Endemic Kaposi’s Sarcoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Epidemiology
2.1. Geographical Disparities
2.2. Age and Gender Distribution
2.3. Epidemiological Impact of HIV
3. Kaposi’s Sarcoma Etiopathogenesis and Other Predisposing Factors
3.1. The Etiologic Role of HHV-8
3.2. HHV-8 Salivary Transmission
3.3. Infectious Agents
3.4. Socioeconomic Status
4. Diagnosis
5. Prognosis, Staging, and Workup
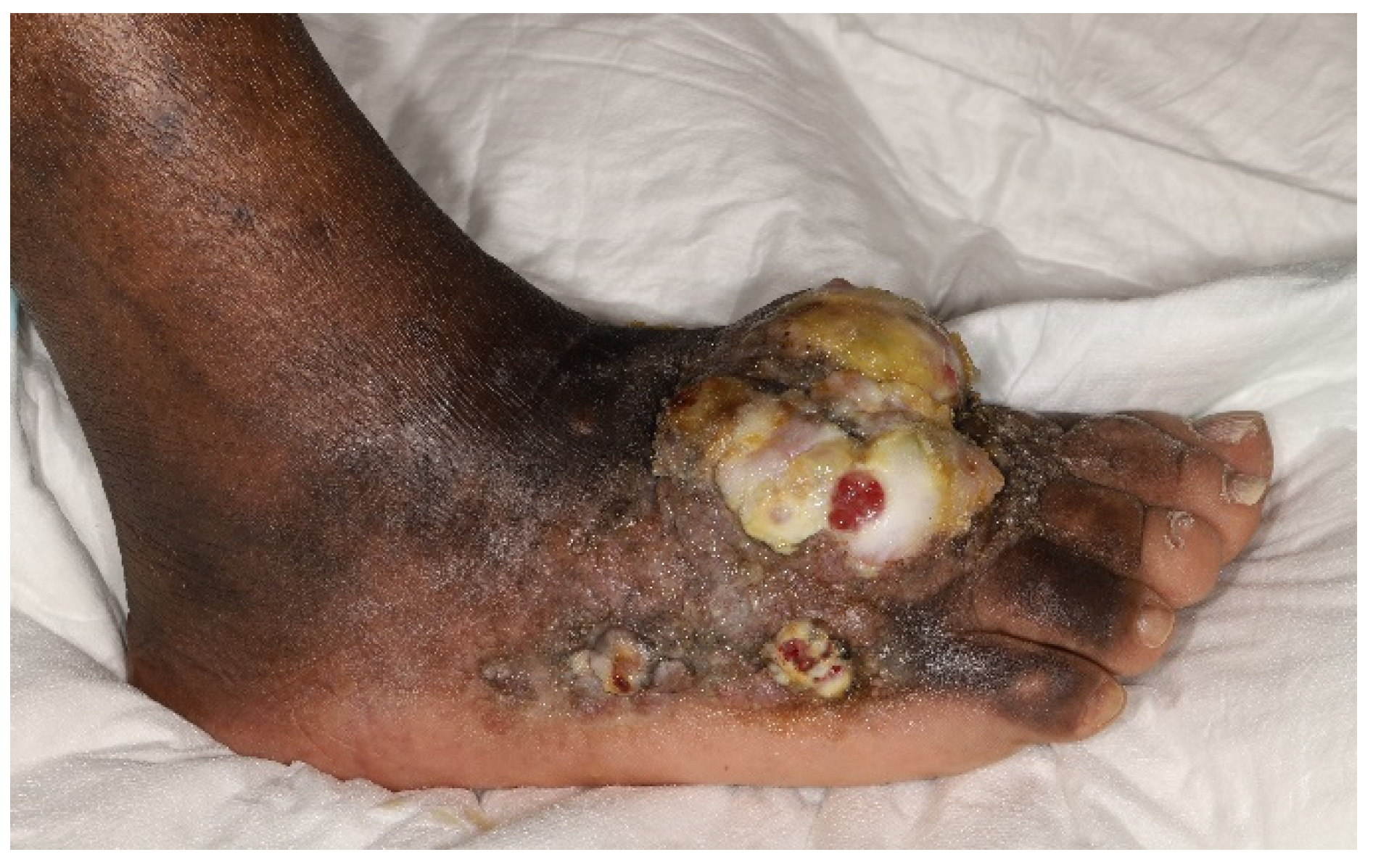
6. Endemic Kaposi’s Sarcoma Presentation and Variants
6.1. Clinical Features
6.2. Pediatric Variant
6.3. Musculoskeletal Skeletal Involvement
6.4. Diagnostic Challenges in Resource-Limited Settings
7. Therapeutic Management
7.1. Treatment Strategy for Adult Form: European Standards
7.2. Therapeutic Considerations in Resource-Limited Settings
7.3. Treatment Strategy for Pediatric Forms
7.4. Current and Future Perspectives
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moore, P.S.; Chang, Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and those without HIV infection. N. Engl. J. Med. 1995, 332, 1181–1185. [Google Scholar] [CrossRef] [Green Version]
- Stebbing, J.; Portsmouth, S.; Gotch, F.; Gazzard, B. Kaposi’s sarcoma—An update. Int. J. STD AIDS 2003, 14, 225–227. [Google Scholar] [CrossRef]
- Mesri, E.A.; Cesarman, E.; Boshoff, C. Kaposi’s sarcoma and its associated herpesvirus. Nat. Rev. Cancer 2010, 10, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Dupin, N. Update on oncogenesis and therapy for Kaposi sarcoma. Curr. Opin. Oncol. 2020, 32, 122–128. [Google Scholar] [CrossRef]
- Friedman-Kien, A.E.; Saltzman, B.R. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J. Am. Acad. Dermatol. 1990, 22, 1237–1250. [Google Scholar] [CrossRef]
- Penn, I. Kaposi’s sarcoma in transplant recipients. Transplantation 1997, 64, 669–673. [Google Scholar] [CrossRef]
- Hymes, K.; Greene, J.; Marcus, A.; William, D.; Cheung, T.; Prose, N.; Ballard, H.; Laubenstein, L. Kaposi’s sarcoma in homosexual men—A report of eight cases. Lancet 1981, 318, 598–600. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Newton, R.; Grulich, A.; Beral, V.; Sindikubwabo, B.; Ngilimana, P.J.; Nganyira, A.; Parkin, D.M. Cancer and HIV infection in Rwanda. Lancet 1995, 345, 1378–1379. [Google Scholar] [CrossRef]
- Sitas, F.; Newton, R. Kaposi’s sarcoma in South Africa. J. Natl. Cancer Inst. Monogr. 2001, 2000, 1–4. [Google Scholar] [CrossRef]
- Sitas, F.; Bezwoda, W.R.; Levin, V.; Ruff, P.; Kew, M.C.; Hale, M.J.; Carrara, H.; Beral, V.; Fleming, G.; Odes, R.; et al. Association between human immunodeficiency virus type 1 infection and cancer in the black population of Johannesburg and Soweto, South Africa. Br. J. Cancer 1997, 75, 1704–1707. [Google Scholar] [CrossRef] [Green Version]
- Cook-Mozaffari, P.; Newton, R.; Beral, V.; Burkitt, D.P. The geographical distribution of Kaposi’s sarcoma and of lymphomas in Africa before the AIDS epidemic. Br. J. Cancer 1998, 78, 1521–1528. [Google Scholar] [CrossRef] [Green Version]
- Dedicoat, M.; Newton, R. Review of the distribution of Kaposi’s sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi’s sarcoma. Br. J. Cancer 2003, 88, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Simonart, T. Role of environmental factors in the pathogenesis of classic and African-endemic Kaposi sarcoma. Cancer Lett. 2006, 244, 1–7. [Google Scholar] [CrossRef]
- Montella, M.; Serraino, D.; Crispo, A.; Rezza, G.; Carbone, S.; Tamburini, M. Is volcanic soil a cofactor for classic Kaposi’s sarcoma? Eur. J. Epidemiol. 2000, 16, 1185–1186. [Google Scholar] [CrossRef]
- Ziegler, J.L. Endemic Kaposi’s sarcoma in Africa and local volcanic soils. Lancet 1993, 342, 1348–1351. [Google Scholar] [CrossRef]
- Pelser, C.; Dazzi, C.; Graubard, B.I.; Lauria, C.; Vitale, F.; Goedert, J.J. Risk of classic Kaposi sarcoma with residential exposure to volcanic and related soils in Sicily. Ann. Epidemiol. 2009, 19, 597–601. [Google Scholar] [CrossRef] [Green Version]
- De Bruin, G.P.; Stefan, D.C. Children with kaposi sarcoma in two southern african hospitals: Clinical presentation, management, and outcome. J. Trop. Med. 2013, 2013, 213490. [Google Scholar] [CrossRef] [Green Version]
- El-Mallawany, N.K.; Kamiyango, W.; Slone, J.S.; Villiera, J.; Kovarik, C.L.; Cox, C.M.; Dittmer, D.P.; Ahmed, S.; Schutze, G.E.; Scheurer, M.E.; et al. Clinical Factors Associated with Long-Term Complete Remission versus Poor Response to Chemotherapy in HIV-Infected Children and Adolescents with Kaposi Sarcoma Receiving Bleomycin and Vincristine: A Retrospective Observational Study. PLoS ONE 2016, 11, e0153335. [Google Scholar] [CrossRef] [Green Version]
- El-Mallawany, N.K.; Villiera, J.; Kamiyango, W.; Peckham-Gregory, E.C.; Scheurer, M.E.; Allen, C.E.; McAtee, C.L.; Legarreta, A.; Dittmer, D.P.; Kovarik, C.L.; et al. Endemic Kaposi sarcoma in HIV-negative children and adolescents: An evaluation of overlapping and distinct clinical features in comparison with HIV-related disease. Infect. Agents Cancer 2018, 13, 33. [Google Scholar] [CrossRef]
- Mittermayer-Vassallo, K.; Banda, K.; Molyneux, E.M. Kaposi sarcoma in HIV-seronegative children presenting to the paediatric oncology ward in The Queen Elizabeth Central Hospital, Blantyre, Malawi during 2002–2014. Trop. Doct. 2016, 46, 138–142. [Google Scholar] [CrossRef]
- Olweny, C.L.; Kaddumukasa, A.; Atine, I.; Owor, R.; Magrath, I.; Ziegler, J.L. Childhood Kaposi’s sarcoma: Clinical features and therapy. Br. J. Cancer 1976, 33, 555–560. [Google Scholar] [CrossRef] [Green Version]
- Lulat, A.G. African Kaposi’s sarcoma. Trans. R Soc. Trop. Med. Hyg. 1989, 83, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Cesarman, E.; Damania, B.; Krown, S.E.; Martin, J.; Bower, M.; Whitby, D. Kaposi sarcoma. Nat. Rev. Dis. Prim. 2019, 5, 9. [Google Scholar] [CrossRef]
- Dutz, W.; Stout, A.P. Kaposi’s sarcoma in infants and children. Cancers 1960, 13, 684–694. [Google Scholar] [CrossRef]
- Ziegler, J.L.; Katongole-Mbidde, E.; Wabinga, H.; Dollbaum, C.M. Absence of sex-hormone receptors in Kaposi’s sarcoma. Lancet 1995, 345, 925. [Google Scholar] [CrossRef]
- Parkin, D.M.; Chingonzoh, T.; Vuma, S.; Liu, B.; Chokunonga, E.; Ndlovu, N.; Borok, M. Changes in the Incidence of Cancer in Bulawayo, Zimbabwe over a 50-Year Period. Cancer Epidemiol. Biomark. Prev. 2021, 30, 867–873. [Google Scholar] [CrossRef]
- Parkin, D.M.; Sitas, F.; Chirenje, M.; Stein, L.; Abratt, R.; Wabinga, H. Part I: Cancer in Indigenous Africans-burden, distribution, and trends. Lancet Oncol. 2008, 9, 683–692. [Google Scholar] [CrossRef]
- Wabinga, H.R.; Parkin, D.M.; Wabwire-Mangen, F.; Mugerwa, J.W. Cancer in Kampala, Uganda, in 1989-91: Changes in incidence in the era of AIDS. Int. J. Cancer 1993, 54, 26–36. [Google Scholar] [CrossRef]
- Dollard, S.C.; Butler, L.M.; Jones, A.M.G.; Mermin, J.H.; Chidzonga, M.; Chipato, T.; Shiboski, C.H.; Brander, C.; Mosam, A.; Kiepiela, P.; et al. Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: Insights on the origin of the “Kaposi’s sarcoma belt. ” Int. J. Cancer 2010, 127, 2395–2401. [Google Scholar] [CrossRef]
- Rogena, E.A.; Simbiri, K.O.; De Falco, G.; Leoncini, L.; Ayers, L.; Nyagol, J. A review of the pattern of AIDS defining, HIV associated neoplasms and premalignant lesions diagnosed from 2000–2011 at Kenyatta National Hospital, Kenya. Infect. Agents Cancer 2015, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Carrilho, C.; Ferro, J.; Lorenzoni, C.; Sultane, T.; Silva-Matos, C.; Lunet, N. A contribution for a more accurate estimation of the incidence of Kaposi sarcoma in Mozambique. Int. J. Cancer 2013, 132, 988–989. [Google Scholar] [CrossRef] [Green Version]
- Amir, H.; Kaaya, E.E.; Manji, K.P.; Kwesigabo, G.; Biberfeld, P. Kaposi’s sarcoma before and during a human immunodeficiency virus epidemic in Tanzanian children. Pediatr. Infect. Dis. J. 2001, 20, 518–521. [Google Scholar] [CrossRef]
- Sinfield, R.L.; Molyneux, E.M.; Banda, K.; Borgstein, E.; Broadhead, R.; Hesseling, P.; Newton, R.; Casabonne, D.; Mkandawire, N.; Nkume, H.; et al. Spectrum and presentation of pediatric malignancies in the HIV era: Experience from Blantyre, Malawi, 1998–2003. Pediatr. Blood Cancer 2007, 48, 515–520. [Google Scholar] [CrossRef]
- El-Mallawany, N.K.; Villiera, J.; Kamiyango, W.; Mhango, J.; Slone, J.S.; Mehta, P.S.; Kazembe, P.N.; Scheurer, M.E. Increasing Numbers of New Kaposi Sarcoma Diagnoses in HIV-Infected Children and Adolescents Despite the Wide Availability of Antiretroviral Therapy in Malawi. Clin. Infect. Dis. 2017, 64, 818–819. [Google Scholar] [CrossRef] [Green Version]
- Stefan, D.C. Patterns of distribution of childhood cancer in Africa. J. Trop. Pediatr. 2015, 61, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Tukei, V.J.; Kekitiinwa, A.; Beasley, R.P. Prevalence and outcome of HIV-associated malignancies among children. AIDS 2011, 25, 1789–1793. [Google Scholar] [CrossRef]
- Mutalima, N.; Molyneux, E.M.; Johnston, W.T.; Jaffe, H.W.; Kamiza, S.; Borgstein, E.; Mkandawire, N.; Liomba, G.N.; Batumba, M.; Carpenter, L.M.; et al. Impact of infection with human immunodeficiency virus-1 (HIV) on the risk of cancer among children in Malawi-preliminary findings. Infect. Agents Cancer 2010, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Irira, M.; Ngocho, J.S.; Youze, J.; Shayo, I.; Komba, V.; Minja, L.; Karia, F.P.; Bartlett, J.; Mmbaga, B.T. Prevalence and Outcome of HIV-associated Malignancies Among HIV-infected Children Enrolled into Care at Kilimanjaro Christian Medical Center 2006 to 2014: A Hospital-based Retrospective Analytical Study. J. Pediatr. Hematol. Oncol. 2020, 42, 69–73. [Google Scholar] [CrossRef]
- zur Hausen, H. Oncogenic DNA viruses. Oncogene 2001, 20, 7820–7823. [Google Scholar] [CrossRef]
- Dittmer, D.P.; Damania, B. Kaposi sarcoma-associated herpesvirus: Immunobiology, oncogenesis, and therapy. J. Clin. Investig. 2016, 126, 3165–3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.F.; Kuppermann, B.D.; Wolitz, R.A.; Palestine, A.G.; Li, H.; Robinson, C.A. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N. Engl. J. Med. 1999, 340, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Ensoli, B.; Stürzl, M.; Monini, P. Reactivation and role of HHV-8 in Kaposi’s sarcoma initiation. Adv. Cancer Res. 2001, 81, 161–200. [Google Scholar] [PubMed]
- Butler, L.M.; Were, W.A.; Balinandi, S.; Downing, R.; Dollard, S.; Neilands, T.B.; Gupta, S.; Rutherford, G.W.; Mermin, J. Human Herpesvirus 8 Infection in Children and Adults in a Population-based Study in Rural Uganda. J. Infect. Dis. 2011, 203, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenthal, M.J.; Schutz, C.; Barr, D.; Locketz, M.; Marshall, V.; Whitby, D.; Katz, A.A.; Uldrick, T.; Meintjes, G.; Schäfer, G. The Contribution of Kaposi’s Sarcoma-Associated Herpesvirus to Mortality in Hospitalized Human Immunodeficiency Virus-Infected Patients Being Investigated for Tuberculosis in South Africa. J. Infect. Dis. 2019, 220, 841–851. [Google Scholar] [CrossRef]
- Gao, S.J.; Kingsley, L.; Li, M.; Zheng, W.; Parravicini, C.; Ziegler, J.; Newton, R.; Rinaldo, C.R.; Saah, A.; Phair, J.; et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat. Med. 1996, 2, 925–928. [Google Scholar] [CrossRef]
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Sciences 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [Green Version]
- Miles, S.A. Pathogenesis of HIV-related Kaposi’s sarcoma. Curr. Opin. Oncol. 1994, 6, 497–502. [Google Scholar] [CrossRef]
- Ye, F.C.; Blackbourn, D.J.; Mengel, M.; Xie, J.P.; Qian, L.W.; Greene, W.; Yeh, I.T.; Graham, D.; Gao, S.J. Kaposi’s sarcoma-associated herpesvirus promotes angiogenesis by inducing angiopoietin-2 expression via AP-1 and Ets1. J. Virol. 2007, 81, 3980–3991. [Google Scholar] [CrossRef] [Green Version]
- Sharma-Walia, N.; Paul, A.G.; Bottero, V.; Sadagopan, S.; Veettil, M.V.; Kerur, N.; Chandran, B. Kaposi’s sarcoma associated herpes virus (KSHV) induced COX-2: A key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 2010, 6, e1000777. [Google Scholar] [CrossRef]
- Miles, S.A.; Martínez-Maza, O.; Rezai, A.; Magpantay, L.; Kishimoto, T.; Nakamura, S.; Radka, S.F.; Linsley, P.S. Oncostatin M as a potent mitogen for AIDS-Kaposi’s sarcoma-derived cells. Science 1992, 255, 1432–1434. [Google Scholar] [CrossRef] [PubMed]
- Naidu, Y.M.; Rosen, E.M.; Zitnick, R.; Goldberg, I.; Park, M.; Naujokas, M.; Polverini, P.J.; Nickoloff, B.J. Role of scatter factor in the pathogenesis of AIDS-related Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 1994, 91, 5281–5285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masood, R.; Cai, J.; Zheng, T.; Smith, D.L.; Naidu, Y.; Gill, P.S. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 1997, 94, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moses, A.V.; Fish, K.N.; Ruhl, R.; Smith, P.P.; Strussenberg, J.G.; Zhu, L.; Chandran, B.; Nelson, J.A. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 1999, 73, 6892–6902. [Google Scholar] [CrossRef] [Green Version]
- Delgado, T.; Carroll, P.A.; Punjabi, A.S.; Margineantu, D.; Hockenbery, D.M.; Lagunoff, M. Induction of the Warburg effect by Kaposi’s sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl. Acad. Sci. USA 2010, 107, 10696–10701. [Google Scholar] [CrossRef] [Green Version]
- Flore, O.; Rafii, S.; Ely, S.; O’Leary, J.J.; Hyjek, E.M.; Cesarman, E. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature 1998, 394, 588–592. [Google Scholar] [CrossRef]
- Ciufo, D.M.; Cannon, J.S.; Poole, L.J.; Wu, F.Y.; Murray, P.; Ambinder, R.F.; Hayward, G.S. Spindle cell conversion by Kaposi’s sarcoma-associated herpesvirus: Formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 2001, 75, 5614–5626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.K.; Foreman, K.; Shin, J.W.; Hirakawa, S.; Curry, C.L.; Sage, D.R.; Libermann, T.; Dezube, B.J.; Fingeroth, J.D.; Detmar, M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 2004, 36, 683–685. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.W.; Trotter, M.W.B.; Lagos, D.; Bourboulia, D.; Henderson, S.; Mäkinen, T.; Elliman, S.; Flanagan, A.M.; Alitalo, K.; Boshoff, C. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 2004, 36, 687–693. [Google Scholar] [CrossRef]
- Chang, H.; Dittmer, D.P.; Shin, Y.C.; Chul, S.Y.; Hong, Y.; Jung, J.U. Role of Notch signal transduction in Kaposi’s sarcoma-associated herpesvirus gene expression. J. Virol. 2005, 79, 14371–14382. [Google Scholar] [CrossRef]
- Okuno, T.; Jiang, Y.B.; Ueda, K.; Nishimura, K.; Tamura, T.; Yamanishi, K. Activation of human herpesvirus 8 open reading frame K5 independent of ORF50 expression. Virus Res. 2002, 90, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Chandriani, S.; Ganem, D. Array-based transcript profiling and limiting-dilution reverse transcription-PCR analysis identify additional latent genes in Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2010, 84, 5565–5573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krown, S.E.; Dittmer, D.P.; Cesarman, E. Pilot study of oral valganciclovir therapy in patients with classic Kaposi sarcoma. J. Infect. Dis. 2011, 203, 1082–1086. [Google Scholar] [CrossRef] [Green Version]
- Staudt, M.R.; Kanan, Y.; Jeong, J.H.; Papin, J.F.; Hines-Boykin, R.; Dittmer, D.P. The tumor microenvironment controls primary effusion lymphoma growth in vivo. Cancer Res. 2004, 64, 4790–4799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitas, F.; Carrara, H.; Beral, V.; Newton, R.; Reeves, G.; Bull, D.; Jentsch, U.; Pacella-Norman, R.; Bourboulia, D.; Whitby, D.; et al. Antibodies against human herpesvirus 8 in black South African patients with cancer. N. Engl. J. Med. 1999, 340, 1863–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosam, A.; Aboobaker, J.; Shaik, F. Kaposi’s sarcoma in sub-Saharan Africa: A current perspective. Curr. Opin. Infect. Dis. 2010, 23, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Sheldon, J.; Gilks, C.F.; Schulz, T.F. Prevalence of infection with human herpesvirus 8/Kaposi’s sarcoma herpesvirus in rural South Africa. S. Afr. Med. J. 1999, 89, 554–557. [Google Scholar]
- He, J.; Bhat, G.; Kankasa, C.; Chintu, C.; Mitchell, C.; Duan, W.; Wood, C. Seroprevalence of human herpesvirus 8 among Zambian women of childbearing age without Kaposi’s sarcoma (KS) and mother-child pairs with KS. J. Infect. Dis. 1998, 178, 1787–1790. [Google Scholar] [CrossRef] [Green Version]
- Davidovici, B.; Karakis, I.; Bourboulia, D.; Ariad, S.; Zong, J.; Benharroch, D.; Dupin, N.; Weiss, R.; Hayward, G.; Sarov, B.; et al. Seroepidemiology and molecular epidemiology of Kaposi’s sarcoma-associated herpesvirus among Jewish population groups in Israel. J. Natl. Cancer Inst. 2001, 93, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Mbulaiteye, S.M.; Biggar, R.J.; Pfeiffer, R.M.; Bakaki, P.M.; Gamache, C.; Owor, A.M.; Katongole-Mbidde, E.; Ndugwa, C.M.; Goedert, J.J.; Whitby, D.; et al. Water, socioeconomic factors, and human herpesvirus 8 infection in Ugandan children and their mothers. J. Acquir. Immune. Defic. Syndr. 2005, 38, 474–479. [Google Scholar] [CrossRef]
- Sitas, F.; Newton, R.; Boshoff, C. Increasing probability of mother-to-child transmission of HHV-8 with increasing maternal antibody titer for HHV-8. N. Engl. J. Med. 1999, 340, 1923. [Google Scholar] [CrossRef] [PubMed]
- Bourboulia, D.; Whitby, D.; Boshoff, C.; Newton, R.; Beral, V.; Carrara, H.; Lane, A.; Sitas, F. Serologic evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus infection. JAMA 1998, 280, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Dorsey, G.; Hladik, W.; Rosenthal, P.J.; Brander, C.; Neilands, T.B.; Mbisa, G.; Whitby, D.; Kiepiela, P.; Mosam, A.; et al. Kaposi sarcoma-associated herpesvirus (KSHV) seroprevalence in population-based samples of African children: Evidence for at least 2 patterns of KSHV transmission. J. Infect. Dis. 2009, 200, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Henke-Gendo, C.; Schulz, T.F. Transmission and disease association of Kaposi’s sarcoma-associated herpesvirus: Recent developments. Curr. Opin. Infect. Dis. 2004, 17, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Hengge, U.R.; Ruzicka, T.; Tyring, S.K.; Stuschke, M.; Roggendorf, M.; Schwartz, R.A.; Seeber, S. Update on Kaposi’s sarcoma and other HHV8 associated diseases. Part 1: Epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect. Dis. 2002, 2, 281–292. [Google Scholar] [CrossRef]
- Butler, L.M.; Neilands, T.B.; Mosam, A.; Mzolo, S.; Martin, J.N. A population-based study of how children are exposed to saliva in KwaZulu-Natal Province, South Africa: Implications for the spread of saliva-borne pathogens to children. Trop. Med. Int. Health 2010, 15, 442–453. [Google Scholar] [CrossRef] [Green Version]
- Wakeham, K.; Webb, E.L.; Sebina, I.; Muhangi, L.; Miley, W.; Johnson, W.T.; Ndibazza, J.; Elliott, A.M.; Whitby, D.; Newton, R. Parasite infection is associated with Kaposi’s sarcoma associated herpesvirus (KSHV) in Ugandan women. Infect. Agents Cancer 2011, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- McHardy, J.; Williams, E.H.; Geser, A.; de-Thé, G.; Beth, E.; Giraldo, G. Endemic Kaposi’s sarcoma: Incidence and risk factors in the West Nile District of Uganda. Int. J. Cancer 1984, 33, 203–212. [Google Scholar] [CrossRef]
- Wojcicki, J.M.; Newton, R.; Urban, M.; Stein, L.; Hale, M.; Patel, M.; Ruff, P.; Sur, R.; Bourboulia, D.; Sitas, F. Low socioeconomic status and risk for infection with human herpesvirus 8 among HIV-1 negative, South African black cancer patients. Epidemiol. Infect. 2004, 132, 1191–1197. [Google Scholar] [CrossRef]
- Pawloski, L.R. Growth and development of adolescent girls from the Segou Region of Mali (West Africa). Am. J. Phys. Anthropol. 2002, 117, 364–372. [Google Scholar] [CrossRef]
- Olsen, S.J.; Chang, Y.; Moore, P.S.; Biggar, R.J.; Melbye, M. Increasing Kaposi’s sarcoma-associated herpesvirus seroprevalence with age in a highly Kaposi’s sarcoma endemic region, Zambia in 1985. AIDS 1998, 12, 1921–1925. [Google Scholar] [CrossRef] [PubMed]
- Forrestel, A.K.; Naujokas, A.; Martin, J.N.; Maurer, T.A.; McCalmont, T.H.; Laker-Opwonya, M.O.; Mulyowa, G.; Busakhala, N.; Amerson, E.H. Bacillary angiomatosis masquerading as Kaposi’s sarcoma in East Africa. J. Int. Assoc. Provid. AIDS Care 2015, 14, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Lebbe, C.; Garbe, C.; Stratigos, A.J.; Harwood, C.; Peris, K.; Marmol, V.D.; Malvehy, J.; Zalaudek, I.; Hoeller, C.; Dummer, R. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organisation for Research and Treatment of Cancer (EORTC); et al. Diagnosis and treatment of Kaposi’s sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). Eur. J. Cancer 2019, 114, 117–127. [Google Scholar] [PubMed] [Green Version]
- Amerson, E.; Woodruff, C.M.; Forrestel, A.; Wenger, M.; McCalmont, T.; LeBoit, P.; Maurer, T.; Laker-Oketta, M.; Muyindike, W.; Bwana, M.; et al. Accuracy of Clinical Suspicion and Pathologic Diagnosis of Kaposi Sarcoma in East Africa. J. Acquir. Immune. Defic. Syndr. 2016, 71, 295–301. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, C.; Liu, D.; Yu, W.; Chen, W.; Wang, Y.; Shi, S.; Yuan, Y. Evidence for Kaposi Sarcoma Originating from Mesenchymal Stem Cell through KSHV-induced Mesenchymal-to-Endothelial Transition. Cancer Res. 2018, 78, 230–245. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Ding, Y.; Liu, D.; Lu, Z.; Wang, Y.; Yuan, Y. Kaposi’s sarcoma-associated herpesvirus vFLIP promotes MEndT to generate hybrid M/E state for tumorigenesis. PLoS Pathogens. 2021, 17, e1009600. [Google Scholar] [CrossRef]
- Dupin, N.; Jary, A.; Boussouar, S.; Syrykh, C.; Gandjbakhche, A.; Bergeret, S.; Palich, R. Current and Future Tools for Diagnosis of Kaposi’s Sarcoma. Cancers 2021, 13, 5927. [Google Scholar] [CrossRef]
- Jakob, L.; Metzler, G.; Chen, K.M.; Garbe, C. Non-AIDS associated Kaposi’s sarcoma: Clinical features and treatment outcome. PLoS ONE 2011, 6, e18397. [Google Scholar] [CrossRef]
- Pellet, C.; Kerob, D.; Dupuy, A.; Carmagnat, M.V.; Mourah, S.; Podgorniak, M.P.; Toledano, C.; Morel, P.; Vérola, O.; Dosquet, C.; et al. Kaposi’s Sarcoma-Associated Herpesvirus Viremia is Associated with the Progression of Classic and Endemic Kaposi’s Sarcoma. J. Investig. Dermatol. 2006, 126, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Radu, O.; Pantanowitz, L. Kaposi sarcoma. Arch. Pathol. Lab. Med. 2013, 137, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, C.S.; Ocazionez, D. Kaposi’s sarcoma: Imaging overview. Semin. Ultrasound CT MR 2011, 32, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.F.; Templeton, A.C.; Vogel, C.L.; Ziegler, J.L.; Kyalwazi, S.K. Kaposi’s sarcoma in Uganda: A clinico-pathological study. Int. J. Cancer 1971, 8, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Pesqué, L.; Delyon, J.; Lheure, C.; Baroudjian, B.; Battistella, M.; Merlet, P.; Lebbé, C.; Vercellino, L. Yield of FDG PET/CT for Defining the Extent of Disease in Patients with Kaposi Sarcoma. Cancers 2022, 14, 2189. [Google Scholar] [CrossRef] [PubMed]
- Chapalain, M.; Goldman-Lévy, G.; Kramkimel, N.; Carlotti, A.; Franck, N.; Lheure, C.; Audard, V.; Avril, M.F.; Marcelin, A.G.; Damotte, D.; et al. Anaplastic Kaposi’s sarcoma: 5 cases of a rare and aggressive type of Kaposi’s sarcoma. Ann. Dermatol. Venereol. 2018, 145, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, M.; Diop, A.; Berthé, S.; Diallo, M.; Mjahed, S.; Diatta, B.A.; Diadie, S.; Seck, N.B.; Diallo, S.; Ndiaye, M.T.; et al. Endemic Kaposi’s sarcoma in Dakar: 29 cases. Mali Med. 2014, 29, 10–14. [Google Scholar] [PubMed]
- Pantanowitz, L.; Duke, W.H. Lymphoedematous variants of Kaposi’s sarcoma. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, V.; Schwartz, R.A.; Ruocco, E. Lymphedema: An immunologically vulnerable site for development of neoplasms. J. Am. Acad. Dermatol. 2002, 47, 124–127. [Google Scholar] [CrossRef]
- Hengge, U.R.; Stocks, K.; Goos, M. Acquired immune deficiency syndrome-related hyperkeratotic Kaposi’s sarcoma with severe lymphoedema: Report of five cases. Br. J. Dermatol. 2000, 142, 501–505. [Google Scholar] [CrossRef]
- Mohanlal, R.D.; Pather, S. Kaposi’s sarcoma, a South African perspective: Demographic and pathological features. S. Afr. Med. J. 2015, 105, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Arkin, L.M.; Cox, C.M.; Kovarik, C.L. Kaposi’s Sarcoma in the Pediatric Population: The Critical Need for a Tissue Diagnosis. Pediatr. Infect. Dis. J. 2009, 28, 426–428. [Google Scholar] [CrossRef]
- Vaz, P.; Macassa, E.; Jani, I.; Thome, B.; Mahagaja, E.; Madede, T.; Muando, V.; Biberfeld, G.; Anderson, S.; Blanche, S. Treatment of Kaposi Sarcoma in Human Immunodeficiency Virus-1-infected Mozambican Children With Antiretroviral Drugs and Chemotherapy. Pediatr. Infect. Dis. J. 2011, 30, 891–893. [Google Scholar] [CrossRef]
- Gantt, S.; Kakuru, A.; Wald, A.; Walusansa, V.; Corey, L.; Casper, C.; Orem, J. Clinical presentation and outcome of epidemic Kaposi sarcoma in Ugandan children. Pediatr. Blood Cancer 2010, 54, 670–674. [Google Scholar] [CrossRef] [Green Version]
- El-Mallawany, N.K.; McAtee, C.L.; Campbell, L.R.; Kazembe, P.N. Pediatric Kaposi sarcoma in context of the HIV epidemic in sub-Saharan Africa: Current perspectives. Pediatric. Health Med. Ther. 2018, 9, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Campbell, L.R.; El-Mallawany, N.K.; Slone, J.S.; Malingoti, B.M.; Mehta, P.S.; Scheurer, M.E.; Bacha, J.M.; Peckham-Gregory, E.C. Clinical characteristics and successful treatment outcomes of children and adolescents with Kaposi sarcoma in Southwestern Tanzania. Pediatr. Hematol. Oncol. 2022, 39, 28–47. [Google Scholar] [CrossRef]
- El-Mallawany, N.K.; Kamiyango, W.; Villiera, J.; Slone, J.S.; Kovarik, C.L.; Campbell, L.R.; Agrawal, A.K.; Dittmer, D.P.; Eason, A.B.; Ahmed, S.; et al. Proposal of a Risk-Stratification Platform to Address Distinct Clinical Features of Pediatric Kaposi Sarcoma in Lilongwe, Malawi. J. Glob. Oncol. 2018, 4, 1–7. [Google Scholar] [CrossRef]
- Braun, M. Classics in Oncology. Idiopathic multiple pigmented sarcoma of the skin by Kaposi. CA Cancer J. Clin. 1982, 32, 340–347. [Google Scholar] [CrossRef]
- Caponetti, G.; Dezube, B.J.; Restrepo, C.S.; Pantanowitz, L. Kaposi sarcoma of the musculoskeletal system: A review of 66 patients. Cancer. 2007, 109, 1040–1052. [Google Scholar] [CrossRef]
- Kaminer, B.; Murray, J.F. Sarcoma idiopathicum multiplex haemorrhagicum of Kaposi, with special reference to its incidence in the South African Negro, and two case reports. S. Afr. J. Clin. Sci. 1950, 1, 1–25. [Google Scholar]
- Simon, F.; Chouc, P.Y.; Chouc-Larriviere, C.; Normand, P.; Jeandel, P. Bone lesions due to endemic African Kaposi sarcoma: A case report. Med. Trop. 1997, 57, 174–176. [Google Scholar]
- Rwomushana, R.J.; Bailey, I.C.; Kyalwazi, S.K. Kaposi’s sarcoma of the brain. A case report with necropsy findings. Cancer 1975, 36, 1127–1131. [Google Scholar] [CrossRef]
- Udayakumar, H.; Indiran, V.; Muthu, P.M. Unanticipated diagnosis of skeletal muscle Kaposi sarcoma: A case report. Pan. Afr. Med. J. 2020, 37, 158. [Google Scholar] [CrossRef] [PubMed]
- Lee Morgan, C.; Gehweiler, J.A. Kaposi’s sarcoma in bone: A case report with unusual radiographic findings and an abnormal radioisotope scan. Rev. Interam. Radiol. 1976, 1, 37–41. [Google Scholar] [PubMed]
- Lausten, L.L.; Ferguson, B.L.; Barker, B.F.; Cobb, C.M. Oral Kaposi sarcoma associated with severe alveolar bone loss: Case report and review of the literature. J. Periodontol. 2003, 74, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.A.; Kuhlman, J.E.; Fishman, E.K. Kaposi sarcoma involving bone: CT demonstration in a patient with AIDS. J. Comput. Assist. Tomogr. 1990, 14, 161–162. [Google Scholar] [CrossRef]
- Restrepo, C.S.; Martínez, S.; Lemos, J.A.; Carrillo, J.A.; Lemos, D.F.; Ojeda, P.; Koshy, P. Imaging manifestations of Kaposi sarcoma. Radiographics 2006, 26, 1169–1185. [Google Scholar] [CrossRef]
- Lombardo, M.; Girolomoni, G.; Spina, V.; Zambruno, G.; Giannetti, A. Kaposi’s sarcoma with primary bone lesions in an HIV-seronegative man. Br. J. Dermatol. 1996, 134, 966–969. [Google Scholar] [CrossRef]
- Isenbarger, D.W.; Aronson, N.E. Lytic vertebral lesions: An unusual manifestation of AIDS-associated Kaposi’s sarcoma. Clin. Infect. Dis. 1994, 19, 751–755. [Google Scholar] [CrossRef]
- Nguyen, C.; Lander, P.; Begin, L.R.; Jarzem, P.; Grad, R. AIDS-related Kaposi sarcoma involving the tarsal bones. Skeletal Radiol. 1996, 25, 100–102. [Google Scholar] [CrossRef]
- Cengiz, A.; Şavk, E.; Tataroğlu, C.; Yürekli, Y. 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Imaging in a Patient with HIV (-) Kaposi Sarcoma. Mol. Imaging Radionucl. Ther. 2016, 25, 140–142. [Google Scholar] [CrossRef]
- Sathekge, M.; Warwick, J.M.; Doruyter, A.; Vorster, M. Appropriate indications for positron emission tomography/computed tomography: College of Nuclear Physicians of the Colleges of Medicine of South Africa. S. Afr. Med. J. 2015, 105, 894–896. [Google Scholar] [CrossRef] [Green Version]
- Benajiba, L.; Lambert, J.; La Selva, R.; Cochereau, D.; Baroudjian, B.; Roux, J.; Le Goff, J.; Pages, C.; Battistella, M.; Delyon, J. Systemic Treatment Initiation in Classical and Endemic Kaposi’s Sarcoma: Risk Factors and Global Multi-State Modelling in a Monocentric Cohort Study. Cancers 2021, 13, 2519. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.; Rakowsky, E.; Katz, A.; Gutman, H.; Sulkes, A.; Schacter, J.; Fenig, E. Tailoring treatment for classical Kaposi’s sarcoma: Comprehensive clinical guidelines. Int. J. Oncol. 1999, 14, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Von Roenn, J.H.; Cianfrocca, M. Treatment of Kaposi’s sarcoma. Cancer Treat. Res. 2001, 104, 127–148. [Google Scholar] [PubMed]
- Brambilla, L.; Romanelli, A.; Bellinvia, M.; Ferrucci, S.; Vinci, M.; Boneschi, V.; Miedico, A.; Tedeschi, L. Weekly paclitaxel for advanced aggressive classic Kaposi sarcoma: Experience in 17 cases. Br. J. Dermatol. 2008, 158, 1339–1344. [Google Scholar] [CrossRef]
- Weshler, Z.; Loewinger, E.; Loewenthal, E.; Levinson, R.; Fuks, Z. Megavoltage radiotherapy using water bolus in the treatment of Kaposi’s sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 1986, 12, 2029–2032. [Google Scholar] [CrossRef]
- Régnier-Rosencher, E.; Guillot, B.; Dupin, N. Treatments for classic Kaposi sarcoma: A systematic review of the literature. J. Am. Acad. Dermatol. 2013, 68, 313–331. [Google Scholar] [CrossRef]
- Denis, D.; Régnier-Rosencher, E.; Kramkimel, N.; Jafari, A.; Avril, M.F.; Dupin, N. First-line treatment with paclitaxel for non-HIV-related Kaposi sarcoma: Experience in 10 cases. Br. J. Dermatol. 2016, 174, 905–908. [Google Scholar] [CrossRef]
- Northfelt, D.W.; Dezube, B.J.; Thommes, J.A.; Miller, B.J.; Fischl, M.A.; Friedman-Kien, A.; Kaplan, L.D.; Du Mond, C.; Mamelok, R.D.; Henry, D.H. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: Results of a randomized phase III clinical trial. J. Clin. Oncol. 1998, 16, 2445–2451. [Google Scholar] [CrossRef]
- Vacca, A.; Ribatti, D.; Iurlaro, M.; Merchionne, F.; Nico, B.; Ria, R.; Dammacco, F. Docetaxel versus paclitaxel for antiangiogenesis. J. Hematother. Stem Cell Res. 2002, 11, 103–118. [Google Scholar] [CrossRef]
- Kibria, G.; Hatakeyama, H.; Sato, Y.; Harashima, H. Anti-tumor effect via passive anti-angiogenesis of PEGylated liposomes encapsulating doxorubicin in drug resistant tumors. Int. J. Pharm. 2016, 509, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Şen, H.S.; Ateş, B.T.; Yılmazbaş, P.; Ocak, S.; Kırımlıoğlu, H.; Gökçe, S.; Acarlı, K. Successful treatment of pediatric post-liver transplant Kaposi‘s sarcoma with paclitaxel. Turk. J. Pediatr. 2020, 62, 858–862. [Google Scholar] [CrossRef]
- Cianfrocca, M.; Lee, S.; Von Roenn, J.; Tulpule, A.; Dezube, B.J.; Aboulafia, D.M.; Ambinder, R.F.; Lee, J.Y.; Krown, S.E.; Sparano, J.A. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma. Cancers 2010, 116, 3969–3977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fardet, L.; Stoebner, P.E.; Bachelez, H.; Descamps, V.; Kerob, D.; Meunier, L.; Dandurand, M.; Morel, P.; Lebbe, C. Treatment with taxanes of refractory or life-threatening Kaposi sarcoma not associated with human immunodeficiency virus infection. Cancers 2006, 106, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Tourlaki, A.; Germiniasi, F.; Rossi, L.C.; Veraldi, S.; Brambilla, L. Paclitaxel as first- or second-line treatment for HIV-negative Kaposi’s sarcoma: A retrospective study of 58 patients. J. Dermatolog. Treat. 2020, 31, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Kreuter, A.; Di Trolio, R.; Guarini, A.; Romano, C.; Montesarchio, V.; Brockmeyer, N.H.; De Placido, S.; Bower, M.; Dezube, B.J. Activity and safety of pegylated liposomal doxorubicin as first-line therapy in the treatment of non-visceral classic Kaposi’s sarcoma: A multicenter study. J. Invest. Dermatol. 2008, 128, 1578–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa da Cunha, C.S.; Lebbe, C.; Rybojad, M.; Agbalika, F.; Ferchal, F.; Rabian, C.; Vignon-Pennamen, M.D.; Calvo, F.; Morel, P. Long-term follow-up of non-HIV Kaposi’s sarcoma treated with low-dose recombinant interferon alfa-2b. Arch. Dermatol. 1996, 132, 285–290. [Google Scholar] [CrossRef]
- Rybojad, M.; Borradori, L.; Verola, O.; Zeller, J.; Puissant, A.; Morel, P. Non-AIDS-associated Kaposi’s sarcoma (classical and endemic African types): Treatment with low doses of recombinant interferon-alpha. J. Invest. Dermatol. 1990, 95 (Suppl. S6), 176S–179S. [Google Scholar] [CrossRef] [PubMed]
- Delyon, J.; Bizot, A.; Battistella, M.; Madelaine, I.; Vercellino, L.; Lebbé, C. PD-1 blockade with nivolumab in endemic Kaposi sarcoma. Ann. Oncol. 2018, 29, 1067–1069. [Google Scholar] [CrossRef]
- Delyon, J.; Biard, L.; Renaud, M.; Resche-Rigon, M.; Le Goff, J.; Dalle, S.; Heidelberger, V.; Da Meda, L.; Toullec, L.; Carcelain, G.; et al. PD-1 blockade with pembrolizumab in classic or endemic Kaposi’s sarcoma: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2022, 23, 491–500. [Google Scholar] [CrossRef]
- Ramaswami, R.; Polizzotto, M.N.; Lurain, K.; Wyvill, K.M.; Widell, A.; George, J.; Goncalves, P.; Steinberg, S.M.; Whitby, D.; Uldrick, T.S.; et al. Safety, Activity, and Long-term Outcomes of Pomalidomide in the Treatment of Kaposi Sarcoma among Individuals with or without HIV Infection. Clin. Cancer Res. 2021, 28, 840–850. [Google Scholar] [CrossRef]
- Research C for DE and FDA Grants Accelerated Approval to Pomalidomide for Kaposi Sarcoma. 2020. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pomalidomide-kaposi-sarcoma (accessed on 21 February 2022).
- Oncology, T.L. Access to cancer medicine in low-resource settings. Lancet Oncol. 2013, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Galindo, C.; Friedrich, P.; Morrissey, L.; Frazier, L. Global challenges in pediatric oncology. Curr. Opin. Pediatr. 2013, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Stefan, D.C. Cancer Care in Africa: An Overview of Resources. J. Glob. Oncol. 2015, 1, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Krown, S.E.; Moser, C.B.; MacPhail, P.; Matining, R.M.; Godfrey, C.; Caruso, S.R.; Hosseinipour, M.C.; Samaneka, W.; Nyirenda, M.; Busakhala, N.W. Treatment of advanced AIDS-associated Kaposi sarcoma in resource-limited settings: A three-arm, open-label, randomised, non-inferiority trial. Lancet 2020, 395, 1195–1207. [Google Scholar] [CrossRef]
- Slavin, G.; Cameron, H.M.; Forbes, C.; Mitchell, R.M. Kaposi’s sarcoma in East African children: A report of 51 cases. J. Pathol. 1970, 100, 187–199. [Google Scholar] [CrossRef]
- Cox, C.M.; El-Mallawany, N.K.; Kabue, M.; Kovarik, C.; Schutze, G.E.; Kazembe, P.N.; Mehta, P.S. Clinical characteristics and outcomes of HIV-infected children diagnosed with kaposi sarcoma in malawi and botswana. Pediatr. Blood Cancer 2013, 60, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A.; Abbas, S.; Davies, M.A.; Bunupuradah, T.; Sohn, A.H.; Technau, K.G.; Renner, L.; Leroy, V.; Edmonds, A.; Yotebieng, M.; et al. Brief Report: Pediatric Cancer Burden and Treatment Resources Within the Pediatric IeDEA Consortium. J. Acquir. Immune. Defic. Syndr. 2017, 76, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, D.A.; Henley, S.J.; Li, J.; Pollack, L.A.; Van Dyne, E.A.; White, A. Rates and Trends of Pediatric Acute Lymphoblastic Leukemia-United States, 2001–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 950–954. [Google Scholar] [CrossRef] [Green Version]
- Adinani, H.; Campbell, L.; El-Mallawany, N.K.; Slone, J.; Mehta, P.; Bacha, J. Use of Paclitaxel to Successfully Treat Children, Adolescents, and Young Adults with Kaposi Sarcoma in Southwestern Tanzania. Children 2021, 8, 275. [Google Scholar] [CrossRef]
- Robertson, J.; Barr, R.; Shulman, L.N.; Forte, G.B.; Magrini, N. Essential medicines for cancer: WHO recommendations and national priorities. Bull. World Health Organ. 2016, 94, 735–742. [Google Scholar] [CrossRef]
- WHO Model List of Essential Medicines-22nd List. 2021. Available online: https://www.who.int/publications-detail-redirect/WHO-MHP-HPS-EML-2021.02 (accessed on 27 May 2022).
- Allied Against Cancer. Available online: https://www.alliedagainstcancer.org/access-partnership (accessed on 27 May 2022).

| Clinical Presentation | Therapeutic Strategy |
|---|---|
| Adult Kaposi Sarcoma Indolent asymptomatic slow progressing disease Localized cutaneous/oral disease | Therapeutic abstinence or Local therapies:
|
| Adult Kaposi Sarcoma Advanced rapidly progressive disease Disseminated cutaneous/oral disease Lymph node disease Visceral localization Soft tissue extension +/− severe lymphedema +/− painful lesions | Systemic chemotherapy
Immune-modulating agents
Immune checkpoint inhibitors (in selected cases)
Anti-angiogenic therapies
+/− Local therapies (radiotherapy or surgical resection) Common Dosing Adjustments for Systemic Chemotherapy in LMIC settings
|
| Pediatric Kaposi Sarcoma | BV regimen (4–6 cycles at two-week intervals for induction treatment then additional 4–6 cycles at monthly basis for consolidation treatment) +/− HAART
ABV intensified regimen (6–8 cycles at three-week intervals) +/− HAART
Paclitaxel (6 cycles at four-week intervals)
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeinaty, P.E.; Lebbé, C.; Delyon, J. Endemic Kaposi’s Sarcoma. Cancers 2023, 15, 872. https://doi.org/10.3390/cancers15030872
Zeinaty PE, Lebbé C, Delyon J. Endemic Kaposi’s Sarcoma. Cancers. 2023; 15(3):872. https://doi.org/10.3390/cancers15030872
Chicago/Turabian StyleZeinaty, Perla El, Céleste Lebbé, and Julie Delyon. 2023. "Endemic Kaposi’s Sarcoma" Cancers 15, no. 3: 872. https://doi.org/10.3390/cancers15030872
APA StyleZeinaty, P. E., Lebbé, C., & Delyon, J. (2023). Endemic Kaposi’s Sarcoma. Cancers, 15(3), 872. https://doi.org/10.3390/cancers15030872






