
| No | 105 (90.5) | 50 (87.7) | 54 (91.5) |
| Yes | 11 (9.5) | 7 (12.3) | 5 (8.5) |
| CHCs | |||
| No | 60 (51.7) | 27 (47.4) | 33 (55.9) |
| Yes | 53 (45.7) | 28 (49.1) | 25 (42.4) |
| Endocrine # | 8 (6.9) | 1 (1.8) | 7 (11.9) |
| Metabolic # | 27 (23.3) | 13 (22.8) | 14 (23.7) |
| Renal # | 12 (10.3) | 12 (21.1) | 0 (0) |
| Cardiovascular # | 8 (6.9) | 6 (10.5) | 2 (3.4) |
| Pulmonary # | 3 (2.6) | 0 (0) | 3 (5.1) |
| Other CHCs: | |||
| Hearing | 14 (12.1) | 11 (19.3) | 3 (5.1) |
| Behavioral | 8 (6.9) | 3 (5.3) | 5 (8.5) |
| Second cancer | 4 (3.4) | 3 (5.3) | 1 (1.7) |
| Musculoskeletal | 4 (3.4) | 1 (1.8) | 3 (5.1) |
| Vision | 4 (3.4) | 0 (0) | 4 (6.8) |
| Gastrointestinal | 4 (3.4) | 1 (1.8) | 3 (5.1) |
| Neurological | 5 (4.3) | 0 (0) | 5 (8.5) |
| Liver | 3 (2.6) | 1 (1.8) | 2 (3.4) |
| Body mass index (years) * | 21.7 (3.9) | 21.6 (3.6) | 21.7 (4.1) |
| Underweight | 16 (13.8) | 9 (15.8) | 7 (11.9) |
| Normal | 73 (62.9) | 30 (52.6) | 30 (50.8) |
| Overweight/obese | 27 (23.3) | 13 (22.8) | 14 (23.7) |
| Treatment characteristics | |||
| Chemotherapy | |||
| No | 21 (18.1) | 5 (8.8) | 16 (27.1) |
| Yes | 95 (81.9) | 52(91.2) | 43 (72.9) |
| Doxorubicin | 72 (62.1) | 51 (89.5) | 21 (35.6) |
| Cisplatin | 51 (44.0) | 49 (86.0) | 2 (3.4) |
| High-dose methotrexate | 50 (43.1) | 49 (86.0) | 1 (1.7) |
| Ifosamide | 46 (39.7) | 27 (47.4) | 19 (32.2) |
| Etoposide | 38 (32.8) | 26 (45.6) | 12 (20.3) |
| Vincristine | 35 (30.2) | 1 (1.8) | 34 (57.6) |
| Actinomycin D | 28 (24.1) | 1 (1.8) | 27 (45.8) |
| Cyclophosamide | 22 (19.0) | 3 (5.3) | 19 (32.2) |
| Carboplain | 6 (5.2) | 4 (7.0) | 2 (3.4) |
| Bleomycin | 2 (1.7) | 1 (1.8) | 1 (1.7) |
| Radiation | |||
| No | 78 (67.2) | 52 (91.2) | 26 (44.1) |
| Yes | 36 (31.0) | 4 (7.0) | 32 (54.2) |
| Cranial area | 8 (6.9) | 0 (0) | 8 (13.6) |
| Non-cranial areas (other body sites) | 28 (24.1) | 4 (7.0) | 24 (40.7) |
| Surgery | |||
| No | 5 (4.3) | 0 (0) | 5 (8.47) |
| Yes | 110 (94.8) | 57 (100) | 53 (89.8) |
| Treatment combinations | |||
| Surgery only | 14 (12.1) | 4 (7.0) | 10 (16.9) |
| Surgery and chemotherapy | 63 (54.3) | 48 (84.2) | 15 (25.4) |
| Surgery, chemotherapy and radiation | 27 (23.3) | 3 (5.3) | 24 (40.7) |
| Others | 12 (10.3) | 2 (3.5) | 10 16.9) |
| Neurocognitive Outcomes | ||||
|---|---|---|---|---|
| Mean (SD) T-Scores * | Impaired% ^ | 95% CI ^ | p† | |
| Attention(Conners Continuous Performance Test-III) | ||||
| Inattentiveness: | ||||
| Omission | 54.4 (4.9) | 4.3 | 0–8.0 | 0.0003 |
| Detectability | 54.0 (8.6) | 13.0 | 5.4–16.9 | 0.0003 |
| Variability | 55.3 (6.9) | 4.3 | 0–8.0 | 0.0003 |
| HRT SD | 57.2 (7.0) | 9.4 | 4.1–14.8 | 0.0003 |
| Impulsivity: | ||||
| Perseverations | 52.4 (5.4) | 0.8 | 0–2.5 | 1.00 |
| Commission | 50.6 (9.8) | 0.8 | 0–2.5 | 0.51 |
| Vigilance: | ||||
| HRT ISI change | 51.2 (7.6) | 1.7 | 0–4.1 | 0.13 |
| Sustained attention: | ||||
| HRT block change | 49.3 (8.5) | 0.8 | 0–2.5 | 0.47 |
| Memory(Modified Taylor Complex Figure) | ||||
| Visual memory (Immediate recall) | 52.6 (13.7) | 16.4 | 9.6–23.1 | 0.067 |
| Visual memory (Delayed recall) | 53.1 (13.4) | 19.0 | 11.8–26.1 | 0.025 |
| Motor processing speed | ||||
| Visual search (TMT-A) | 48.7 (9.4) | 6.0 | 1.7–10.4 | 0.17 |
| Motor processing speed (GPB) | 62.3 (17.2) | 34.5 | 25.8–43.1 | 0.003 |
| Cognitive flexibility(TMT-B) | 53.5 (13.9) | 18.1 | 11.1–25.1 | 0.018 |
| Lifestyle Factors | Number (%) |
|---|---|
| Physical activity * median (IQR) CUHK-PARCY score | 5 (2–6) points |
| Low level (0 to 3 points) | 50 (43.1%) |
| Moderate level (4 to 6 points) | 40 (34.5%) |
| High level (7 to 10 points) | 26 (22.4%) |
| Sleep | |
| Self-reported average hours of actual sleep † | |
| <7 h/day | 42 (37.5%) |
| ≥7 h/day | 70 (62.5%) |
| Median (IQR) sleep–rest fatigue score ^ | 62.5 (50.0–70.8) |
| Low level of fatigue | 48 (41.4%) |
| High level of fatigue | 68 (58.6%) |
| Working hours † | |
| Self-reported average working hours/day | |
| ≤9 h/day | 66 (81.5%) |
| >9 h/day | 15 (18.5%) |
| Alcohol consumption | |
| Non-drinker | 104 (89.7%) |
| Drinkers Ɨ | 12 (10.3%) |
| No. of heavy drinking days during the past 6 months (range) | (1–20) |
| Smoking | |
| Never smokers | 109 (94.0%) |
| Current smoker | 7 (6.0%) |
| Average no. of cigarettes/day during the past 6 months (range) | (2–20) |
| Substance abuse | |
| No | 115 (99.1%) |
| Yes | 1 (0.9%) |
| CPT Detectability * | CPT Omissions * | CPT HRTSD * | CPT Variability * | Visual Memory (Delayed Recall) * | Motor Processing Speed * | Cognitive Flexibility * | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p | |
| Physical activity ^ | −0.88 | 0.28 | 0.002 | −0.97 | 0.32 | 0.003 | −0.18 | 0.23 | 0.44 | −0.39 | 0.22 | 0.084 | −0.66 | 0.45 | 0.14 | −0.13 | 0.56 | 0.82 | −0.61 | 0.46 | 0.19 |
| Smoking | |||||||||||||||||||||
| Current/ever | 6.69 | 3.39 | 0.048 | 8.96 | 3.81 | 0.020 | −0.71 | 2.77 | 0.79 | −0.79 | 2.71 | 0.77 | 7.44 | 5.29 | 0.16 | −6.55 | 6.41 | 0.31 | −4.08 | 5.52 | 0.46 |
| Never | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||||||||
| Alcohol | |||||||||||||||||||||
| Drinkers | 4.12 | 2.69 | 0.13 | 6.65 | 3.00 | 0.029 | 0.40 | 2.19 | 0.85 | 1.82 | 2.13 | 0.39 | −0.92 | 4.36 | 0.83 | 9.98 | 5.18 | 0.056 | 5.76 | 4.33 | 0.18 |
| Non-drinkers | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||||||||
| Sleep | |||||||||||||||||||||
| Sleep hours | |||||||||||||||||||||
| <7 h/day | 1.90 | 1.69 | 0.26 | 3.24 | 1.92 | 0.095 | 0.44 | 1.40 | 0.75 | 0.75 | 1.36 | 0.58 | 4.30 | 2.62 | 0.10 | 2.00 | 2.98 | 0.50 | 1.33 | 2.78 | 0.63 |
| ≥7 h/day | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||||||||
| Sleep–rest fatigue † | −0.07 | 0.04 | 0.08 | −0.05 | 0.04 | 0.28 | −0.06 | 0.03 | 0.041 | −0.08 | 0.03 | 0.005 | −0.03 | 0.06 | 0.64 | 0.03 | 0.08 | 0.70 | 0.01 | 0.06 | 0.84 |
| Working hours Ɨ | |||||||||||||||||||||
| >9 h/day | 5.42 | 2.33 | 0.023 | 5.94 | 2.61 | 0.025 | 2.88 | 2.24 | 0.20 | 2.74 | 2.16 | 0.21 | 0.13 | 3.95 | 0.97 | 6.27 | 4.62 | 0.17 | 5.22 | 1.80 | 0.005 |
| ≤9 h/day | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheung, Y.T.; Ma, C.T.; Li, M.C.H.; Zhou, K.R.; Loong, H.H.F.; Chan, A.S.Y.; Wong, K.C.; Li, C.K. Associations between Lifestyle Factors and Neurocognitive Impairment among Chinese Adolescent and Young Adult (AYA) Survivors of Sarcoma. Cancers 2023, 15, 799. https://doi.org/10.3390/cancers15030799
Cheung YT, Ma CT, Li MCH, Zhou KR, Loong HHF, Chan ASY, Wong KC, Li CK. Associations between Lifestyle Factors and Neurocognitive Impairment among Chinese Adolescent and Young Adult (AYA) Survivors of Sarcoma. Cancers. 2023; 15(3):799. https://doi.org/10.3390/cancers15030799
Chicago/Turabian StyleCheung, Yin Ting, Chung Tin Ma, Michael Can Heng Li, Keary Rui Zhou, Herbert Ho Fung Loong, Agnes Sui Yin Chan, Kwok Chuen Wong, and Chi Kong Li. 2023. "Associations between Lifestyle Factors and Neurocognitive Impairment among Chinese Adolescent and Young Adult (AYA) Survivors of Sarcoma" Cancers 15, no. 3: 799. https://doi.org/10.3390/cancers15030799
APA StyleCheung, Y. T., Ma, C. T., Li, M. C. H., Zhou, K. R., Loong, H. H. F., Chan, A. S. Y., Wong, K. C., & Li, C. K. (2023). Associations between Lifestyle Factors and Neurocognitive Impairment among Chinese Adolescent and Young Adult (AYA) Survivors of Sarcoma. Cancers, 15(3), 799. https://doi.org/10.3390/cancers15030799







