Management of Recurrent Well-Differentiated Thyroid Carcinoma in the Neck: A Comprehensive Review †
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Recommended Diagnostic Work-Up
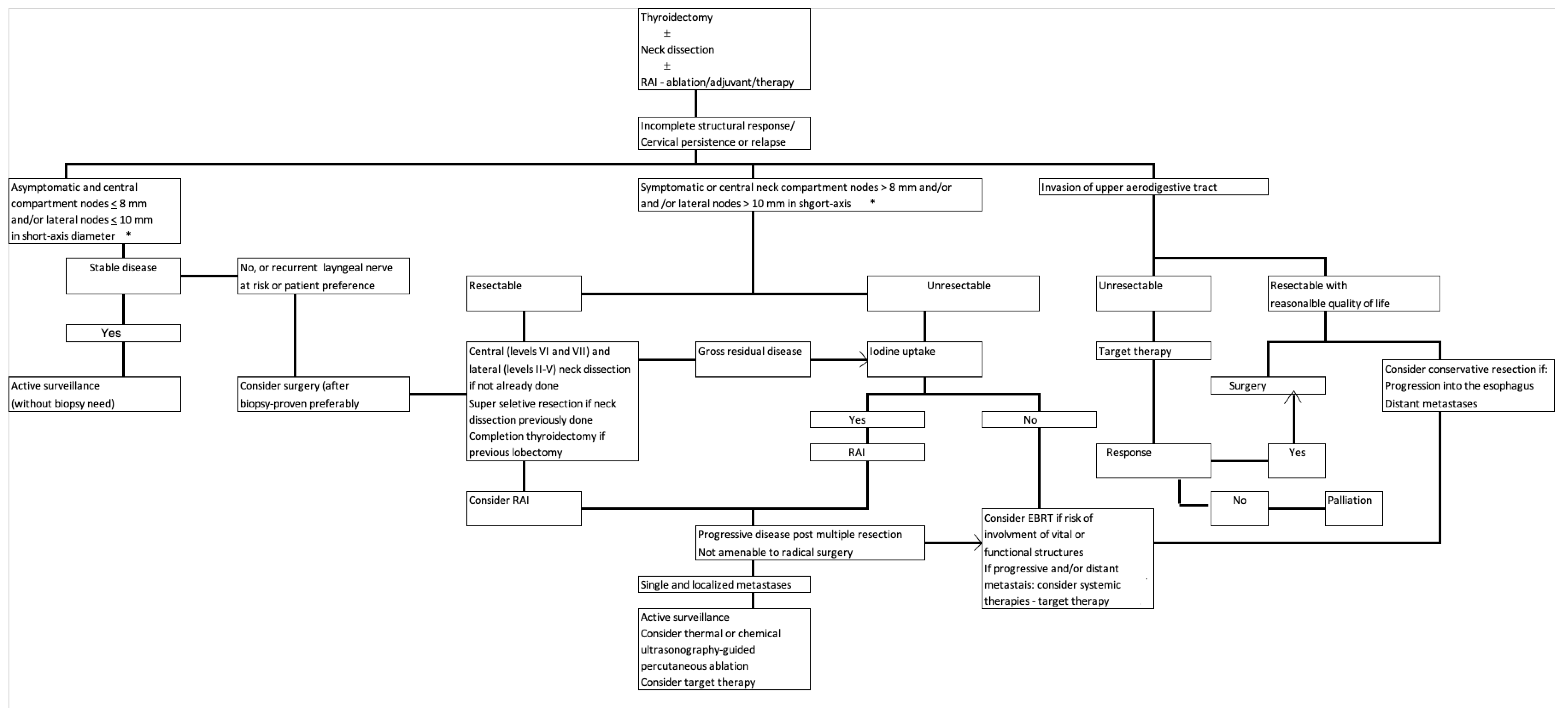
4. The Basis for Therapeutic Decision Making
5. Current Treatment Modalities
5.1. Surgery
5.2. Active Surveillance
5.3. Radioactive Iodine
5.4. External Beam Radiotherapy
5.5. Ultrasonography-Guided Percutaneous Ablation
5.6. Systemic Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology. Thyroid Carcinoma. Natl. Compr. Cancer Netw., Version 2; 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf (accessed on 8 August 2022).
- Filetti, S.; Durante, C.; Hartl, D.; Leboulleux, S.; Locati, L.D.; Newbold, K.; Papotti, M.G.; Berruti, A.; ESMO Guidelines Committee. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1856–1883. [Google Scholar] [CrossRef]
- Ito, Y.; Onoda, N.; Okamoto, T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: Core questions and recommendations for treatments of thyroid cancer. Endocr. J. 2020, 67, 669–717. [Google Scholar] [CrossRef] [PubMed]
- Cody, H.S., 3rd; Shah, J.P. Locally invasive, well-differentiated thyroid cancer. 22 years’ experience at Memorial Sloan-Kettering Cancer Center. Am. J. Surg. 1981, 142, 480–483. [Google Scholar] [CrossRef]
- Mazzaferri, E.L.; Jhiang, S.M. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am. J. Med. 1994, 97, 418–428, Erratum in Am. J. Med. 1995, 98, 215. [Google Scholar] [CrossRef]
- Cavalheiro, B.G.; de Matos, L.L.; Leite, A.K.N.; Kulcsar, M.A.V.; Cernea, C.R.; Kowalski, L.P. Survival in differentiated thyroid carcinoma: Comparison between the 7th and 8th editions of the AJCC/UICC TNM staging system and the ATA initial risk stratification system. Head Neck 2021, 43, 2913–2922. [Google Scholar] [CrossRef]
- Randolph, G.W.; Duh, Q.Y.; Heller, K.S.; LiVolsi, V.A.; Mandel, S.J.; Steward, D.L.; Tufano, R.P.; Tuttle, R.M.; American Thyroid Association Surgical Affairs Committee’s Taskforce on Thyroid Cancer Nodal Surgery. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012, 22, 1144–1152. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer (AJCC); Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; et al. Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Cao, H.S.T.; Johnston, L.E.; Chang, D.C.; Bouvet, M. A critical analysis of the American Joint Committee on Cancer (AJCC) staging system for differentiated thyroid carcinoma in young patients on the basis of the Surveillance, Epidemiology, and End Results (SEER) registry. Surgery 2012, 152, 145–151. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Tala, H.; Shah, J.; Leboeuf, R.; Ghossein, R.; Gonen, M.; Brokhin, M.; Omry, G.; Fagin, J.A.; Shaha, A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010, 20, 1341–1349. [Google Scholar] [CrossRef]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur. Thyroid J. 2019, 8, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Mauri, G.; Hegedüs, L.; Bandula, S.; Cazzato, R.L.; Czarniecka, A.; Dudeck, O.; Fugazzola, L.; Netea-Maier, R.; Russ, G.; Wallin, G.; et al. European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe, 2021 Clinical Practice Guideline for the Use of Minimally Invasive Treatments in Malignant Thyroid Lesions. Eur. Thyroid J. 2021, 10, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Fuhrer, D.; Elisei, R.; Handkiewicz-Junak, D.; Leboulleux, S.; Luster, M.; Schlumberger, M.; Smit, J.W. 2022 ETA Consensus Statement: What are the indications for post-surgical radioiodine therapy in differentiated thyroid cancer? Eur. Thyroid J. 2022, 11, e210046. [Google Scholar] [CrossRef]
- Elisei, R.; Agate, L.; Viola, D.; Matrone, A.; Biagini, A.; Molinaro, E. How to manage patients with differentiated thyroid cancer and a rising serum thyroglobulin level. Endocrinol. Metab. Clin. N. Am. 2014, 43, 331–344. [Google Scholar] [CrossRef]
- Pacini, F.; Agate, L.; Elisei, R.; Capezzone, M.; Ceccarelli, C.; Lippi, F.; Molinaro, E.; Pinchera, A. Outcome of differentiated thyroid cancer with detectable serum Tg and negative diagnostic 131I whole body scan: Comparison of patients treated with high (131)I activities versus untreated patients. J. Clin. Endocrinol. Metab. 2001, 86, 4092–4097. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Shaha, A.R. Locally advanced thyroid cancer. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 112–116. [Google Scholar] [CrossRef]
- Schlumberger, M.; Brose, M.; Elisei, R.; Leboulleux, S.; Luster, M.; Pitoia, F.; Pacini, F. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. 2014, 2, 356–358. [Google Scholar] [CrossRef]
- Asimakopoulos, P.; Shaha, A.R.; Nixon, I.J.; Shah, J.P.; Randolph, G.W.; Angelos, P.; Zafereo, M.E.; Kowalski, L.P.; Hartl, D.M.; Olsen, K.D.; et al. Management of the Neck in Well-Differentiated Thyroid Cancer. Curr. Oncol. Rep. 2020, 23, 1. [Google Scholar] [CrossRef]
- Shokoohi, A.; Berthelet, E.; Gill, S.; Prisman, E.; Sexsmith, G.; Tran, E.; White, A.; Wiseman, S.M.; Wu, J.; Ho, C. Treatment for Recurrent Differentiated Thyroid Cancer: A Canadian Population Based Experience. Cureus 2020, 12, e7122. [Google Scholar] [CrossRef]
- Ito, Y.; Kudo, T.; Takamura, Y.; Kobayashi, K.; Miya, A.; Miyauchi, A. Lymph node recurrence in patients with N1b papillary thyroid carcinoma who underwent unilateral therapeutic modified radical neck dissection. World J. Surg. 2012, 36, 593–597. [Google Scholar] [CrossRef]
- Chinn, S.B.; Zafereo, M.E.; Waguespack, S.G.; Edeiken, B.S.; Roberts, D.B.; Clayman, G.L. Long-Term Outcomes of Lateral Neck Dissection in Patients with Recurrent or Persistent Well-Differentiated Thyroid Cancer. Thyroid 2017, 27, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.; Amit, M.; Boonsripitayanon, M.; Cabanillas, M.E.; Busaidy, N.L.; Gunn, G.B.; Lai, S.Y.; Gross, N.D.; Sturgis, E.M.; Zafereo, M.E. Adjuvant External Beam Radiotherapy in Locally Advanced Differentiated Thyroid Cancer. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1244–1251. [Google Scholar] [CrossRef]
- Lamartina, L.; Borget, I.; Mirghani, H.; Al Ghuzlan, A.; Berdelou, A.; Bidault, F.; Deandreis, D.; Baudin, E.; Travagli, J.P.; Schlumberger, M.; et al. Surgery for Neck Recurrence of Differentiated Thyroid Cancer: Outcomes and Risk Factors. J. Clin. Endocrinol. Metab. 2017, 102, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Tufano, R.P.; Bishop, J.; Wu, G. Reoperative central compartment dissection for patients with recurrent/persistent papillary thyroid cancer: Efficacy, safety, and the association of the BRAF mutation. Laryngoscope 2012, 122, 1634–1640. [Google Scholar] [CrossRef]
- Hughes, D.T.; Laird, A.M.; Miller, B.S.; Gauger, P.G.; Doherty, G.M. Reoperative lymph node dissection for recurrent papillary thyroid cancer and effect on serum thyroglobulin. Ann. Surg. Oncol. 2012, 19, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Fundakowski, C.E.; Hales, N.W.; Agrawal, N.; Barczyński, M.; Camacho, P.M.; Hartl, D.M.; Kandil, E.; Liddy, W.E.; McKenzie, T.J.; Morris, J.C.; et al. Surgical management of the recurrent laryngeal nerve in thyroidectomy: American Head and Neck Society Consensus Statement. Head Neck 2018, 40, 663–675. [Google Scholar] [CrossRef]
- Kihara, M.; Miyauchi, A.; Yabuta, T.; Higashiyama, T.; Fukushima, M.; Ito, Y.; Kobayashi, K.; Miya, A. Outcome of vocal cord function after partial layer resection of the recurrent laryngeal nerve in patients with invasive papillary thyroid cancer. Surgery 2014, 155, 184–189. [Google Scholar] [CrossRef]
- Brooks, J.A.; Abdelhamid Ahmed, A.H.; Al-Qurayshi, Z.; Kamani, D.; Kyriazidis, N.; Hammon, R.J.; Ma, H.; Sritharan, N.; Wasserman, I.; Trinh, L.N.; et al. Recurrent Laryngeal Nerve Invasion by Thyroid Cancer: Laryngeal Function and Survival Outcomes. Laryngoscope 2022, 132, 2285–2292. [Google Scholar] [CrossRef]
- Simó, R.; Nixon, I.J.; Rovira, A.; Poorten, V.V.; Sanabria, A.; Zafereo, M.; Hartl, D.M.; Kowalski, L.P.; Randolph, G.W.; Kamani, D.; et al. Immediate Intraoperative Repair of the Recurrent Laryngeal Nerve in Thyroid Surgery. Laryngoscope 2021, 131, 1429–1435. [Google Scholar] [CrossRef]
- Piazza, C.; Lancini, D.; Tomasoni, M.; D’Cruz, A.; Hartl, D.M.; Kowalski, L.P.; Randolph, G.W.; Rinaldo, A.; Shah, J.P.; Shaha, A.R.; et al. Tracheal and Cricotracheal Resection with End-to-End Anastomosis for Locally Advanced Thyroid Cancer: A Systematic Review of the Literature on 656 Patients. Front. Endocrinol. 2021, 12, 779999. [Google Scholar] [CrossRef]
- Matsumoto, F.; Ikeda, K. Surgical Management of Tracheal Invasion by Well-Differentiated Thyroid Cancer. Cancers 2021, 13, 797. [Google Scholar] [CrossRef] [PubMed]
- Su, S.Y.; Milas, Z.L.; Bhatt, N.; Roberts, D.; Clayman, G.L. Well-differentiated thyroid cancer with aerodigestive tract invasion: Long-term control and functional outcomes. Head Neck 2016, 38, 72–78. [Google Scholar] [CrossRef]
- Rondeau, G.; Fish, S.; Hann, L.E.; Fagin, J.A.; Tuttle, R.M. Ultrasonographically detected small thyroid bed nodules identified after total thyroidectomy for differentiated thyroid cancer seldom show clinically significant structural progression. Thyroid 2011, 21, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Lepoutre-Lussey, C.; Deandreis, D.; Leboulleux, S.; Schlumberger, M. Postoperative radioactive iodine administration for differentiated thyroid cancer patients. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef]
- Deandreis, D.; Al Ghuzlan, A.; Leboulleux, S.; Lacroix, L.; Garsi, J.P.; Talbot, M.; Lumbroso, J.; Baudin, E.; Caillou, B.; Bidart, J.M.; et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr. Relat. Cancer 2011, 18, 159–169. [Google Scholar] [CrossRef]
- Kiess, A.P.; Agrawal, N.; Brierley, J.D.; Duvvuri, U.; Ferris, R.L.; Genden, E.; Wong, R.J.; Tuttle, R.M.; Lee, N.Y.; Randolph, G.W. External-beam radiotherapy for differentiated thyroid cancer locoregional control: A statement of the American Head and Neck Society. Head Neck 2016, 38, 493–498. [Google Scholar] [CrossRef]
- Terezakis, S.A.; Lee, K.S.; Ghossein, R.A.; Rivera, M.; Tuttle, R.M.; Wolden, S.L.; Zelefsky, M.J.; Wong, R.J.; Patel, S.G.; Pfister, D.G.; et al. Role of external beam radiotherapy in patients with advanced or recurrent nonanaplastic thyroid cancer: Memorial Sloan-Kettering Cancer Center experience. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 795–801. [Google Scholar] [CrossRef]
- Jacomina, L.E.; Jacinto, J.K.M.; Co, L.B.A.; Yu, K.K.L.; Agas, R.A.F.; Co, J.L.; Mejia, M.B.A. The Role of postoperative external beam radiotherapy for differentiated thyroid carcinoma: A systematic review and meta-analysis. Head Neck 2020, 42, 2181–2193. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Rondeau, G.; Lee, N.Y. A risk-adapted approach to the use of radioactive iodine and external beam radiation in the treatment of well-differentiated thyroid cancer. Cancer Control 2011, 18, 89–95. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoo, W.S.; Park, Y.J.; Park, D.J.; Yun, T.J.; Choi, S.H.; Sohn, C.H.; Lee, K.E.; Sung, M.W.; Youn, Y.K.; et al. Efficacy and Safety of Radiofrequency Ablation for Treatment of Locally Recurrent Thyroid Cancers Smaller than 2 cm. Radiology 2015, 276, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Offi, C.; Misso, C.; Antonelli, G.; Esposito, M.G.; Brancaccio, U.; Spiezia, S. Laser Ablation Treatment of Recurrent Lymph Node Metastases from Papillary Thyroid Carcinoma. J. Clin. Med. 2021, 10, 5295. [Google Scholar] [CrossRef]
- Hay, I.D.; Lee, R.A.; Davidge-Pitts, C.; Reading, C.C.; Charboneau, J.W. Long-term outcome of ultrasound-guided percutaneous ethanol ablation of selected “recurrent” neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery 2013, 154, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Heilo, A.; Sigstad, E.; Fagerlid, K.H.; Håskjold, O.I.; Grøholt, K.K.; Berner, A.; Bjøro, T.; Jørgensen, L.H. Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, 2750–2755. [Google Scholar] [CrossRef] [PubMed]
- Guenette, J.P.; Monchik, J.M.; Dupuy, D.E. Image-guided ablation of postsurgical locoregional recurrence of biopsy-proven well-differentiated thyroid carcinoma. J. Vasc. Interv. Radiol. 2013, 24, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Baek, J.H.; Choi, Y.J.; Lee, J.H. Efficacy and Safety of Radiofrequency and Ethanol Ablation for Treating Locally Recurrent Thyroid Cancer: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 420–428. [Google Scholar] [CrossRef]
- Lorusso, L.; Cappagli, V.; Valerio, L.; Giani, C.; Viola, D.; Puleo, L.; Gambale, C.; Minaldi, E.; Campopiano, M.C.; Matrone, A.; et al. Thyroid Cancers: From Surgery to Current and Future Systemic Therapies through Their Molecular Identities. Int. J. Mol. Sci. 2021, 22, 3117. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef]
- Stewart, K.E.; Strachan, M.W.J.; Srinivasan, D.; MacNeill, M.; Wall, L.; Nixon, I.J. Tyrosine Kinase Inhibitor Therapy in Locally Advanced Differentiated Thyroid Cancer: A Case Report. Eur. Thyroid J. 2019, 8, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Shen, T.; Terzyan, S.S.; Liu, X.; Hu, X.; Patel, K.P.; Hu, M.; Cabanillas, M.; Behrang, A.; Meric-Bernstam, F.; et al. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann. Oncol. 2021, 32, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Waguespack, S.G.; Drilon, A.; Lin, J.J.; Brose, M.S.; McDermott, R.; Almubarak, M.; Bauman, J.; Casanova, M.; Krishnamurthy, A.; Kummar, S.; et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur. J. Endocrinol. 2022, 186, 631–643. [Google Scholar] [CrossRef] [PubMed]
| Variables Related to the Patient | Age | Variables Related to the Tumor | Initial Staging (AJCC-UICC TNM) [10] |
|---|---|---|---|
| Comorbidities | Tumor histological subtype | ||
| Life expectancy | Possibility of undifferentiated carcinoma | ||
| Previous treatments related or not with thyroid tumor | Disease extent | ||
| Symptoms | Size and location of the lesions | ||
| Initial risk classification (ATA IRSS) [1] | Rate of tumor progression | ||
| Dynamic risk stratification [12] | Presence of molecular markers associated with aggressive behavior | ||
| Worries, wishes, values | Iodine avidity | ||
| 18FDG avidity | |||
| Variables related to the treatment | Appropriateness of the initial surgical resection | Presence of druggable mutations for targeted therapy | |
| Permanent complication | Initial staging (AJCC-UICC TNM) [10] | ||
| Response to initial treatment [1] | Tumor histological subtype | ||
| Current surgical resectability | Possibility of undifferentiated carcinoma | ||
| Expected sequelae of the current therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalheiro, B.G.; Shah, J.P.; Randolph, G.W.; Medina, J.E.; Tufano, R.P.; Zafereo, M.; Hartl, D.M.; Nixon, I.J.; Guntinas-Lichius, O.; Vander Poorten, V.; et al. Management of Recurrent Well-Differentiated Thyroid Carcinoma in the Neck: A Comprehensive Review. Cancers 2023, 15, 923. https://doi.org/10.3390/cancers15030923
Cavalheiro BG, Shah JP, Randolph GW, Medina JE, Tufano RP, Zafereo M, Hartl DM, Nixon IJ, Guntinas-Lichius O, Vander Poorten V, et al. Management of Recurrent Well-Differentiated Thyroid Carcinoma in the Neck: A Comprehensive Review. Cancers. 2023; 15(3):923. https://doi.org/10.3390/cancers15030923
Chicago/Turabian StyleCavalheiro, Beatriz G., Jatin P. Shah, Gregory W. Randolph, Jesus E. Medina, Ralph P. Tufano, Mark Zafereo, Dana M. Hartl, Iain J. Nixon, Orlando Guntinas-Lichius, Vincent Vander Poorten, and et al. 2023. "Management of Recurrent Well-Differentiated Thyroid Carcinoma in the Neck: A Comprehensive Review" Cancers 15, no. 3: 923. https://doi.org/10.3390/cancers15030923
APA StyleCavalheiro, B. G., Shah, J. P., Randolph, G. W., Medina, J. E., Tufano, R. P., Zafereo, M., Hartl, D. M., Nixon, I. J., Guntinas-Lichius, O., Vander Poorten, V., López, F., Khafif, A. H., Owen, R. P., Shaha, A., Rodrigo, J. P., Rinaldo, A., Mäkitie, A. A., Silver, C. E., Sanabria, A., ... Ferlito, A. (2023). Management of Recurrent Well-Differentiated Thyroid Carcinoma in the Neck: A Comprehensive Review. Cancers, 15(3), 923. https://doi.org/10.3390/cancers15030923











