Forecasting Molecular Features in IDH-Wildtype Gliomas: The State of the Art of Radiomics Applied to Neurosurgery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search of the Literature
2.2. Study Selection
2.3. Eligibility Criteria
2.4. Data Extraction
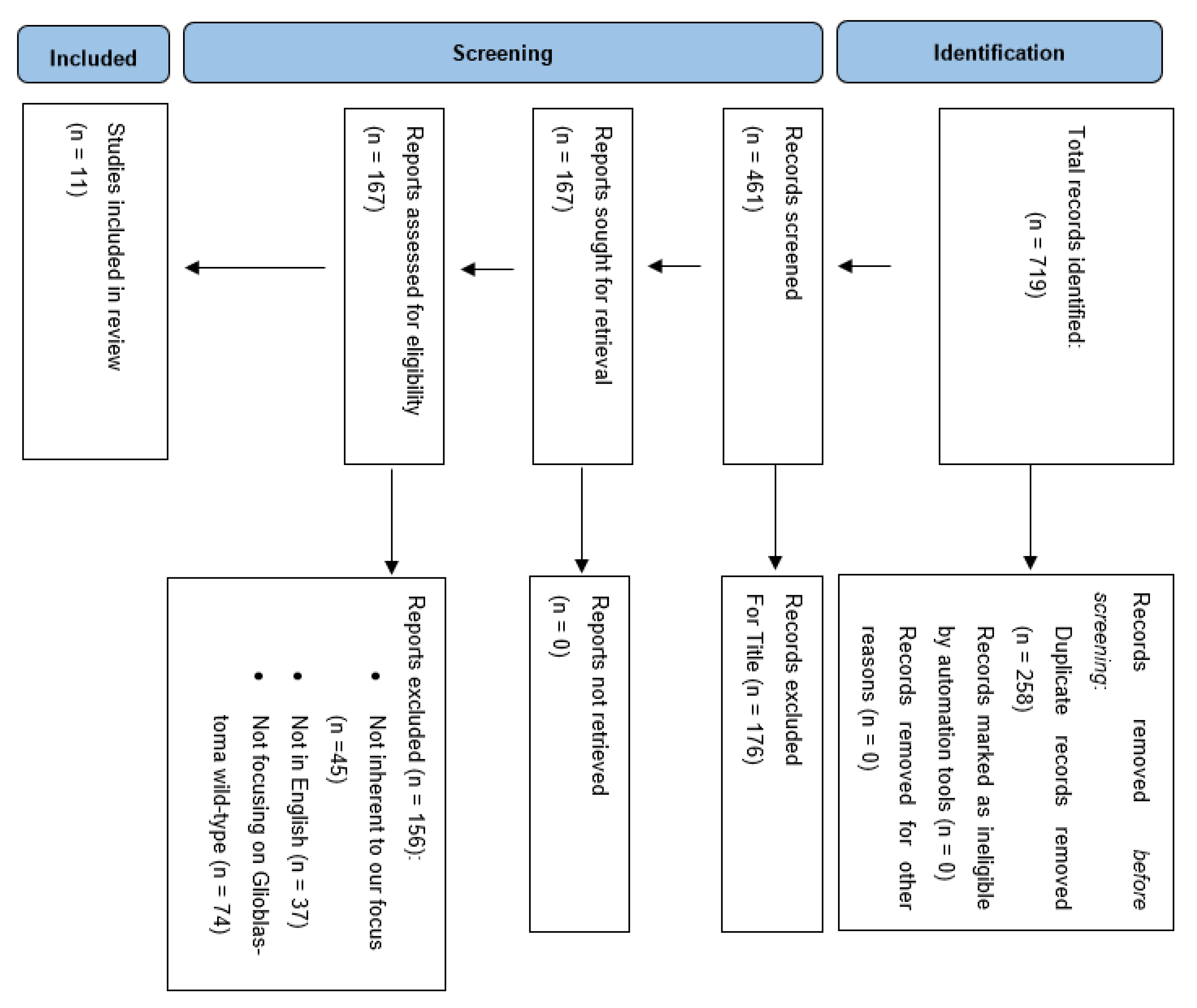
3. Results
3.1. Data Selection
3.2. Patients’ Demographic Data and Study Characteristics
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, B.; Yu, Z.; Liang, R. Effect of long-term adjuvant temozolomide chemotherapy on primary glioblastoma patient survival. BMC Neurol. 2021, 21, 424. [Google Scholar] [CrossRef] [PubMed]
- Ylanan, A.M.D.; Pascual, J.S.G.; Cruz-Lim, E.M.D.; Ignacio, K.H.D.; Cañal, J.P.A.; Khu, K.J.O. Intraoperative radiotherapy for glioblastoma: A systematic review of techniques and outcomes. J. Clin. Neurosci. 2021, 93, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xi, K.; Fu, X.; Sun, H.; Wang, H.; Yu, D.; Li, Z.; Ma, Y.; Liu, X.; Huang, B.; et al. Versatile metal-phenolic network nanoparticles for multitargeted combination therapy and magnetic resonance tracing in glioblastoma. Biomaterials 2021, 278, 121163. [Google Scholar] [CrossRef]
- Gallego, L.; Ceña, V. Nanoparticle-mediated therapeutic compounds delivery to glioblastoma. Expert Opin. Drug Deliv. 2020, 17, 1541–1554. [Google Scholar] [CrossRef]
- Pino, M.A.; Imperato, A.; Musca, I.; Maugeri, R.; Giammalva, G.R.; Costantino, G.; Graziano, F.; Meli, F.; Francaviglia, N.; Iacopino, D.G.; et al. New Hope in Brain Glioma Surgery: The Role of Intraoperative Ultrasound. A Review. Brain Sci. 2018, 8, 202. [Google Scholar] [CrossRef]
- Maugeri, R.; Villa, A.; Pino, M.; Imperato, A.; Giammalva, G.R.; Costantino, G.; Graziano, F.; Gulì, C.; Meli, F.; Francaviglia, N.; et al. With a Little Help from My Friends: The Role of Intraoperative Fluorescent Dyes in the Surgical Management of High-Grade Gliomas. Brain Sci. 2018, 8, 31. [Google Scholar] [CrossRef]
- Barone, F.; Alberio, N.; Iacopino, D.G.; Giammalva, G.R.; D’Arrigo, C.; Tagnese, W.; Graziano, F.; Cicero, S.; Maugeri, R. Brain Mapping as Helpful Tool in Brain Glioma Surgical Treatment-Toward the “Perfect Surgery”? Brain Sci. 2018, 8, 192. [Google Scholar] [CrossRef]
- Umana, G.E.; Scalia, G.; Graziano, F.; Maugeri, R.; Alberio, N.; Barone, F.; Crea, A.; Fagone, S.; Giammalva, G.R.; Brunasso, L.; et al. Navigated Transcranial Magnetic Stimulation Motor Mapping Usefulness in the Surgical Management of Patients Affected by Brain Tumors in Eloquent Areas: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 644198. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Musso, S.; Salvaggio, G.; Pino, M.A.; Gerardi, R.M.; Umana, G.E.; Midiri, M.; Iacopino, D.G.; Maugeri, R. Coplanar Indirect-Navigated Intraoperative Ultrasound: Matching Un-navigated Probes With Neuronavigation During Neurosurgical Procedures. How We Do It. Oper. Neurosurg. 2021, 21, 485–490. [Google Scholar] [CrossRef]
- Francaviglia, N.; Iacopino, D.G.; Costantino, G.; Villa, A.; Impallaria, P.; Meli, F.; Maugeri, R. Fluorescein for resection of high-grade gliomas: A safety study control in a single center and review of the literature. Surg. Neurol. Int. 2017, 8, 145. [Google Scholar] [CrossRef] [Green Version]
- La Torre, D.; Maugeri, R.; Angileri, F.F.; Pezzino, G.; Conti, A.; Cardali, S.M.; Calisto, A.; Sciarrone, G.; Misefari, A.; Germanò, A.; et al. Human leukocyte antigen frequency in human high-grade gliomas: A case-control study in Sicily. Neurosurgery 2009, 64, 1082–1088; discussion 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Giammalva, G.R.; Iacopino, D.G.; Azzarello, G.; Gaggiotti, C.; Graziano, F.; Gulì, C.; Pino, M.A.; Maugeri, R. End-of-Life Care in High-Grade Glioma Patients. The Palliative and Supportive Perspective. Brain Sci. 2018, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ugiliweneza, B.; Wang, D.; Hsin, G.; Boakye, M.; Skirboll, S. Trends and outcomes of early and late palliative care consultation for adult patients with glioblastoma: A SEER-Medicare retrospective study. Neuro-Oncol. Pract. 2022, 9, 299–309. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xing, L.; Napel, S.; Rubin, D.L. (Eds.) Radiomics and Radiogenomics: Technical Basis and Clinical Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Aabedi, A.A.; Young, J.S.; Zhang, Y.; Ammanuel, S.; Morshed, R.A.; Dalle Ore, C.; Brown, D.; Phillips, J.J.; Oberheim Bush, N.A.; Taylor, J.W.; et al. Association of Neurological Impairment on the Relative Benefit of Maximal Extent of Resection in Chemoradiation-Treated Newly Diagnosed Isocitrate Dehydrogenase Wild-Type Glioblastoma. Neurosurgery 2022, 90, 124–130. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef]
- Gerritsen, J.K.W.; Broekman, M.L.D.; De Vleeschouwer, S.; Schucht, P.; Jungk, C.; Krieg, S.M.; Nahed, B.V.; Berger, M.S.; Vincent, A.J.P.E. Decision making and surgical modality selection in glioblastoma patients: An international multicenter survey. J. Neurooncol. 2022, 156, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Khalafallah, A.M.; Rakovec, M.; Bettegowda, C.; Jackson, C.M.; Gallia, G.L.; Weingart, J.D.; Lim, M.; Esquenazi, Y.; Zacharia, B.E.; Goldschmidt, E.; et al. A Crowdsourced Consensus on Supratotal Resection Versus Gross Total Resection for Anatomically Distinct Primary Glioblastoma. Neurosurgery 2021, 89, 712–719. [Google Scholar] [CrossRef]
- Incekara, F.; Koene, S.; Vincent, A.J.P.E.; van den Bent, M.J.; Smits, M. Association Between Supratotal Glioblastoma Resection and Patient Survival: A Systematic Review and Meta-Analysis. World Neurosurg. 2019, 127, 617–624.e2. [Google Scholar] [CrossRef]
- Jackson, C.; Choi, J.; Khalafallah, A.M.; Price, C.; Bettegowda, C.; Lim, M.; Gallia, G.; Weingart, J.; Brem, H.; Mukherjee, D. A systematic review and meta-analysis of supratotal versus gross total resection for glioblastoma. J. Neurooncol. 2020, 148, 419–431. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Brunasso, L.; Costanzo, R.; Paolini, F.; Umana, G.E.; Scalia, G.; Gagliardo, C.; Gerardi, R.M.; Basile, L.; Graziano, F.; et al. Brain Mapping-Aided SupraTotal Resection (SpTR) of Brain Tumors: The Role of Brain Connectivity. Front. Oncol. 2021, 11, 645854. [Google Scholar] [CrossRef] [PubMed]
- Giammalva, G.R.; Ferini, G.; Musso, S.; Salvaggio, G.; Pino, M.A.; Gerardi, R.M.; Brunasso, L.; Costanzo, R.; Paolini, F.; Di Bonaventura, R.; et al. Intraoperative Ultrasound: Emerging Technology and Novel Applications in Brain Tumor Surgery. Front. Oncol. 2022, 12, 818446. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef]
- Scherrer, H.J. Cerebral astrocytomas and their derivates. Am. J. Cancer 1940, 40, 159–198. [Google Scholar]
- Rong, Y.; Durden, D.L.; Van Meir, E.G.; Brat, D.J. ’Pseudopalisading’ necrosis in glioblastoma: A familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol. 2006, 65, 529–539. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Yamasaki, K.; Matsushita, Y.; Nakamura, T.; Shimokawa, A.; Takami, H.; Tanaka, S.; Mukasa, A.; Shirahata, M.; Shimizu, S.; et al. A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol. Commun. 2016, 4, 79. [Google Scholar] [CrossRef]
- Ramos-Fresnedo, A.; Pullen, M.W.; Perez-Vega, C.; Domingo, R.A.; Akinduro, O.O.; Almeida, J.P.; Suarez-Meade, P.; Marenco-Hillembrand, L.; Jentoft, M.E.; Bendok, B.R.; et al. The survival outcomes of molecular glioblastoma IDH-wildtype: A multicenter study. J. Neurooncol. 2022, 157, 177–185. [Google Scholar] [CrossRef]
- Brunasso, L.; Ferini, G.; Bonosi, L.; Costanzo, R.; Musso, S.; Benigno, U.E.; Gerardi, R.M.; Giammalva, G.R.; Paolini, F.; Umana, G.E.; et al. A Spotlight on the Role of Radiomics and Machine-Learning Applications in the Management of Intracranial Meningiomas: A New Perspective in Neuro-Oncology: A Review. Life 2022, 12, 586. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kaiser, L.; Holzgreve, A.; Ruf, V.C.; Suchorska, B.; Wenter, V.; Quach, S.; Herms, J.; Bartenstein, P.; Tonn, J.C.; et al. Prediction of TERTp-mutation status in IDH-wildtype high-grade gliomas using pre-treatment dynamic. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4415–4425. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, B.; Zhang, S.; Cheng, J.; Liu, X.; Wang, W.; Dong, Y.; Zhang, L.; Mo, X.; Chen, Q.; et al. Quantitative MRI-based radiomics for noninvasively predicting molecular subtypes and survival in glioma patients. NPJ Precis. Oncol. 2021, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wu, H.; Wu, G.; Xu, G. Noninvasive Prediction of TERT Promoter Mutations in High-Grade Glioma by Radiomics Analysis Based on Multiparameter MRI. BioMed Res. Int. 2020, 2020, 3872314. [Google Scholar] [CrossRef] [PubMed]
- Kihira, S.; Tsankova, N.M.; Bauer, A.; Sakai, Y.; Mahmoudi, K.; Zubizarreta, N.; Houldsworth, J.; Khan, F.; Salamon, N.; Hormigo, A.; et al. Multiparametric MRI texture analysis in prediction of glioma biomarker status: Added value of MR diffusion. Neurooncol. Adv. 2021, 3, vdab051. [Google Scholar] [CrossRef]
- Rathore, S.; Akbari, H.; Rozycki, M.; Abdullah, K.G.; Nasrallah, M.P.; Binder, Z.A.; Davuluri, R.V.; Lustig, R.A.; Dahmane, N.; Bilello, M.; et al. Radiomic MRI signature reveals three distinct subtypes of glioblastoma with different clinical and molecular characteristics, offering prognostic value beyond IDH1. Sci. Rep. 2018, 8, 5087. [Google Scholar] [CrossRef]
- Akbari, H.; Bakas, S.; Pisapia, J.M.; Nasrallah, M.P.; Rozycki, M.; Martinez-Lage, M.; Morrissette, J.J.D.; Dahmane, N.; O’Rourke, D.M.; Davatzikos, C. In vivo evaluation of EGFRvIII mutation in primary glioblastoma patients via complex multiparametric MRI signature. Neuro-Oncology 2018, 20, 1068–1079. [Google Scholar] [CrossRef]
- Pasquini, L.; Napolitano, A.; Lucignani, M.; Tagliente, E.; Dellepiane, F.; Rossi-Espagnet, M.C.; Ritrovato, M.; Vidiri, A.; Villani, V.; Ranazzi, G.; et al. AI and High-Grade Glioma for Diagnosis and Outcome Prediction: Do All Machine Learning Models Perform Equally Well? Front. Oncol. 2021, 11, 601425. [Google Scholar] [CrossRef]
- Sohn, B.; An, C.; Kim, D.; Ahn, S.S.; Han, K.; Kim, S.H.; Kang, S.G.; Chang, J.H.; Lee, S.K. Radiomics-based prediction of multiple gene alteration incorporating mutual genetic information in glioblastoma and grade 4 astrocytoma, IDH-mutant. J. Neurooncol. 2021, 155, 267–276. [Google Scholar] [CrossRef]
- Zinn, P.O.; Singh, S.K.; Kotrotsou, A.; Abrol, S.; Thomas, G.; Mosley, J.; Elakkad, A.; Hassan, I.; Kumar, A.; Colen, R.R. Distinct Radiomic Phenotypes Define Glioblastoma TP53-PTEN-EGFR Mutational Landscape. Neurosurgery 2017, 64, 203–210. [Google Scholar] [CrossRef]
- Bakas, S.; Akbari, H.; Pisapia, J.; Martinez-Lage, M.; Rozycki, M.; Rathore, S.; Dahmane, N.; O’Rourke, D.M.; Davatzikos, C. Detection of EGFRvIII in Glioblastoma via Perfusion Magnetic Resonance Imaging Signature Consistent with Deep Peritumoral Infiltration: The ϕ-IndexIn Vivo EGFRvIII Detection in Glioblastoma via MRI Signature. Clin. Cancer Res. 2017, 23, 4724–4734. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, E.; Villanueva-Meyer, J.E.; Cha, S. A fully automated artificial intelligence method for non-invasive, imaging-based identification of genetic alterations in glioblastomas. Sci. Rep. 2020, 10, 11852. [Google Scholar] [CrossRef]
- Siegal, T. Clinical Relevance of Prognostic and Predictive Molecular Markers in Gliomas. Adv. Tech. Stand. Neurosurg. 2016, 43, 91–108. [Google Scholar] [CrossRef]
- Silantyev, A.S.; Falzone, L.; Libra, M.; Gurina, O.I.; Kardashova, K.S.; Nikolouzakis, T.K.; Nosyrev, A.E.; Sutton, C.W.; Mitsias, P.D.; Tsatsakis, A. Current and Future Trends on Diagnosis and Prognosis of Glioblastoma: From Molecular Biology to Proteomics. Cells 2019, 8, 863. [Google Scholar] [CrossRef] [PubMed]
- Graziano, F.; Bavisotto, C.C.; Gammazza, A.M.; Rappa, F.; de Macario, E.C.; Macario, A.J.L.; Cappello, F.; Campanella, C.; Maugeri, R.; Iacopino, D.G. Chaperonology: The Third Eye on Brain Gliomas. Brain Sci. 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Bonosi, L.; Ferini, G.; Giammalva, G.R.; Benigno, U.E.; Porzio, M.; Giovannini, E.A.; Musso, S.; Gerardi, R.M.; Brunasso, L.; Costanzo, R.; et al. Liquid Biopsy in Diagnosis and Prognosis of High-Grade Gliomas; State-of-the-Art and Literature Review. Life 2022, 12, 407. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Graziano, F.; Rappa, F.; Marino Gammazza, A.; Logozzi, M.; Fais, S.; Maugeri, R.; Bucchieri, F.; Conway de Macario, E.; Macario, A.J.L.; et al. Exosomal Chaperones and miRNAs in Gliomagenesis: State-of-Art and Theranostics Perspectives. Int. J. Mol. Sci. 2018, 19, 2626. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Azevedo, A.; Esteves, S.; Marques, A.R.; Martins, C.; Costa, I.; Mafra, M.; Bravo Marques, J.M.; Roque, L.; Pojo, M. Clinical insights gained by refining the 2016 WHO classification of diffuse gliomas with: EGFR amplification, TERT mutations, PTEN deletion and MGMT methylation. BMC Cancer 2019, 19, 968. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.J.; Gleeson, J.P.; O’Reilly, S.; Bambury, R.M. Management of newly diagnosed glioblastoma multiforme: Current state of the art and emerging therapeutic approaches. Med. Oncol. 2022, 39, 129. [Google Scholar] [CrossRef]
- Wykes, V.; Zisakis, A.; Irimia, M.; Ughratdar, I.; Sawlani, V.; Watts, C. Importance and Evidence of Extent of Resection in Glioblastoma. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2021, 82, 75–86. [Google Scholar] [CrossRef]
- Bette, S.; Barz, M.; Wiestler, B.; Huber, T.; Gerhardt, J.; Buchmann, N.; Combs, S.E.; Schmidt-Graf, F.; Delbridge, C.; Zimmer, C.; et al. Prognostic Value of Tumor Volume in Glioblastoma Patients: Size Also Matters for Patients with Incomplete Resection. Ann. Surg. Oncol. 2018, 25, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, M.W.; Stupp, R.; Sonabend, A.M. Role of Resection in Glioblastoma Management. Neurosurg. Clin. N. Am. 2021, 32, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Shinohara, R.T.; Akbari, H.; Davatzikos, C. Combining generative models for multifocal glioma segmentation and registration. Med. Image Comput. Comput. Assist. Interv. 2014, 17, 763–770. [Google Scholar] [CrossRef]
- Bakas, S.; Shukla, G.; Akbari, H.; Erus, G.; Sotiras, A.; Rathore, S.; Sako, C.; Min Ha, S.; Rozycki, M.; Shinohara, R.T.; et al. Overall survival prediction in glioblastoma patients using structural magnetic resonance imaging (MRI): Advanced radiomic features may compensate for lack of advanced MRI modalities. J. Med. Imaging 2020, 7, 031505. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Viola, A.; Maugeri, R.; Giardina, K.; Di Bonaventura, R.; Musso, S.; Brunasso, L.; Cepeda, S.; Della Pepa, G.M.; Scerrati, A.; et al. Intraoperative Evaluation of Brain-Tumor Microvascularization through MicroV IOUS: A Protocol for Image Acquisition and Analysis of Radiomic Features. Cancers 2022, 14, 5335. [Google Scholar] [CrossRef]
- Chato, L.; Latifi, S. Machine Learning and Radiomic Features to Predict Overall Survival Time for Glioblastoma Patients. J. Pers. Med. 2021, 11, 1336. [Google Scholar] [CrossRef]
- Cepeda, S.; Pérez-Nuñez, A.; García-García, S.; García-Pérez, D.; Arrese, I.; Jiménez-Roldán, L.; García-Galindo, M.; González, P.; Velasco-Casares, M.; Zamora, T.; et al. Predicting Short-Term Survival after Gross Total or Near Total Resection in Glioblastomas by Machine Learning-Based Radiomic Analysis of Preoperative MRI. Cancers 2021, 13, 5047. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, X.; Li, Y.; Pang, P.; Shu, Z.; Gong, X. The Nomogram of MRI-based Radiomics with Complementary Visual Features by Machine Learning Improves Stratification of Glioblastoma Patients: A Multicenter Study. J. Magn. Reson. Imaging 2021, 54, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, H.; Tian, Q.; Feng, N.; Yin, L.; Xu, X.; Du, P.; Liu, Y. A radiomics nomogram based on multiparametric MRI might stratify glioblastoma patients according to survival. Eur. Radiol. 2019, 29, 5528–5538. [Google Scholar] [CrossRef] [PubMed]
- Labussière, M.; Di Stefano, A.L.; Gleize, V.; Boisselier, B.; Giry, M.; Mangesius, S.; Bruno, A.; Paterra, R.; Marie, Y.; Rahimian, A.; et al. TERT promoter mutations in gliomas, genetic associations and clinico-pathological correlations. Br. J. Cancer 2014, 111, 2024–2032. [Google Scholar] [CrossRef]
- Vinagre, J.; Almeida, A.; Pópulo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita, H.; Narita, Y.; Takami, H.; Fukushima, S.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Shibui, S.; Ichimura, K. TERT promoter mutations rather than methylation are the main mechanism for TERT upregulation in adult gliomas. Acta Neuropathol. 2013, 126, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Narita, Y.; Fukushima, S.; Tateishi, K.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Collins, V.P.; Kawahara, N.; et al. Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013, 126, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef]
- Simon, M.; Hosen, I.; Gousias, K.; Rachakonda, S.; Heidenreich, B.; Gessi, M.; Schramm, J.; Hemminki, K.; Waha, A.; Kumar, R. TERT promoter mutations: A novel independent prognostic factor in primary glioblastomas. Neuro-Oncology 2015, 17, 45–52. [Google Scholar] [CrossRef]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.K.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. cIMPACT-NOW update 3: Recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018, 136, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Wesseling, P.; Aldape, K.; Brat, D.J.; Capper, D.; Cree, I.A.; Eberhart, C.; Figarella-Branger, D.; Fouladi, M.; Fuller, G.N.; et al. cIMPACT-NOW update 6: New entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020, 30, 844–856. [Google Scholar] [CrossRef]
- Jiang, C.; Kong, Z.; Zhang, Y.; Liu, S.; Liu, Z.; Chen, W.; Liu, P.; Liu, D.; Wang, Y.; Lyu, Y.; et al. Conventional magnetic resonance imaging-based radiomic signature predicts telomerase reverse transcriptase promoter mutation status in grade II and III gliomas. Neuroradiology 2020, 62, 803–813. [Google Scholar] [CrossRef]
- Park, C.J.; Han, K.; Kim, H.; Ahn, S.S.; Choi, D.; Park, Y.W.; Chang, J.H.; Kim, S.H.; Cha, S.; Lee, S.K. MRI Features May Predict Molecular Features of Glioblastoma in. AJNR Am. J. Neuroradiol. 2021, 42, 448–456. [Google Scholar] [CrossRef]
- Fang, S.; Fan, Z.; Sun, Z.; Li, Y.; Liu, X.; Liang, Y.; Liu, Y.; Zhou, C.; Zhu, Q.; Zhang, H.; et al. Radiomics Features Predict. Front. Oncol. 2020, 10, 606741. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Kinoshita, M.; Kawaguchi, A.; Takahashi, M.; Narita, Y.; Terakawa, Y.; Tsuyuguchi, N.; Okita, Y.; Nonaka, M.; Moriuchi, S.; et al. Lesion location implemented magnetic resonance imaging radiomics for predicting IDH and TERT promoter mutations in grade II/III gliomas. Sci. Rep. 2018, 8, 11773. [Google Scholar] [CrossRef] [PubMed]
- Aghi, M.; Gaviani, P.; Henson, J.W.; Batchelor, T.T.; Louis, D.N.; Barker, F.G. Magnetic resonance imaging characteristics predict epidermal growth factor receptor amplification status in glioblastoma. Clin. Cancer Res. 2005, 11, 8600–8605. [Google Scholar] [CrossRef] [PubMed]
- Barker, F.G.; Simmons, M.L.; Chang, S.M.; Prados, M.D.; Larson, D.A.; Sneed, P.K.; Wara, W.M.; Berger, M.S.; Chen, P.; Israel, M.A.; et al. EGFR overexpression and radiation response in glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, J.; Merdes, A.; Stumm, G.; Albert, F.K.; Forsting, M.; Hynes, N.; Kiessling, M. Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int. J. Cancer 1994, 56, 72–77. [Google Scholar] [CrossRef]
- Lal, A.; Glazer, C.A.; Martinson, H.M.; Friedman, H.S.; Archer, G.E.; Sampson, J.H.; Riggins, G.J. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002, 62, 3335–3339. [Google Scholar]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef]
- Crespo, I.; Vital, A.L.; Nieto, A.B.; Rebelo, O.; Tão, H.; Lopes, M.C.; Oliveira, C.R.; French, P.J.; Orfao, A.; Tabernero, M.D. Detailed characterization of alterations of chromosomes 7, 9, and 10 in glioblastomas as assessed by single-nucleotide polymorphism arrays. J. Mol. Diagn. 2011, 13, 634–647. [Google Scholar] [CrossRef]
- Lopez-Gines, C.; Cerda-Nicolas, M.; Gil-Benso, R.; Pellin, A.; Lopez-Guerrero, J.A.; Callaghan, R.; Benito, R.; Roldan, P.; Piquer, J.; Llacer, J.; et al. Association of chromosome 7, chromosome 10 and EGFR gene amplification in glioblastoma multiforme. Clin. Neuropathol. 2005, 24, 209–218. [Google Scholar]
| Author/Year | Study Design | Patients Enrolled | Mean Age | Molecular Finding (TERT, EGFR, Aneuploidy) | Imaging Techniques | Sequences | Best Sens/Spec/AUC Reached |
|---|---|---|---|---|---|---|---|
| Z. Li et al., 2021 [33] | Randomized controlled trial | 159 | 60.2 | TERTp mutations | PET | Dynamic [18F]FET PET | 0.921/NA/0.82 |
| J. Yan et al., 2021 [34] | Retrospective study | 357 | N/A | TERTp mutations | MRI | CE-T1w, DWI (using ADC) | 0.944/0.400/0.811 |
| H. Tian et al., 2020 [35] | Retrospective study | 126 | N/A | TERTp mutations | MRI | CE-T1w, T1w, T2w, T2-FLAIR, MRS | 0.947/0.840/0.955 |
| S. Kihira et al., 2021 [36] | Retrospective study | 111 | 57.0 | EGFR amplification | MRI | CE-T1W, T2–FLAIR, DWI. | 0.65/0.68/0.83 |
| S. Rathore et al., 2018 [37] | Retrospective study | 208 | N/A | EGFRvIII | MRI | CE-T1w, T1w, T2w, T2-FLAIR, DSC MRI | NA |
| H. Akbari et al., 2018 [38] | Retrospective study | 129 | 59.3 | EGFRvIII | MRI | CE-T1w, T1w, T2w, T2-FLAIR, DTI, DSC MRI | 0.786/0.90/0.86 |
| Pasquini L. et al., 2021 [39] | Retrospective study | 156 | N/A | EGFR amplification | MRI | MPRAGE, T1w, T2w, T2-FLAIR, DWI, DSC MRI | NOTE: accuracy 81%; ROC 74.3%. |
| B. Sohn et al., 2021 [40] | Retrospective study | 418 | 60.1 | EGFR amplification | MRI | CE-T1w, T1w, T2w, T2-FLAIR | 0.812/0.585/0.743 |
| O. Zinn et al., 2017 [41] | Retrospective study | 29 | N/A | EGFR | MRI | CE-T1w, T1w, T2w, T2-FLAIR | NA |
| S. Bakas et al., 2017 [42] | Retrospective study | 142 | 59.82 | EGFRvIII | MRI | CE-T1w, T2-FLAIR, DSC MRI | 0.8377/0.9235/0.8869 |
| Calabrese E. et al., 2020 [43] | Retrospective study | 199 | N/A | Aneuploidy | MRI | T2w, T2-FLAIR, SWI, DWI, CE-T1w, T1w, ASL perfusion images, HARDI | 0.90/0.88/0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerardi, R.M.; Cannella, R.; Bonosi, L.; Vernuccio, F.; Ferini, G.; Viola, A.; Zagardo, V.; Buscemi, F.; Costanzo, R.; Porzio, M.; et al. Forecasting Molecular Features in IDH-Wildtype Gliomas: The State of the Art of Radiomics Applied to Neurosurgery. Cancers 2023, 15, 940. https://doi.org/10.3390/cancers15030940
Gerardi RM, Cannella R, Bonosi L, Vernuccio F, Ferini G, Viola A, Zagardo V, Buscemi F, Costanzo R, Porzio M, et al. Forecasting Molecular Features in IDH-Wildtype Gliomas: The State of the Art of Radiomics Applied to Neurosurgery. Cancers. 2023; 15(3):940. https://doi.org/10.3390/cancers15030940
Chicago/Turabian StyleGerardi, Rosa Maria, Roberto Cannella, Lapo Bonosi, Federica Vernuccio, Gianluca Ferini, Anna Viola, Valentina Zagardo, Felice Buscemi, Roberta Costanzo, Massimiliano Porzio, and et al. 2023. "Forecasting Molecular Features in IDH-Wildtype Gliomas: The State of the Art of Radiomics Applied to Neurosurgery" Cancers 15, no. 3: 940. https://doi.org/10.3390/cancers15030940
APA StyleGerardi, R. M., Cannella, R., Bonosi, L., Vernuccio, F., Ferini, G., Viola, A., Zagardo, V., Buscemi, F., Costanzo, R., Porzio, M., Giovannini, E. A., Paolini, F., Brunasso, L., Giammalva, G. R., Umana, G. E., Scarpitta, A., Iacopino, D. G., & Maugeri, R. (2023). Forecasting Molecular Features in IDH-Wildtype Gliomas: The State of the Art of Radiomics Applied to Neurosurgery. Cancers, 15(3), 940. https://doi.org/10.3390/cancers15030940














