Groin Surveillance by Serial Ultrasonography Rather Than Sentinel Node Biopsy or Inguinofemoral Lymphadenectomy for Patients with Vulvar Cancer: A Pilot Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Considerations
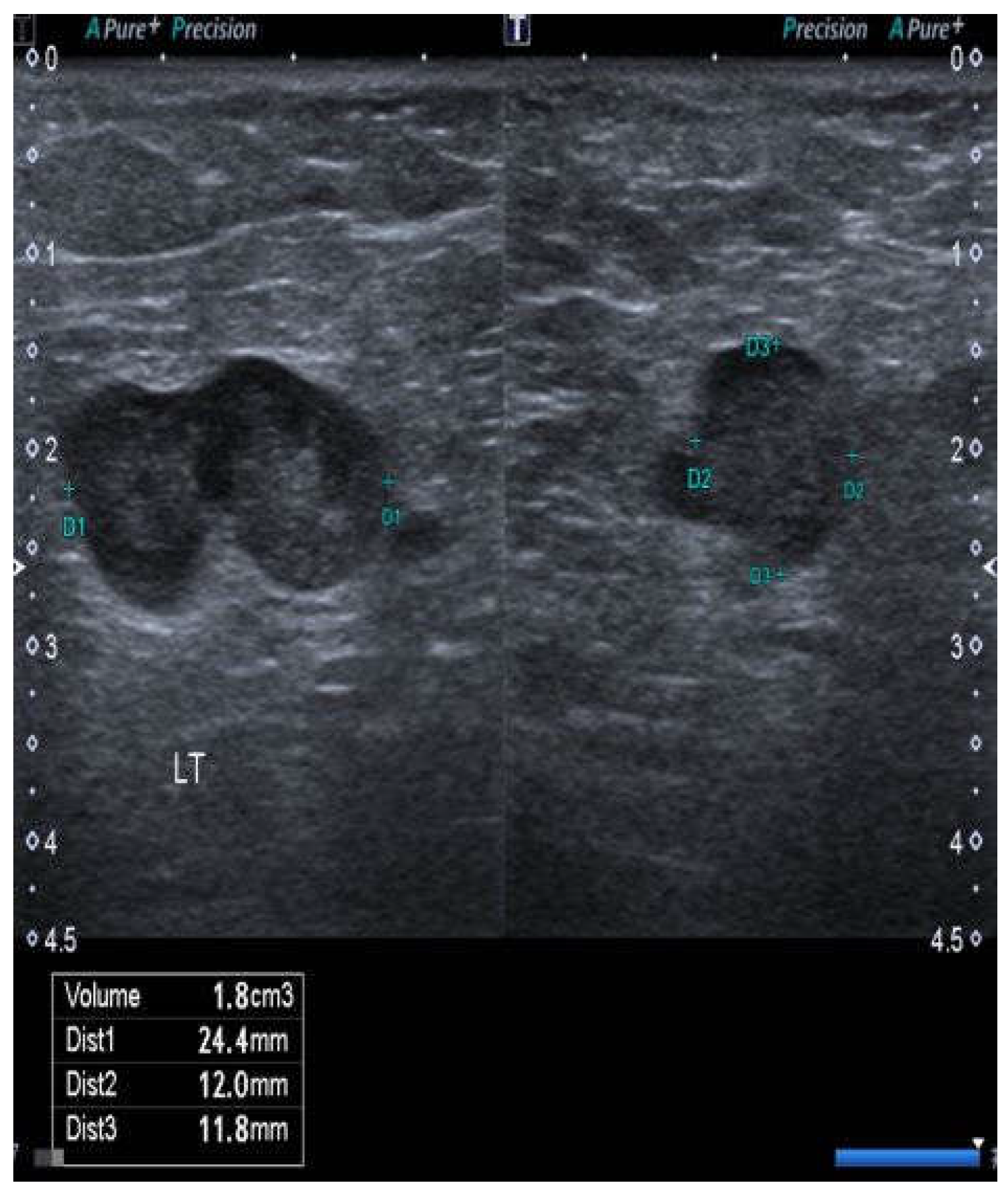
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2020 Submission Data (1999–2018): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; Centers for Disease Control and Prevention and National Cancer Institute: Bethseda, MD, USA, 2021. Available online: www.cdc.gov/cancer/dataviz (accessed on 20 August 2021).
- National Comprehensive Cancer Network (NCNN) Clinical Practice Guidelines in Oncology—Vulvar Cancer (Squamous Cell Carcinoma). Version 3. 2020. Available online: https://www.ncnn.org/professionals/physician_gls/pdf/vulvar.pdf (accessed on 20 August 2021).
- Homesley, H.D.; Bundy, B.N.; Sedlis, A.; Adcock, L. Radiotherapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet. Gynecol. 1986, 68, 733–740. [Google Scholar]
- van der Velden, J.; Pleunis, P.; Barlow, E.; Zijlmans, H.; de Hullu, J.; Hacker, N.F.; Fons, G. Radiotherapy is not indicated in patients with vulvar squamous cell carcinoma and only one occult intracapsular groin node metastasis. Gynecol. Oncol. 2021, 160, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Barlow, E.L.; Donoghoe, M.W.; Hacker, N.F. Morbidity related to the groin lymph node dissection in vulvar cancer. Int. J. Gynecol. Clin. Pract. 2019, 6, 149. [Google Scholar] [CrossRef]
- Gaarenstroom, K.N.; Kenter, G.G.; Trimbos, J.B.; Agous, I.; Amant, F.; Peters, A.A.; Vergote, I. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int. J. Gynecol. Cancer 2003, 13, 522–527. [Google Scholar] [CrossRef]
- Hinten, F.; van den Einden, L.C.; Hendriks, J.C.; van der Zee, A.G.J.; Bulten, J.; Massuger, L.F.A.G.; van de Nieuwenhof, H.P.; de Hullu, J.A. Risk factors for short and long-term complications after groin surgery in vulvar cancer. Brit. J. Cancer 2011, 105, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Scott, E.; Sukumvanich, P.; Smith, A.; Olawaiye, A.; Comerci, J.; Kelley, J.L.; Beriwal, S.; Huang, M. The effect of groin treatment modality and sequence on clinically significant chronic lymphedema in patients with vulvar carcinoma. Int. J. Gynecol. Cancer 2015, 25, 119–124. [Google Scholar] [CrossRef]
- Carlson, J.W.; Kauderer, J.; Walker, J.L.; Gold, M.A.; O’Malley, D.; Tuller, E.; Clarke–Pearson, D.L. A randomised phase III trial of VH fibrin sealant to reduce lymphedema after inguinal lymph node dissection. A Gynecologic Oncology Group study. Gynecol. Oncol. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Serrado, M.A.; Horta, M.; Cunha, T.M. State of the art in vulvar imaging. Radio Bras. 2019, 52, 316–324. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.; Zobbe, V.; Thranov, I.R.; Damgaard Pederson, K. Relevance of computerized tomography in the preoperative evaluation of patients with vulvar cancer: A prospective study. Cancer Imaging 2015, 15, 8. [Google Scholar] [CrossRef] [Green Version]
- Bipat, S.; Fransen, G.A.; Spijkerboer, A.M.; van der Velden, J.; Bossuyt, P.M.M.; Zwinderman, A.H.; Stoker, J. Is there a role for magnetic resonance imaging of inguinal lymph node metastases in patients with vulvar carcinoma? Gynecol. Oncol. 2006, 103, 1001–1006. [Google Scholar] [CrossRef]
- Dolanbay, M.; Ozcelik, B.; Abdulrezzak, U.; Serin, S.I.; Kutuk, M.S.; Uludag, S. F-18 fluoro-D-glucose (FDG)-positron emission tomography (PET)/computed tomography (CT) in planning of surgery and sentinel lymph node screening in vulvar cancers. Arch. Gynecol. Obstet. 2016, 293, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Triumbari, E.K.A.; de Koster, E.J.; Rufini, V.; Fragomeni, S.M.; Garganese, G.; Collarino, A. 18F-FDG PET and 18F-FDG PET/CT in vulvar cancer. A systematic review and meta-analysis. Clin. Nucl. Med. 2021, 46, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.B.; Barton, D.P.J.; Trott, P.A.; Nasiri, N.; Shepherd, J.H.; Thomas, J.M.; Moskovic, E.C. The role of ultrasound-guided cytology of groin lymph nodes in the management of squamous cell carcinoma of the vulva: 5-year experience in 44 patients. Clin. Radiol. 2003, 58, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Garganese, G.; Fragomeni, S.M.; Pasciuto, T.; Leombroni, M.; Moro, F.; Evangelista, M.T.; Bove, S.; Gentileschi, S.; Tagliaferri, L.; Paris, I.; et al. Ultrasound morphometric and cytologic preoperative assessment of inguinal lymph-node status in women with vulvar cancer: MorphoNode study. Ultrasound Obstet. Gynecol. 2020, 55, 401–410. [Google Scholar] [CrossRef]
- De Gregorio, N.; Ebner, F.; Schwentner, I.; Friedl, T.W.P.; Deniz, M.; Lato, K.; Kreienberg, R.; Janni, W.; Varga, D. The role of preoperative ultrasound evaluation of inguinal lymph nodes in patients with vulvar malignancy. Gynecol. Oncol. 2013, 131, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Verri, D.; Moro, F.; Fragomeni, S.M.; Zace, D.; Bove, S.; Pozzati, F.; Gui, B.; Scambia, G.; Trsta, A.C.; Garganese, G. The role of ultrasound in the evaluation of inguinal lymph nodes in patients with vulvar cancer: A systematic review and meta-analysis. Cancers 2022, 14, 3082. [Google Scholar] [CrossRef]
- Hacker, N.F.; Eifel, P.J. Vulvar cancer. In Gynecologic Oncology, 7th ed; Berek, J.S., Hacker, N.F., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2021; pp. 503–546. [Google Scholar]
- Te Grootenhuis, N.C.; van der Zee, A.G.; van Doorn, H.C.; van der Velden, J.; Vergote, I.; Zanagnolo, V.; Baldwin, P.; Gaarenstroom, K.N.; van Dorst, E.B.; Trum, J.W.; et al. Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen International Study on Sentinel nodes in Vulvar cancer (GROINSS-V) 1. Gynecol. Oncol. 2016, 140, 8–14. [Google Scholar] [CrossRef]
- Pouwer, A.W.; Mus, R.D.M.; IntHout, J.; van der Zee, A.G.J.; Bulten, J.; Massuger, L.F.A.G.; de Hullu, J.A. The efficacy of ultrasound in the follow-up after a negative sentinel lymph node in women with vulvar cancer: A prospective single-centre study. BJOG 2018, 125, 1461–1468. [Google Scholar] [CrossRef]
- Wilson, E. Probable inference, the law of succession, and statistical inference. J. Amer. Stat. Assoc. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- Quigley, J.; Revie, M. Estimating the probability of rare events: Addressing zero failure data. Risk Analysis 2011, 31, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, A.G.; Oonk, M.H.; De Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; Van Doorst, E.B.; et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J. Clin. Oncol. 2008, 26, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Broach, V.; Abu-Rustim, N.R.; Sonoda, Y.; Brown, C.L.; Jewell, E.; Gardner, G.; Chi, D.S.; Zivanovic, O.; Leitao, M.M., Jr. Evolution and outcomes of sentinel lymph node mapping in vulvar cancer. Int. J. Gynecol. Oncol. 2020, 30, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Fischerova, D.; Garganese, G.; Reina, H.; Fragomeni, S.M.; Cibula, D.; Nanka, O.; Rettenbacher, A.; Testa, A.C.; Epstein, E.; Guiggi, I.; et al. Terms, definitions, and measurements to describe sonographic features of lymph nodes: Consensus opinion from the Vulvar International Tumor Analysis (VITA) group. Ultrasound Obstet. Gynecol. 2021, 57, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.; Gebski, V.; Hacker, N.F. Quality of life after complete lymphadenectomy for vulvar cancer: Do women prefer sentinel node biopsy. Int. J. Gynecol. Cancer 2014, 24, 813–819. [Google Scholar] [CrossRef] [PubMed]

| Patient Number | Dimensions of the Primary Cancer | Nodes | Outcome Months |
|---|---|---|---|
| 1 | Unilateral 6 × 6 mm. Invasion 1.2 mm. | Neg | NED 88 |
| 2 | Midline 8 × 6 mm. Invasion 2.2 mm. | Neg | NED 65 |
| 3 | Unilateral 9 × 9 mm. Invasion 2.7 mm. | Pos | NED 96 |
| 4 | Unilateral 15 ×15 mm. Invasion 1.7 mm. | Neg | NED 49 |
| 5 | Unilateral 10 × 10 mm. Invasion 1.7 mm. | Neg | NED 36 |
| 6 | Midline 8 × 8 mm. Invasion 1.3 mm. | Neg | NED 34 |
| 7 | Midline 15 × 15 mm. Invasion 1.4 mm. | Neg | NED 26 |
| 8 | Midline 12 × 7 mm. Invasion 2.5 mm. | Neg | NED 24 |
| 9 | Unilateral 8 × 6 mm. Invasion 2.2 mm. | Neg | NED 23 |
| 10 | Midline 15 × 15 mm. Invasion 3.5 mm. | Pos | DOD 6 * |
| 11 | Unilateral 10 × 10 mm. Invasion 2 mm. | Neg | NED 22 |
| 12 | Unilateral 6 × 6 mm. Invasion 2.4 mm. | Neg | NED 20 |
| 13 | Midline 20 ×12 mm. Invasion 3.0 mm. | Neg | NED 18 |
| 14 | Midline 20 × 20 mm. Invasion 1.8 mm. Unable to identify one sentinel node. | Pos | NED 36 |
| Patient Number | Status of Primary Cancer | Ipsilateral Nodes | Outcome Months |
|---|---|---|---|
| 15 | 18 × 18 mm. Invasion 4.3 mm. | 11 neg | NED 65 |
| 16 | 46 × 20 mm. Invasion 3.4 mm. | 14 neg | NED 74 |
| 17 | 45 × 30 mm. Invasion 5.8 mm. | 12 neg | NED 69 |
| 18 | 18 × 18 mm. Invasion 4.3 mm. | 11 neg | NED 72 |
| 19 | 50 × 40 mm. Invasion 7.2 mm. | 1/12 pos | NED 48 |
| 20 | 55 × 30 mm. Invasion 8 mm. | 4 neg | NED 41 |
| 21 | 60 × 60 mm. Invasion 14 mm. | 2/10 pos | NED 38 |
| 22 | 45 × 55 mm. Invasion 9 mm. | 12 neg | NED 36 |
| 23 | 45 × 30 mm. Invasion 19 mm. | 13 neg | NED 32 |
| 24 | 50 × 30 mm. Invasion 8 mm. | 1/7 pos | NED 30 |
| Patient Number | Status of Primary Cancer | Nodes | Outcome Months |
|---|---|---|---|
| 25 | Bilateral in situ and invasive Paget’s disease. Largest invasive focus 8 × 3 mm. Invasion 2.8 mm. | Neg | NED 72 |
| 26 | Bilateral in situ and invasive SCCs. Largest invasive focus 5 mm. Invasion 1.9 mm. | Neg | NED 49 |
| 27 | Bilateral in situ and invasive SCCs. Largest invasive focus 14 × 12 mm. Invasion 3.3 mm. | Neg | NED 42 |
| 28 | Unilateral in situ and invasive Paget’s disease. Largest invasive focus 16 × 16 mm. Invasion 3 mm. | Neg | NED 39 |
| 29 | Unilateral in situ and invasive Paget’s. Largest invasive focus 12 × 6 mm. Invasion 2 mm. | Neg | NED 32 |
| Patient Number | Status of Primary Cancer | Nodes | Outcome Months |
|---|---|---|---|
| 30 | Ipsilateral SCC, 50 × 30 mm. Invasion 3.5 mm (refused). | Neg | NED 60 |
| 31 | Multifocal SCC. Largest focus 30 × 20 mm. Invasion 1.3 mm (refused). | False pos at 10 months | NED 26 |
| 32 | Ipsilateral SCC 56 × 35 mm. Invasion 19 mm (unfit). | Neg | NED 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hacker, N.F.; Barlow, E.L.; McNally, G.; Morrell, S.; Gebski, V.; Obermair, A. Groin Surveillance by Serial Ultrasonography Rather Than Sentinel Node Biopsy or Inguinofemoral Lymphadenectomy for Patients with Vulvar Cancer: A Pilot Study. Cancers 2023, 15, 831. https://doi.org/10.3390/cancers15030831
Hacker NF, Barlow EL, McNally G, Morrell S, Gebski V, Obermair A. Groin Surveillance by Serial Ultrasonography Rather Than Sentinel Node Biopsy or Inguinofemoral Lymphadenectomy for Patients with Vulvar Cancer: A Pilot Study. Cancers. 2023; 15(3):831. https://doi.org/10.3390/cancers15030831
Chicago/Turabian StyleHacker, Neville F., Ellen L. Barlow, Glenn McNally, Stephen Morrell, Val Gebski, and Andreas Obermair. 2023. "Groin Surveillance by Serial Ultrasonography Rather Than Sentinel Node Biopsy or Inguinofemoral Lymphadenectomy for Patients with Vulvar Cancer: A Pilot Study" Cancers 15, no. 3: 831. https://doi.org/10.3390/cancers15030831





