CA125 for the Diagnosis of Advanced Urothelial Carcinoma of the Bladder: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Statistical Analysis
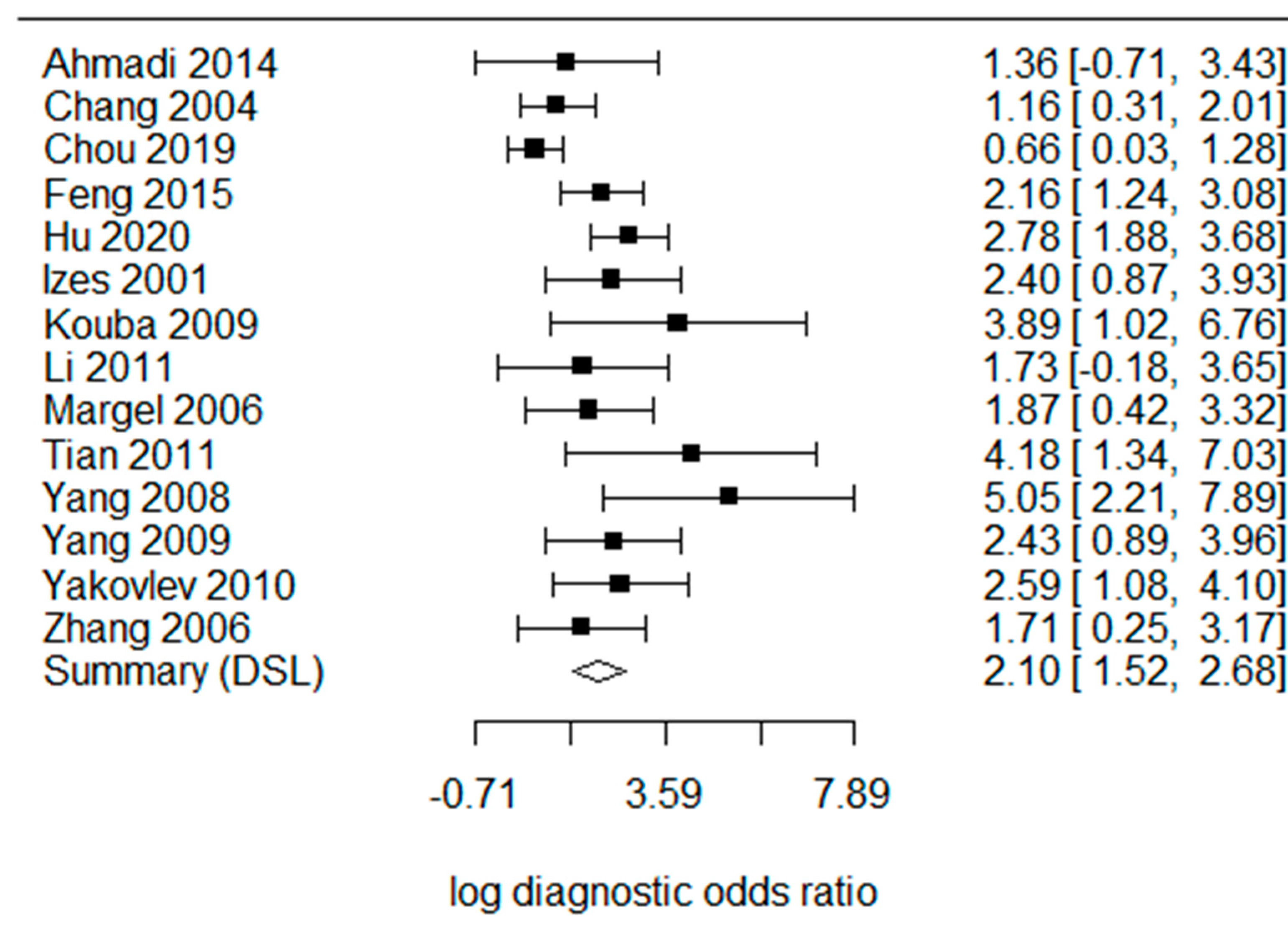
4. Results
5. Discussions and Systematic Review
5.1. CA125 for Advanced UCB
5.2. CA125 as a Prognostic Marker
5.3. Correlation of CA125 with Tumor Stage and Grade
5.4. CA125 for Metastatic UCB
5.5. CA125 in Follow-Up
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Babjuk, M.; Böhle, A.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Hernández, V.; Kaasinen, E.; Palou, J.; Rouprêt, M.; et al. EAU Guidelines on Non–Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur. Urol. 2016, 71, 447–461. [Google Scholar] [CrossRef]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA A Cancer J. Clin. 2017, 68, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef]
- Vargas, H.A.; Akin, O.; Schoder, H.; Olgac, S.; Dalbagni, G.; Hricak, H.; Bochner, B.H. Prospective evaluation of MRI, (1)(1)C-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur. J. Radiol. 2012, 81, 4131–4137. [Google Scholar] [CrossRef]
- Luo, C.; Huang, B.; Wu, Y.; Chen, J.; Chen, L. Use of Vesical Imaging-Reporting and Data System (VI-RADS) for detecting the muscle invasion of bladder cancer: a diagnostic meta-analysis. Eur. Radiol. 2020, 30, 4606–4614. [Google Scholar] [CrossRef]
- Yin, B.W.T.; Lloyd, K.O. Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16. J. Biol. Chem. 2001, 276, 27371–27375. [Google Scholar] [CrossRef]
- Rump, A.; Morikawa, Y.; Tanaka, M.; Minami, S.; Umesaki, N.; Takeuchi, M.; Miyajima, A. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 2004, 279, 9190–9198. [Google Scholar] [CrossRef]
- Comamala, M.; Pinard, M.; Theriault, C.; Matte, I.; Albert, A.; Boivin, M.; Beaudin, J.; Piche, A.; Rancourt, C. Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells. Br. J. Cancer 2011, 104, 989–999. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Duffy, M.J.; Stenman, U.H.; Lilja, H.; Brunner, N.; Chan, D.W.; Babaian, R.; Bast, R.C., Jr.; Dowell, B.; Esteva, F.J.; et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicu-lar, prostate, colorectal, breast, and ovarian cancers. Clin. Chem. 2008, 54, e11–e79. [Google Scholar]
- Cotton, S.; Azevedo, R.; Gaiteiro, C.; Ferreira, D.; Lima, L.; Peixoto, A.; Fernandes, E.; Neves, M.; Neves, D.; Amaro, T.; et al. Targeted Oglycoproteomics explored increased sialylation and identified MUC16 as a poor prognosis biomarker in advanced-stage bladder tumours. Mol. Oncol. 2017, 11, 895–912. [Google Scholar] [CrossRef]
- Margel, D.; Tal, R.; Neuman, A.; Konichezky, M.; Sella, A.; Baniel, J. Prediction of Extravesical Disease by Preoperative Serum Markers in Patients With Clinically Organ Confined Invasive Bladder Cancer. J. Urol. 2006, 175, 1253–1257. [Google Scholar] [CrossRef]
- Benoit, T.; Keller, E.X.; Wolfsgruber, P.; Hermanns, T.; Gunthart, M.; Banzola, I.; Sulser, T.; Provenzano, M.; Poyet, C. High VEGF-D and Low MMP-2 Serum Levels Predict Nodal-Positive Disease in Invasive Bladder Cancer. Med. Sci. Monit. 2015, 21, 2266–2274. [Google Scholar]
- Lin, Y.; Zhang, Y.; Luo, L.; Zhang, X. Clinical effect of robot-assisted radical cystectomy in bladder cancer. Am. J. Transl. Res. 2021, 13, 10545–10553. [Google Scholar]
- Shim, S.R.; Kim, S.J.; Lee, J. Diagnostic test accuracy: application and practice using R software. Epidemiol. Health 2019, 41, e2019007. [Google Scholar] [CrossRef]
- Ahmadi, H.; Djaladat, H.; Cai, J.; Miranda, G.; Daneshmand, S. Precystectomy serum levels of carbohydrate antigen 19-9, carbohydrate antigen 125, and carcinoembryonic antigen: Prognostic value in invasive urothelial carcinoma of the bladder. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 648–656. [Google Scholar] [CrossRef]
- Chang, A.; Cai, J.; Miranda, G.; Groshen, S.; Skinner, D.; Stein, J.P. USEFULNESS OF CA 125 AS A PREOPERATIVE PROGNOSTIC MARKER FOR TRANSITIONAL CELL CARCINOMA OF THE BLADDER. J. Urol. 2004, 172, 2182–2186. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Shu, K.-H.; Chen, H.-C.; Wang, M.-C.; Chang, C.-C.; Hsu, B.-G.; Chen, T.-W.; Chen, C.-L.; Huang, C.-C. Development and validation of a nomogram for urothelial cancer in patients with chronic kidney disease. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Li, D.L.; Zhang, W. Clinical significance of VEGF and CA125. Med. Inf. (In Chinese). 2011, 24, 118. [Google Scholar]
- Feng, R.; Li, Z.X.; Shen, B.; Wang, X.; Ge, G.C.; Wu, D.; Jia, Y.J. Detection of serum CA125 in patients with bladder urothelial carci-noma and the clinical significance. Hainan Med. J. 2015, 26, 3018–3020. [Google Scholar]
- Yang, H.W.; Liu, L. Clinical significance of serum CA125 in patients with transitional cell carcinoma of the bladder. Shenyang Budui Yiyao 2009, 22, 391–392. (In Chinese) [Google Scholar]
- Hu, J.D.; Zhang, J.J.; Yan, H.Q.; Xu, Y.G.; Shi, R.J.; Cheng, Z.; Wang, S.H. Predictive value of serum VEGF, TSGF and CA125 for prognosis in patients with invasive bladder cancer. J. Minim. Invasive Urol. 2020, 9, 400–405. [Google Scholar]
- Izes, J.K.; Dyer, M.W.; Callum, M.G.; Bankes, P.; Libertino, J.A.; Caffrey, J.A. Ca 125 as a marker of tumor activity in advanced urothelial malignancy. J. Urol. 2001, 165, 1908–1913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Expression of E-cadherin in transitional cell carcinoma of bladder and its relationship with serum CA125. Xiandai Miniao Waike Zazhi 2006, 11, 83–85. [Google Scholar]
- Yang, J.Y.; Wei, W.; Liu, J.G.; Hu, X.Q.; Lin, Y.; Zhou, J.; Jiang, H.; Jiang, X.J. Clinical significance of elevation of serum CA125 level in patients with metastatic bladder carcinoma. Chin. Ger. J. Clin. Oncol. 2008, 7, 477–479. [Google Scholar] [CrossRef]
- Kouba, E.J.; Lentz, A.; Wallen, E.M.; Pruthi, R.S. Clinical use of serum CA-125 levels in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder. Urol. Oncol. Semin. Orig. Investig. 2009, 27, 486–490. [Google Scholar] [CrossRef]
- Tian, X.Q.; Xing, N.X. Clinical significance of CA125 in advanced bladder cancer. Chin. J. Postgrad. Med. 2011, 34, 32–34. [Google Scholar]
- Yakovlev, P.G.; Sakalo, V.S.; Mrachkovskiy, V.V.; Kondratenko, A.V.; Hrygorenko, V.M.; Olijnichenko, G.P. Sensitivity and specificity of serum marker CA-125 in patients with urothelial carcinoma. Eur. Urol. Suppl. 2010, 9, 544. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Lu, Y.; Jiang, H.; Zhang, L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J. Cell Biochem. 2019, 120, 5852–5859. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Harel, A.; Yossepowitch, O.; Baniel, J. A novel algorithm to improve pathologic stage prediction of clinically or-gan-confined muscle-invasive bladder cancer. Cancer 2009, 115, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Tal, R.; Baniel, J. Serum tumor markers may predict overall and disease specific survival in patients with clinically organ confined invasive bladder cancer. J. Urol. 2007, 178, 2297–2300. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, S.T.; Clifford, T.G.; Djaladat, H.; Schuckman, A.K.; Wayne, K.; Miranda, G.; Cai, J.; Sadeghi, S.; Dorff, T.; Quinn, D.I.; et al. Association between precystectomy epithelial tumor marker response to neoadjuvant chemotherapy and oncological out-comes in urothelial bladder cancer. Urol. Oncol. 2019, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Bostrom, P.; Baniel, J.; Yossepowitch, O.; Zlotta, A.; Fleshner, N. External Validation of a Biomarker Based Pre-Cystectomy Algorithm to Predict Nonorgan Confined Urothelial Cancers. J. Urol. 2012, 187, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. A PROSPECTIVE ANALYSIS OF THE DIAGNOSTIC YIELD RESULTING FROM THE ATTENDANCE OF 4020 PATIENTS AT A PROTOCOL-DRIVEN HAEMATURIA CLINIC. BJU Int. 2007, 99, 699. [Google Scholar] [CrossRef]
- Faiena, I.; Rosser, C.J.; Chamie, K.; Furuya, H. Diagnostic biomarkers in non-muscle invasive bladder cancer. World J. Urol. 2018, 37, 2009–2016. [Google Scholar] [CrossRef]
- Xie, Z.H.; Qi, H.G.; Zhang, S.W.; Yao, X.P. The study on the seven tumor markers including serum CK19 and AFP in the com-bined diagnosis of bladder cancer. China Mod. Dr. 2017, 55, 23–27. [Google Scholar]
- Zhang, Z.; Fan, W.; Deng, Q.; Tang, S.; Wang, P.; Xu, P.; Wang, J.; Yu, M. The prognostic and diagnostic value of circulating tumor cells in bladder cancer and upper tract urothelial carcinoma: A meta-analysis of 30 published studies. Oncotarget 2017, 8, 59527–59538. [Google Scholar] [CrossRef]
- Christensen, E.; Birkenkamp-Demtröder, K.; Sethi, H.; Shchegrova, S.; Salari, R.; Nordentoft, I.K.; Wu, H.-T.; Knudsen, M.; Lamy, P.; Lindskrog, S.V.; et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. J. Clin. Oncol. 2019, 37, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Huddart, R.A.; Jay, G.; Norman, A.; Dearnaley, D.P.; Horwich, A. Horwich, The utility of tumour markers in assessing the re-sponse to chemotherapy in advanced bladder cancer. Br. J. Cancer 2000, 82, 1952–1957. [Google Scholar] [PubMed]
- Manvar, A.M.; Wallen, E.M.; Pruthi, R.S.; E Nielsen, M. Prognostic value of CA 125 in transitional cell carcinoma of the bladder. Expert Rev. Anticancer Ther. 2010. 10, 1877–1881. [CrossRef]




| Study | Cut-Off | Advanced | Number | Age | Female (%) |
|---|---|---|---|---|---|
| Ahmadi 2014 | 35 | T3-4N1 | 186 | 36–89 | 36.6 |
| Chang 2004 | 35 | T3-4N1 | 287 | 34–89 | 21.3 |
| Chou 2019 | 35 | T3-4N1 | 169 | 37–90 | 35.5 |
| Feng 2015 | 35 | T3-4N1 | 251 | 21–92 | 48.6 |
| Hu 2020 | 35 | T3-4N1 | 118 | 55–79 | 49.1 |
| Izes 2001 | 35 | T3-4N1M1 | 68 | 43–86 | NA |
| Kouba 2009 | 35 | T3-4N1 | 92 | NA | 31.5 |
| Li 2011 | 35 | T2-4 | 90 | 31–88 | 26.7 |
| Margel 2006 | 35 | T3-4N1 | 91 | 67 ± 9 | 15.4 |
| Tian 2011 | 35 | T3-4N1 | 92 | 47–85 | 40.2 |
| Yang 2008 | 35 | T3-4M1 | 58 | 45–86 | 22.4 |
| Yang 2009 | 35 | T2-4 | 46 | 43–74 | 10.9 |
| Yakovlev 2010 | 30 | N1M1 | 53 | 45–71 | 24.5 |
| Zhang 2006 | 20 | T2-4 | 60 | 41–75 | 30.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-J.; Hu, R.-M.; Chen, H.-C.; Lin, C.-C.; Lee, C.-Y.; Chou, C.-Y. CA125 for the Diagnosis of Advanced Urothelial Carcinoma of the Bladder: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 813. https://doi.org/10.3390/cancers15030813
Lin H-J, Hu R-M, Chen H-C, Lin C-C, Lee C-Y, Chou C-Y. CA125 for the Diagnosis of Advanced Urothelial Carcinoma of the Bladder: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(3):813. https://doi.org/10.3390/cancers15030813
Chicago/Turabian StyleLin, Hsuan-Jen, Rouh-Mei Hu, Hung-Chih Chen, Chung-Chih Lin, Chi-Yu Lee, and Che-Yi Chou. 2023. "CA125 for the Diagnosis of Advanced Urothelial Carcinoma of the Bladder: A Systematic Review and Meta-Analysis" Cancers 15, no. 3: 813. https://doi.org/10.3390/cancers15030813
APA StyleLin, H.-J., Hu, R.-M., Chen, H.-C., Lin, C.-C., Lee, C.-Y., & Chou, C.-Y. (2023). CA125 for the Diagnosis of Advanced Urothelial Carcinoma of the Bladder: A Systematic Review and Meta-Analysis. Cancers, 15(3), 813. https://doi.org/10.3390/cancers15030813





