Development of Novel Murine BRAFV600E-Driven Papillary Thyroid Cancer Cell Lines for Modeling of Disease Progression and Preclinical Evaluation of Therapeutics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Derivation of Murine Thyroid Tumor Cell Lines and Wild-Type Thyrocyte Cultures
2.2. H&E and Trichrome Staining
2.3. RT-PCR Analysis
2.4. Proliferation and Viability Assays
2.5. Western Blot Analysis
2.6. Organoids
2.7. Migration Assay
2.8. Imaging
2.9. In Vivo Tumorigenicity Assay
2.10. Statistical Analysis
3. Results
3.1. Generation of Stable Tumor Cell Lines from BrafV600E Murine Thyroid Tumors
3.2. Expression of Thyroid-Specific Genes, Proliferation Rate, and Activation of MAPK and PI3K Signaling Pathways in Novel BrafV600E Murine Cell Lines
3.3. Evaluation of EMT Properties of BrafV600E Cell Lines
3.4. Growth Suppressive Effect of Pathway-Specific Inhibitors in BrafV600E-Driven Thyroid Tumor Cell Lines in 2D Monolayer and 3D Organoid Cultures
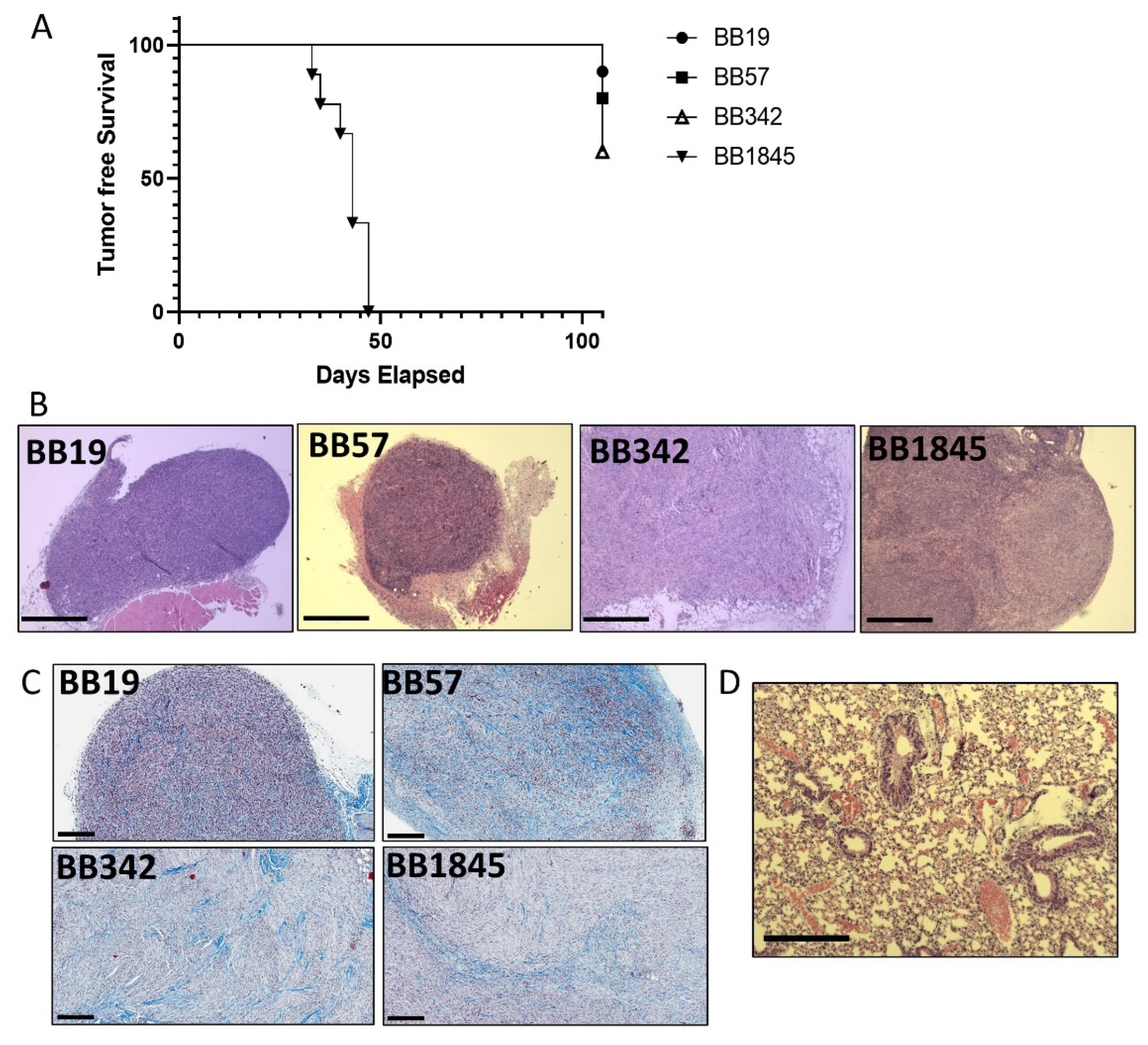
3.5. Development of a Syngeneic Subcutaneous Tumor Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Jakubowski, C.D.; Fedewa, S.A.; Davis, A.; Azad, N.S. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.J.; Jin, M.C.; Meister, K.D.; Megwalu, U.C. Pediatric Thyroid Cancer Incidence and Mortality Trends in the United States, 1973–2013. JAMA Otolaryngol. Neck Surg. 2019, 145, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Saini, S.; Tulla, K.; Maker, A.V.; Burman, K.D.; Prabhakar, B.S. Therapeutic Advances in Anaplastic Thyroid Cancer: A Current Perspective. Mol. Cancer 2018, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Ricarte-Filho, J.C.; Ryder, M.; Chitale, D.A.; Rivera, M.; Heguy, A.; Ladanyi, M.; Janakiraman, M.; Solit, D.; Knauf, J.A.; Tuttle, R.M.; et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009, 69, 4885–4893. [Google Scholar] [CrossRef]
- Abe, I.; Lam, A.K. Anaplastic Thyroid Carcinoma: Current Issues in Genomics and Therapeutics. Curr. Oncol. Rep. 2021, 23, 31. [Google Scholar] [CrossRef]
- Hescheler, D.A.; Hartmann, M.J.M.; Riemann, B.; Michel, M.; Bruns, C.J.; Alakus, H.; Chiapponi, C. Anaplastic thyroid cancer: Genome-based search for new targeted therapy options. Endocr. Connect. 2022, 11, e210624. [Google Scholar] [CrossRef]
- Franco, A.T.; Ricarte-Filho, J.C.; Isaza, A.; Jones, Z.; Jain, N.; Mostoufi-Moab, S.; Surrey, L.; Laetsch, T.W.; Li, M.M.; DeHart, J.C.; et al. Fusion Oncogenes Are Associated with Increased Metastatic Capacity and Persistent Disease in Pediatric Thyroid Cancers. J. Clin. Oncol. 2022, 40, 1081–1090. [Google Scholar] [CrossRef]
- Puxeddu, E.; Moretti, S.; Elisei, R.; Romei, C.; Pascucci, R.; Martinelli, M.; Marino, C.; Avenia, N.; Rossi, E.D.; Fadda, G.; et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J. Clin. Endocrinol. Metab. 2004, 89, 2414–2420. [Google Scholar] [CrossRef]
- Bauer, A.J. Molecular Genetics of Thyroid Cancer in Children and Adolescents. Endocrinol. Metab. Clin. N. Am. 2017, 46, 389–403. [Google Scholar] [CrossRef]
- Xing, M. Braf Mutation in Thyroid Cancer. Endocr. Relat. Cancer 2005, 12, 245–262. [Google Scholar] [CrossRef]
- Xing, M. Molecular Pathogenesis and Mechanisms of Thyroid Cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, E.S.; Kim, Y.S. Clinicopathologic Significance of Braf V600e Mutation in Papillary Carcinomas of the Thyroid: A Meta-Analysis. Cancer 2007, 110, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Ugolini, C.; Viola, D.; Lupi, C.; Biagini, A.; Giannini, R.; Romei, C.; Miccoli, P.; Pinchera, A.; Basolo, F. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 3943–3949. [Google Scholar] [CrossRef] [PubMed]
- Frasca, F.; Nucera, C.; Pellegriti, G.; Gangemi, P.; Attard, M.; Stella, M.; Loda, M.; Vella, V.; Giordano, C.; Trimarchi, F.; et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocr. Relat. Cancer 2008, 15, 191–205. [Google Scholar] [CrossRef]
- Namba, H.; Nakashima, M.; Hayashi, T.; Hayashida, N.; Maeda, S.; Rogounovitch, T.I.; Ohtsuru, A.; Saenko, V.A.; Kanematsu, T.; Yamashita, S. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J. Clin. Endocrinol. Metab. 2003, 88, 4393–4397. [Google Scholar] [CrossRef]
- Kebebew, E.; Weng, J.; Bauer, J.; Ranvier, G.; Clark, O.H.; Duh, Q.Y.; Shibru, D.; Bastian, B.; Griffin, A. The Prevalence and Prognostic Value of Braf Mutation in Thyroid Cancer. Ann. Surg. 2007, 246, 466–471; discussion 70–71. [Google Scholar] [CrossRef]
- Liu, J.; Liu, R.; Shen, X.; Zhu, G.; Li, B.; Xing, M. The Genetic Duet of Braf V600e and Tert Promoter Mutations Robustly Predicts Loss of Radioiodine Avidity in Recurrent Papillary Thyroid Cancer. J. Nucl. Med. 2020, 61, 177–182. [Google Scholar] [CrossRef]
- Pekova, B.; Sykorova, V.; Dvorakova, S.; Vaclavikova, E.; Moravcova, J.; Katra, R.; Astl, J.; Vlcek, P.; Kodetova, D.; Vcelak, J.; et al. Ret, Ntrk, Alk, Braf, and Met Fusions in a Large Cohort of Pediatric Papillary Thyroid Carcinomas. Thyroid 2020, 30, 1771–1780. [Google Scholar] [CrossRef]
- Henke, L.E.; Perkins, S.M.; Pfeifer, J.D.; Ma, C.; Chen, Y.; DeWees, T.; Grigsby, P.W. Braf V600e Mutational Status in Pediatric Thyroid Cancer. Pediatr. Blood Cancer 2014, 61, 1168–1172. [Google Scholar] [CrossRef]
- Givens, D.J.; Buchmann, L.O.; Agarwal, A.M.; Grimmer, J.F.; Hunt, J.P. Braf V600e Does Not Predict Aggressive Features of Pediatric Papillary Thyroid Carcinoma. Laryngoscope 2014, 124, E389–E393. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Liu, D.; Shan, Y.; Hu, S.; Studeman, K.; Condouris, S.; Wang, Y.; Trink, A.; El-Naggar, A.K.; Tallini, G.; et al. Genetic Alterations and Their Relationship in the Phosphatidylinositol 3-Kinase/Akt Pathway in Thyroid Cancer. Clin. Cancer Res. 2007, 13, 1161–1170. [Google Scholar] [CrossRef]
- Santarpia, L.; El-Naggar, A.K.; Cote, G.J.; Myers, J.N.; Sherman, S.I. Phosphatidylinositol 3-Kinase/Akt and Ras/Raf-Mitogen-Activated Protein Kinase Pathway Mutations in Anaplastic Thyroid Cancer. J. Clin. Endocrinol. Metab. 2008, 93, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Jin, M.; Jang, A.; Jeon, M.J.; Song, D.E.; Yoo, H.J.; Kim, W.B.; Shong, Y.K.; Kim, W.G. Mutation in Genes Encoding Key Functional Groups Additively Increase Mortality in Patients with Braf(V600e)-Mutant Advanced Papillary Thyroid Carcinoma. Cancers 2021, 13, 5846. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hou, P.; Ji, M.; Guan, H.; Studeman, K.; Jensen, K.; Vasko, V.; El-Naggar, A.K.; Xing, M. Highly Prevalent Genetic Alterations in Receptor Tyrosine Kinases and Phosphatidylinositol 3-Kinase/Akt and Mitogen-Activated Protein Kinase Pathways in Anaplastic and Follicular Thyroid Cancers. J. Clin. Endocrinol. Metab. 2008, 93, 3106–3116. [Google Scholar] [CrossRef] [PubMed]
- Schweppe, R.E.; Klopper, J.P.; Korch, C.; Pugazhenthi, U.; Benezra, M.; Knauf, J.A.; Fagin, J.A.; Marlow, L.A.; Copland, J.A.; Smallridge, R.C.; et al. Deoxyribonucleic Acid Profiling Analysis of 40 Human Thyroid Cancer Cell Lines Reveals Cross-Contamination Resulting in Cell Line Redundancy and Misidentification. J. Clin. Endocrinol. Metab. 2008, 93, 4331–4341. [Google Scholar] [CrossRef] [PubMed]
- Knauf, J.A.; Luckett, K.A.; Chen, K.Y.; Voza, F.; Socci, N.D.; Ghossein, R.; Fagin, J.A. Hgf/Met Activation Mediates Resistance to Braf Inhibition in Murine Anaplastic Thyroid Cancers. J. Clin. Investig. 2018, 128, 4086–4097. [Google Scholar] [CrossRef]
- Vanden Borre, P.; McFadden, D.G.; Gunda, V.; Sadow, P.M.; Varmeh, S.; Bernasconi, M.; Jacks, T.; Parangi, S. The Next Generation of Orthotopic Thyroid Cancer Models: Immunocompetent Orthotopic Mouse Models of Braf V600e-Positive Papillary and Anaplastic Thyroid Carcinoma. Thyroid 2014, 24, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Caperton, C.O.; Jolly, L.A.; Massoll, N.; Bauer, A.J.; Franco, A.T. Development of Novel Follicular Thyroid Cancer Models Which Progress to Poorly Differentiated and Anaplastic Thyroid Cancer. Cancers 2021, 13, 1094. [Google Scholar] [CrossRef]
- Franco, A.T.; Malaguarnera, R.; Refetoff, S.; Liao, X.H.; Lundsmith, E.; Kimura, S.; Pritchard, C.; Marais, R.; Davies, T.F.; Weinstein, L.S.; et al. Thyrotrophin Receptor Signaling Dependence of Braf-Induced Thyroid Tumor Initiation in Mice. Proc. Natl. Acad. Sci. USA 2011, 108, 1615–1620. [Google Scholar] [CrossRef]
- Jolly, L.A.; Novitskiy, S.; Owens, P.; Massoll, N.; Cheng, N.; Fang, W.; Moses, H.L.; Franco, A.T. Fibroblast-Mediated Collagen Remodeling within the Tumor Microenvironment Facilitates Progression of Thyroid Cancers Driven by Brafv600e and Pten Loss. Cancer Res. 2016, 76, 1804–1813. [Google Scholar] [CrossRef]
- Vilgelm, A.E.; Bergdorf, K.; Wolf, M.; Bharti, V.; Shattuck-Brandt, R.; Blevins, A.; Jones, C.; Phifer, C.; Lee, M.; Lowe, C.; et al. Fine-Needle Aspiration-Based Patient-Derived Cancer Organoids. iScience 2020, 23, 101408. [Google Scholar] [CrossRef]
- Fernandez, L.P.; Lopez-Marquez, A.; Santisteban, P. Thyroid Transcription Factors in Development, Differentiation and Disease. Nat. Rev. Endocrinol. 2015, 11, 29–42. [Google Scholar] [CrossRef]
- Kimura, S. Thyroid-Specific Transcription Factors and Their Roles in Thyroid Cancer. J. Thyroid Res. 2011, 2011, 710213. [Google Scholar] [CrossRef]
- Kanjanahattakij, N.; Chayangsu, P.; Kanoksil, W.; Chontong, S.; Sriphrapradang, C. Pitfall in Immunohistochemical Staining for Thyroglobulin in Case of Thyroid Metastasis from Lung Carcinoma. Cytojournal 2015, 12, 27. [Google Scholar] [CrossRef]
- Liu, T.; Men, Q.; Su, X.; Chen, W.; Zou, L.; Li, Q.; Song, M.; Ouyang, D.; Chen, Y.; Li, Z.; et al. Downregulated Expression of Tshr Is Associated with Distant Metastasis in Thyroid Cancer. Oncol. Lett. 2017, 14, 7506–7512. [Google Scholar] [CrossRef]
- Riesco-Eizaguirre, G.; Rodriguez, I.; De la Vieja, A.; Costamagna, E.; Carrasco, N.; Nistal, M.; Santisteban, P. The Brafv600e Oncogene Induces Transforming Growth Factor Beta Secretion Leading to Sodium Iodide Symporter Repression and Increased Malignancy in Thyroid Cancer. Cancer Res. 2009, 69, 8317–8325. [Google Scholar] [CrossRef]
- Tavares, C.; Coelho, M.J.; Eloy, C.; Melo, M.; da Rocha, A.G.; Pestana, A.; Batista, R.; Ferreira, L.B.; Rios, E.; Selmi-Ruby, S.; et al. Nis Expression in Thyroid Tumors, Relation with Prognosis Clinicopathological and Molecular Features. Endocr. Connect. 2018, 7, 78–90. [Google Scholar] [CrossRef]
- Spitzweg, C.; Bible, K.C.; Hofbauer, L.C.; Morris, J.C. Advanced Radioiodine-Refractory Differentiated Thyroid Cancer: The Sodium Iodide Symporter and Other Emerging Therapeutic Targets. Lancet Diabetes Endocrinol. 2014, 2, 830–842. [Google Scholar] [CrossRef]
- Hou, P.; Bojdani, E.; Xing, M. Induction of Thyroid Gene Expression and Radioiodine Uptake in Thyroid Cancer Cells by Targeting Major Signaling Pathways. J. Clin. Endocrinol. Metab. 2010, 95, 820–828. [Google Scholar] [CrossRef]
- Fu, H.; Cheng, L.; Sa, R.; Jin, Y.; Chen, L. Combined Tazemetostat and Mapki Enhances Differentiation of Papillary Thyroid Cancer Cells Harbouring Braf(V600e) by Synergistically Decreasing Global Trimethylation of H3k27. J. Cell. Mol. Med. 2020, 24, 3336–3345. [Google Scholar] [CrossRef] [PubMed]
- Shakib, H.; Rajabi, S.; Dehghan, M.H.; Mashayekhi, F.J.; Safari-Alighiarloo, N.; Hedayati, M. Epithelial-to-Mesenchymal Transition in Thyroid Cancer: A Comprehensive Review. Endocrine 2019, 66, 435–455. [Google Scholar] [CrossRef]
- Busaidy, N.L.; Konda, B.; Wei, L.; Wirth, L.J.; Devine, C.; Daniels, G.A.; DeSouza, J.A.; Poi, M.; Seligson, N.D.; Cabanillas, M.E.; et al. Dabrafenib Versus Dabrafenib + Trametinib in ≪I≫Braf≪/I≫-Mutated Radioactive Iodine Refractory Differentiated Th205yroid Cancer: Results of a Randomized, Phase 2, Open-Label Multicenter Trial. Thyroid 2022, 32, 1184–1992. [Google Scholar] [CrossRef]
- Kieran, M.W.; Geoerger, B.; Dunkel, I.J.; Broniscer, A.; Hargrave, D.; Hingorani, P.; Aerts, I.; Bertozzi, A.I.; Cohen, K.J.; Hummel, T.R.; et al. A Phase I and Pharmacokinetic Study of Oral Dabrafenib in Children and Adolescent Patients with Recurrent or Refractory Braf V600 Mutation-Positive Solid Tumors. Clin. Cancer Res. 2019, 25, 7294–7302. [Google Scholar] [CrossRef] [PubMed]
- Paulson, V.A.; Rudzinski, E.R.; Hawkins, D.S. Thyroid Cancer in the Pediatric Population. Genes 2019, 10, 723. [Google Scholar] [CrossRef]
- Yang, J.; Weinberg, R.A. Epithelial-Mesenchymal Transition: At the Crossroads of Development and Tumor Metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-Mesenchymal Transitions in Tumour Progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Vasko, V.; Espinosa, A.V.; Scouten, W.; He, H.; Auer, H.; Liyanarachchi, S.; Larin, A.; Savchenko, V.; Francis, G.L.; de la Chapelle, A.; et al. Gene Expression and Functional Evidence of Epithelial-to-Mesenchymal Transition in Papillary Thyroid Carcinoma Invasion. Proc. Natl. Acad. Sci. USA 2007, 104, 2803–2808. [Google Scholar] [CrossRef]
- Olson, A.; Le, V.; Aldahl, J.; Yu, E.J.; Hooker, E.; He, Y.; Lee, D.H.; Kim, W.K.; Cardiff, R.D.; Geradts, J.; et al. The Comprehensive Role of E-Cadherin in Maintaining Prostatic Epithelial Integrity During Oncogenic Transformation and Tumor Progression. PLoS Genet. 2019, 15, e1008451. [Google Scholar] [CrossRef]
- Wiseman, S.M.; Griffith, O.L.; Deen, S.; Rajput, A.; Masoudi, H.; Gilks, B.; Goldstein, L.; Gown, A.; Jones, S.J. Identification of Molecular Markers Altered during Transformation of Differentiated into Anaplastic Thyroid Carcinoma. Arch. Surg. 2007, 142, 717–729; discussion 27–29. [Google Scholar] [CrossRef]
- Knauf, J.A.; Sartor, M.A.; Medvedovic, M.; Lundsmith, E.; Ryder, M.; Salzano, M.; Nikiforov, Y.E.; Giordano, T.J.; Ghossein, R.A.; Fagin, J.A. Progression of Braf-Induced Thyroid Cancer Is Associated with Epithelial-Mesenchymal Transition Requiring Concomitant Map Kinase and Tgfbeta Signaling. Oncogene 2011, 30, 3153–3162. [Google Scholar] [CrossRef]
- Jung, C.W.; Han, K.H.; Seol, H.; Park, S.; Koh, J.S.; Lee, S.S.; Kim, M.J.; Choi, I.J.; Myung, J.K. Expression of Cancer Stem Cell Markers and Epithelial-Mesenchymal Transition-Related Factors in Anaplastic Thyroid Carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 560–568. [Google Scholar]
- Gunda, V.; Gigliotti, B.; Ndishabandi, D.; Ashry, T.; McCarthy, M.; Zhou, Z.; Amin, S.; Freeman, G.J.; Alessandrini, A.; Parangi, S. Combinations of Braf Inhibitor and Anti-Pd-1/Pd-L1 Antibody Improve Survival and Tumour Immunity in an Immunocompetent Model of Orthotopic Murine Anaplastic Thyroid Cancer. Br. J. Cancer 2018, 119, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Ryder, M.; Ghossein, R.A.; Ricarte-Filho, J.C.; Knauf, J.A.; Fagin, J.A. Increased Density of Tumor-Associated Macrophages Is Associated with Decreased Survival in Advanced Thyroid Cancer. Endocr. Relat. Cancer 2008, 15, 1069–1074. [Google Scholar] [CrossRef]
- Zhang, P.; Guan, H.; Yuan, S.; Cheng, H.; Zheng, J.; Zhang, Z.; Liu, Y.; Yu, Y.; Meng, Z.; Zheng, X.; et al. Targeting Myeloid Derived Suppressor Cells Reverts Immune Suppression and Sensitizes Braf-Mutant Papillary Thyroid Cancer to Mapk Inhibitors. Nat. Commun. 2022, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Kreisle, R.A.; Stebler, B.A.; Ershler, W.B. Effect of Host Age on Tumor-Associated Angiogenesis in Mice. J. Natl. Cancer Inst. 1990, 82, 44–47. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the Ageing Microenvironment Influences Tumour Progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef]
- Jackaman, C.; Radley-Crabb, H.G.; Soffe, Z.; Shavlakadze, T.; Grounds, M.D.; Nelson, D.J. Targeting Macrophages Rescues Age-Related Immune Deficiencies in C57bl/6j Geriatric Mice. Aging Cell 2013, 12, 345–357. [Google Scholar] [CrossRef]
- Reed, M.J.; Karres, N.; Eyman, D.; Cruz, A.; Brekken, R.A.; Plymate, S. The Effects of Aging on Tumor Growth and Angiogenesis Are Tumor-Cell Dependent. Int. J. Cancer 2007, 120, 753–760. [Google Scholar] [CrossRef]
- Vincent-Chong, V.K.; DeJong, H.; Rich, L.J.; Patti, A.; Merzianu, M.; Hershberger, P.A.; Seshadri, M. Impact of Age on Disease Progression and Microenvironment in Oral Cancer. J. Dent. Res. 2018, 97, 1268–1276. [Google Scholar] [CrossRef]
- Pettan-Brewer, C.; Morton, J.; Coil, R.; Hopkins, H.; Fatemie, S.; Ladiges, W. B16 Melanoma Tumor Growth Is Delayed in Mice in an Age-Dependent Manner. Pathobiol. Aging Age Relat. Dis. 2012, 2, 19182. [Google Scholar] [CrossRef] [PubMed]
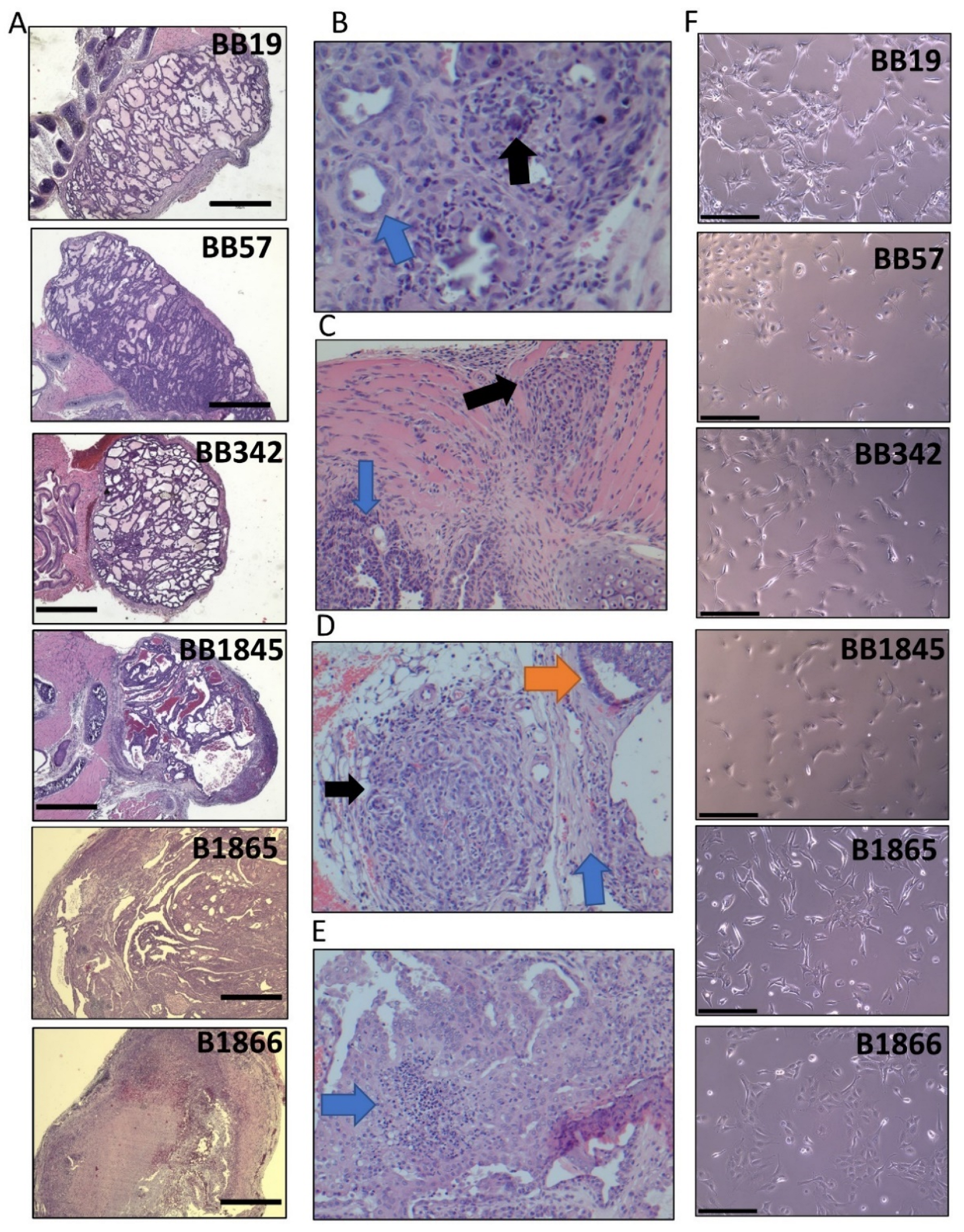
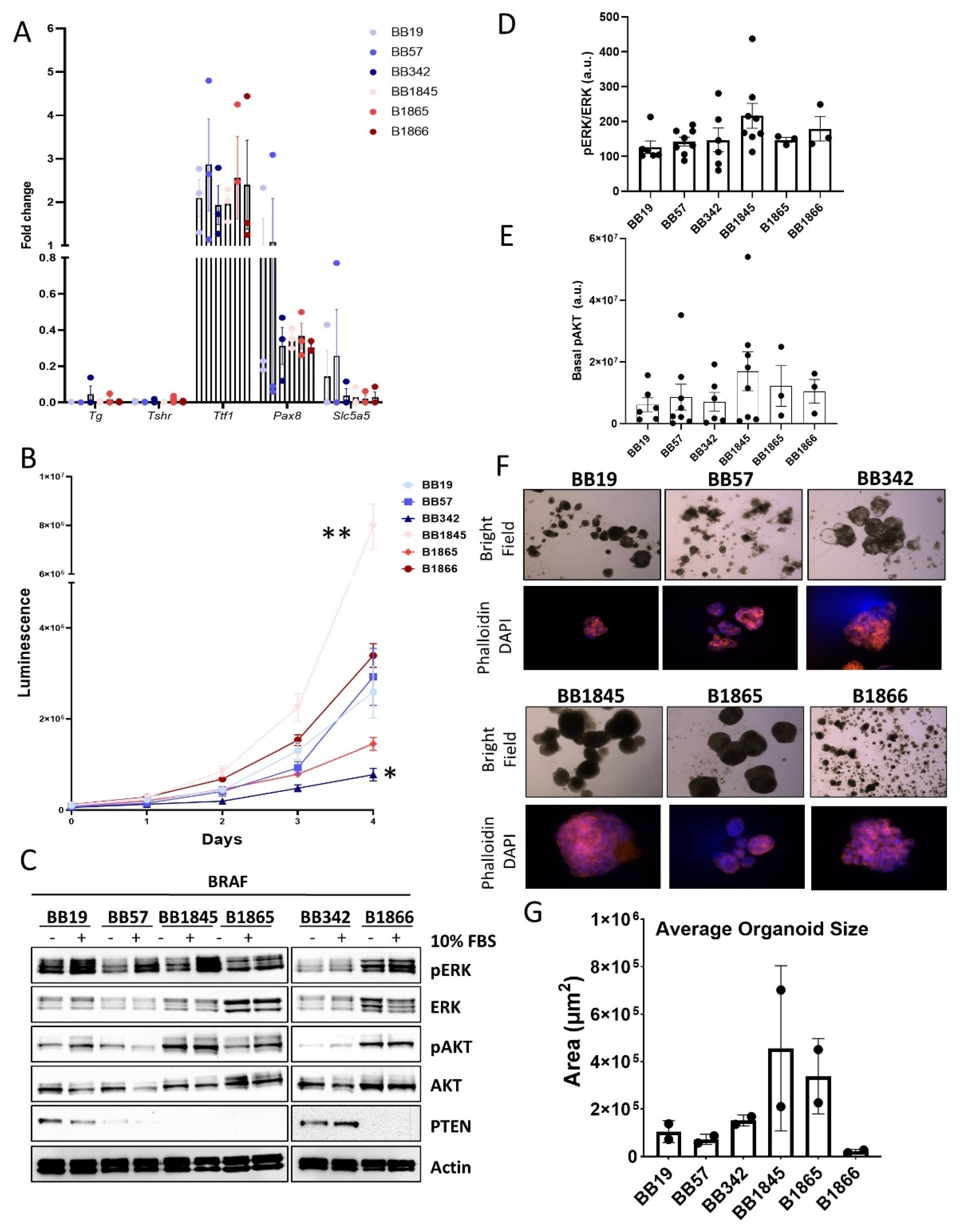
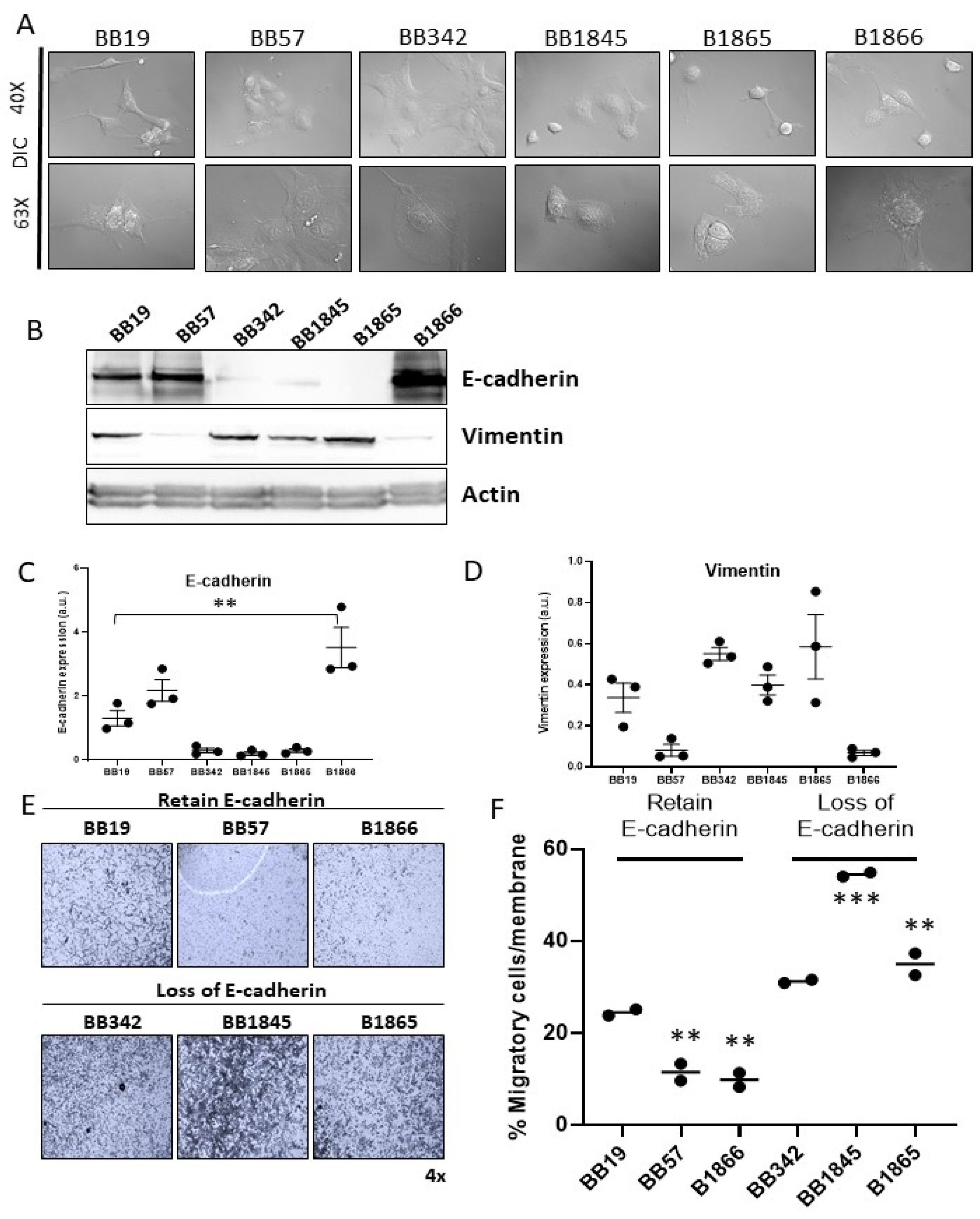
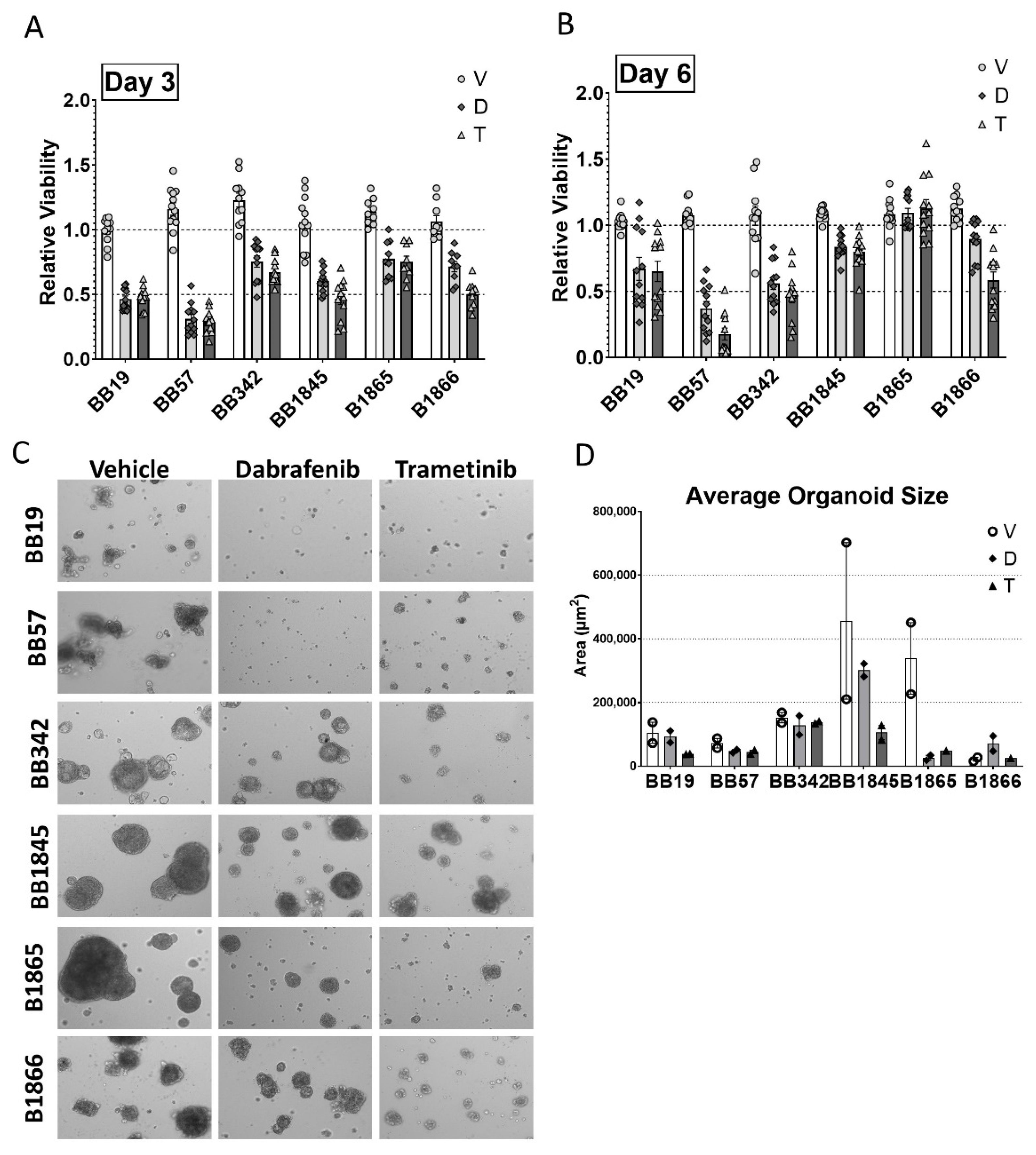
| Cell Line | Genotype | Sex | Age (Weeks) at Collection |
|---|---|---|---|
| BB19 | BrafV600EHet/PtenHet/TPO-Cre+ | Female | 6 |
| BB57 | BrafV600EHet/PtenHet/TPO-Cre+ | Male | 4 |
| BB342 | BrafV600EHet/PtenHet/TPO-Cre+ | Male | 3 |
| BB1845 | BrafV600EHet/PtenHet/TPO-Cre+ | Female | 32 |
| B1865 | BrafV600EHet/PtenHet/TPO-Cre+ | Male | 24 |
| B1866 | BrafV600EHet/PtenHet/TPO-Cre+ | Male | 24 |
| Cell Line | Number of Animals with Tumors | % Penetrance (/N) | Number of Animals with Lung Metastasis |
|---|---|---|---|
| BB19 | 1 | 10% (1/10) | 0 |
| BB57 | 2 | 20% (2/10) | 0 |
| BB342 | 4 | 40% (4/10) | 1 |
| BB1845 | 9 | 100% (9/9) | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branigan, G.P.; Casado-Medrano, V.; O’Neill, A.B.; Ricarte-Filho, J.C.; Massoll, N.; Salwen, M.; Spangler, Z.; Scheerer, M.; Williamson, E.K.; Bauer, A.J.; et al. Development of Novel Murine BRAFV600E-Driven Papillary Thyroid Cancer Cell Lines for Modeling of Disease Progression and Preclinical Evaluation of Therapeutics. Cancers 2023, 15, 879. https://doi.org/10.3390/cancers15030879
Branigan GP, Casado-Medrano V, O’Neill AB, Ricarte-Filho JC, Massoll N, Salwen M, Spangler Z, Scheerer M, Williamson EK, Bauer AJ, et al. Development of Novel Murine BRAFV600E-Driven Papillary Thyroid Cancer Cell Lines for Modeling of Disease Progression and Preclinical Evaluation of Therapeutics. Cancers. 2023; 15(3):879. https://doi.org/10.3390/cancers15030879
Chicago/Turabian StyleBranigan, Grace Purvis, Victoria Casado-Medrano, Alison B. O’Neill, Julio C. Ricarte-Filho, Nicole Massoll, Madeleine Salwen, Zachary Spangler, Michele Scheerer, Edward K. Williamson, Andrew J. Bauer, and et al. 2023. "Development of Novel Murine BRAFV600E-Driven Papillary Thyroid Cancer Cell Lines for Modeling of Disease Progression and Preclinical Evaluation of Therapeutics" Cancers 15, no. 3: 879. https://doi.org/10.3390/cancers15030879
APA StyleBranigan, G. P., Casado-Medrano, V., O’Neill, A. B., Ricarte-Filho, J. C., Massoll, N., Salwen, M., Spangler, Z., Scheerer, M., Williamson, E. K., Bauer, A. J., & Franco, A. T. (2023). Development of Novel Murine BRAFV600E-Driven Papillary Thyroid Cancer Cell Lines for Modeling of Disease Progression and Preclinical Evaluation of Therapeutics. Cancers, 15(3), 879. https://doi.org/10.3390/cancers15030879





