Global Longitudinal Strain in Cardio-Oncology: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Definition of Cardiotoxicity
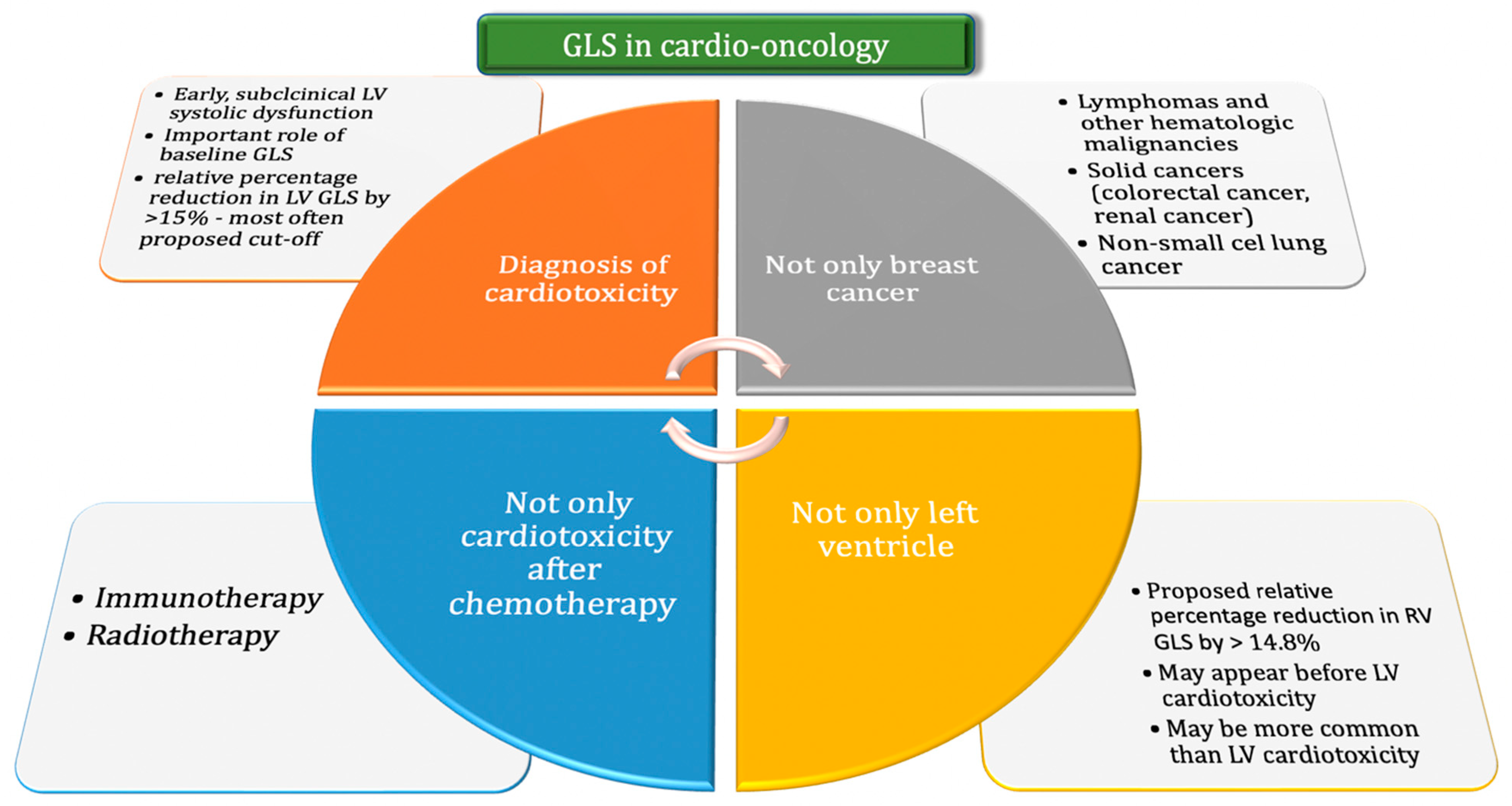
1.2. GLS in the Diagnosis of Cardiotoxicity
1.3. Assessment of GLS in Combination with Cardiac Biomarkers
1.4. GLS vs. Segmental Strain
1.5. The Use of GLS Assessment in Patients with Various Cancers
1.6. GLS Depending on the Method of Anticancer Treatment
1.7. GLS Assessment in the Context of Right Ventricular Cardiotoxicity
1.8. GLS Assessment during Cancer Treatment
1.9. Perspectives of GLS Assessment
1.10. Disadvantages of GLS
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BNP | brain natriuretic peptide |
| cTnT | cardiac troponin T |
| GLS | global longitudinal strain |
| HSCT | hematopoietic stem cell transplantation |
| LVEF | left ventricular ejection fraction |
| MACE | major adverse cardiac events |
| NT-proBNP | N-terminal pro B-type natriuretic peptide |
| RV | right ventricle |
| SR | strain rate |
| STE | speckle tracking echocardiography |
References
- Bohdan, M.; Kowalczys, A.; Mickiewicz, A.; Gruchała, M.; Lewicka, E. Cancer Therapy-Related Cardiovascular Complications in Clinical Practice: Current Perspectives. Clin. Med. 2021, 10, 1647. [Google Scholar] [CrossRef] [PubMed]
- Medvedofsky, D.; Maffessanti, F.; Weinert, L.; Tehrani, D.M.; Narang, A.; Addetia, K.; Mediratta, A.; Besser, S.A.; Maor, M.; Patel, A.R.; et al. 2D and 3D Echocardiography-Derived Indices of Left Ventricular Function and Shape: Relationship with Mortality. JACC Cardiovasc. Imaging 2018, 11, 1569–1579. [Google Scholar] [PubMed]
- Flachskampf, F.A.; Blankstein, R.; Grayburn, P.A.; Kramer, C.M.; Kwong, R.Y.; Marwick, T.H.; Nagel, E.; Sengupta, P.P.; Zoghbi, W.A.; Chandrashekhar, Y. Global Longitudinal Shortening: A Positive Step Towards Reducing Confusion Surrounding Global Longitudinal Strain. JACC Cardiovasc. Imaging 2019, 12, 1566–1567. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, S.; Dahlslett, T.; Grenne, B.; Sjøli, B.; Smiseth, O.; Edvardsen, T.; Brunvand, H. Global longitudinal strain is a more reproducible measure of left ventricular function than ejection fraction regardless of echocardiographic training. Cardiovasc. Ultrasound 2019, 17, 18. [Google Scholar] [CrossRef]
- Belghitia, H.; Brette, S.; Lafitte, S.; Reant, P.; Picard, F.; Serri, K.; Courregelongue, M.; Dos Santos, P.; Douard, H.; Roudaut, R.; et al. Automated function imaging: A new operator-independent strain method for assessing left ventricular function. Arch. Cardiovasc. Dis. 2008, 101, 163–169. [Google Scholar]
- Krishnasamy, R.; Isbel, N.M.; Hawley, C.M.; Pascoe, E.M.; Burrage, M.K.; Leano, R.; Haluska, B.A.; Marwick, T.H.; Stanton, T. Left Ventricular Global Longitudinal Strain (GLS) Is a Superior Predictor of All-Cause and Cardiovascular Mortality When Compared to Ejection Fraction in Advanced Chronic Kidney Disease. PLoS ONE 2015, 10, e0127044. [Google Scholar]
- D’Elia, N.; Caselli, S.; Kosmala, W.; Lancellotti, P.; Morris, D.; Muraru, D.; Takeuchi, M.; van den Bosch, A.; van Grootel, R.W.J.; Villarraga, H.; et al. Normal Global Longitudinal Strain: An Individual Patient Meta-Analysis. JACC Cardiovasc. Imaging 2020, 13, 167–169. [Google Scholar]
- Dobson, R.; Ghosh, A.K.; Ky, B.; Marwick, T.; Stout, M.; Harkness, A.; Steeds, R.; Robinson, S.; Oxborough, D.; Adlam, D.; et al. BSE and BCOS Guideline for Transthoracic Echocardiographic Assessment of Adult Cancer Patients Receiving Anthracyclines and/or Trastuzumab. JACC CardioOncol. 2021, 3, 1–16. [Google Scholar]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.; Asteggiano, R.; Aznar, M.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 41, 4229–4361. [Google Scholar]
- Gripp, E.A.; Oliveira, G.E.; Feijó, L.A.; Garcia, M.I.; Xavier, S.S.; Sousa, A.S. Global Longitudinal Strain Accuracy for Cardiotoxicity Prediction in a Cohort of Breast Cancer Patients During Anthracycline and/or Trastuzumab Treatment. Arq. Bras. Cardiol. 2018, 110, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Kokkinidis, D.G.; Kampaktsis, P.N.; Amir, E.A.; Marwick, T.H.; Gupta, D.; Thavendiranathan, P. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. 2019, 4, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Cocco, L.D.; Chiaparini, A.F.; Saffi, M.A.L.; Leiria, T.L.L. Global Longitudinal Strain for the Early Detection of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. Clin. Oncol. (R. Coll. Radiol.) 2022, 34, 514–525. [Google Scholar] [CrossRef]
- Araujo-Gutierrez, R.; Chitturi, K.R.; Xu, J.; Wang, Y.; Kinder, E.; Senapati, A.; Chebrolu, B.L.; Kassi, M.; Trachtenberg, B.H. Baseline global longitudinal strain predictive of anthracycline-induced cardiotoxicity. Cardiooncology 2021, 7, 4. [Google Scholar]
- Laufer-Perl, M.; Arnold, J.H.; Mor, L.; Amrami, N.; Derakhshesh, M.; Moshkovits, Y.; Sadeh, B.; Arbel, Y.; Topilsky, Y.; Rozenbaum, Z. The association of reduced global longitudinal strain with cancer therapy-related cardiac dysfunction among patients receiving cancer therapy. Clin. Res. Cardiol. 2020, 109, 255–262. [Google Scholar] [CrossRef]
- Negishi, T.; Thavendiranathan, P.; Negishi, K.; Marwick, T.H.; SUCCOUR Investigators. Rationale and Design of the Strain Surveillance of Chemotherapy for Improving Cardiovascular Outcomes: The SUCCOUR Trial. JACC Cardiovasc. Imaging 2018, 11, 1098–1105. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Negishi, T.; Somerset, E.; Negishi, K.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; et al. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J. Am. Coll. Cardiol. 2021, 77, 392–401. [Google Scholar]
- Jordan, J.H.; Sukpraphrute, B.; Meléndez, G.C.; Jolly, M.P.; D’Agostino RBJr Hundley, W.G. Early Myocardial Strain Changes During Potentially Cardiotoxic Chemotherapy May Occur as a Result of Reductions in Left Ventricular End-Diastolic Volume: The Need to Interpret Left Ventricular Strain With Volumes. Circulation 2017, 135, 2575–2577. [Google Scholar] [CrossRef]
- Mele, D.; Malagutti, P.; Indelli, M.; Ferrari, L.; Casadei, F.; Da Ros, L.; Pollina, A.; Fiorencis, A.; Frassoldati, A.; Ferrari, R. Reversibility of Left Ventricle Longitudinal Strain Alterations Induced by Adjuvant Therapy in Early Breast Cancer Patients. Ultrasound Med. Biol. 2016, 42, 125–132. [Google Scholar] [CrossRef]
- Avila, M.; Alves, M.; Ayub-Ferreira, S.; Junior, M.R.D.B.W.; Cruz, F.D.D.; Brandão, S.M.G.; Hajjar, L.A.; Filho, R.K.; Cruz, C.B.B.V.; Abduch, A.M.C.; et al. Global Longitudinal Strain as a Predictor of Chemotherapy-Induced Cardiotoxicity. Authorea 2021, 35, eabc340. [Google Scholar]
- Sulaiman, L.; Hesham, D.; Abdel Hamid, M.; Youssef, G. The combined role of NT-proBNP and LV-GLS in the detection of early subtle chemotherapy-induced cardiotoxicity in breast cancer female patients. Egypt. Heart J. 2021, 73, 20. [Google Scholar]
- Kang, Y.; Xu, X.; Cheng, L.; Li, L.; Sun, M.; Chen, H.; Pan, C.; Shu, X. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur. J. Heart Fail. 2014, 16, 300–308. [Google Scholar] [PubMed]
- Öztürk, C.; Validyev, D.; Becher, U.M.; Weber, M.; Nickenig, G.; Tiyerili, V. A novel scoring system to estimate chemotherapy-induced myocardial toxicity: Risk assessment prior to non-anthracycline chemotherapy regimens. Int. J. Cardiol. Heart Vasc. 2021, 33, 100751. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Negishi, T.; Coté, M.A.; Penicka, M.; Massey, R.; Cho, G.-Y.; Hristova, K.; Vinereanu, D.; Popescu, B.A.; Izumo, M.; et al. Single Versus Standard Multiview Assessment of Global Longitudinal Strain for the Diagnosis of Cardiotoxicity During Cancer Therapy. JACC Cardiovasc. Imaging 2018, 11, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Portugal, G.; Branco, L.M.; Galrinho, A.; Carmo, M.M.; Timoteo, A.T.; Feliciano, J.; Abreu, J.; Oliveira, S.D.; Batarda, L.; Ferreira, R.C. Global and regional patterns of longitudinal strain in screening for chemotherapy-induced cardiotoxicity. Importância da deformação longitudinal na deteção da cardiotoxicidade induzida por quimioterapia e na identificação de padrões específicos de afetação segmentar. Rev. Port. Cardiol. 2017, 36, 9–15. [Google Scholar] [PubMed]
- Astuti, A.; Erwinanto, E.; Akbar, M.R.; Martanto, E.; Badudu, D.F. Global and Regional Longitudinal Strain Reduction in Breast Cancer Patients After First Chemotherapy Cycle With Fluorouracil, Adriamycin, and Cyclophosphamide Regimen. Cardiol. Res. 2021, 12, 238–243. [Google Scholar] [CrossRef]
- Mahjoob, M.P.; Sheikholeslami, S.A.; Dadras, M.; Mansouri, H.; Haghi, M.; Naderian, M.; Sadeghi, L.; Tabary, M.; Khaheshi, I. Prognostic Value of Cardiac Biomarkers Assessment in Combination with Myocardial 2D Strain Echocardiography for Early Detection of Anthracycline-Related Cardiac Toxicity. Cardiovasc. Hematol. Disord.-Drug Targets 2020, 20, 74–83. [Google Scholar]
- Arciniegas Calle, M.C.; Sandhu, N.P.; Xia, H.; Cha, S.S.; Pellikka, P.A.; Ye, Z.; Villarraga, H.R. Two-dimensional speckle tracking echocardiography predicts early subclinical cardiotoxicity associated with anthracycline-trastuzumab chemotherapy in patients with breast cancer. BMC Cancer 2018, 18, 1037. [Google Scholar] [CrossRef]
- Chang, H.Y.; Lee, C.H.; Su, P.L.; Li, S.S.; Chen, M.Y.; Chen, Y.P.; Hsu, Y.T.; Tsai, W.C.; Liu, P.Y.; Chen, T.Y.; et al. Subtle cardiac dysfunction in lymphoma patients receiving low to moderate dose chemotherapy. Sci. Rep. 2021, 11, 7100. [Google Scholar]
- Hatazawa, K.; Tanaka, H.; Nonaka, A.; Takada, H.; Soga, F.; Hatani, Y.; Matsuzoe, H.; Shimoura, H.; Ooka, J.; Sano, H.; et al. Baseline Global Longitudinal Strain as a Predictor of Left Ventricular Dysfunction and Hospitalization for Heart Failure of Patients With Malignant Lymphoma After Anthracycline Therapy. Circ. J. 2018, 82, 2566–2574. [Google Scholar] [CrossRef]
- Gonzalez-Manzanares, R.; Castillo, J.C.; Molina, J.R.; Ruiz-Ortiz, M.; Mesa, D.; Ojeda, S.; Anguita, M.; Pan, M. Automated Global Longitudinal Strain Assessment in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Cancers 2022, 14, 1513. [Google Scholar]
- Oka, T.; Tada, Y.; Oboshi, M.; Kamada, R.; Yasui, T.; Shioyama, W.; Nishikawa, T.; Hino, A.; Ishikawa, J.; Fujita, M. Serial Changes in Cardiac Strain and Contractility after Hematopoietic Stem Cell Transplantation in Patients with Hematologic Malignancies. Int. Heart J. 2021, 62, 575–583. [Google Scholar] [PubMed]
- Sonaglioni, A.; Albini, A.; Fossile, E.; Pessi, M.A.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Ambrosio, G. Speckle-Tracking Echocardiography for Cardioncological Evaluation in Bevacizumab-Treated Colorectal Cancer Patients. Cardiovasc. Toxicol. 2020, 20, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Astarita, A.; Airale, L.; Maffei, I.; Cesareo, M.; Crea, T.; Bruno, G.; Leone, D.; Avenatti, E.; Catarinella, C.; et al. Effects of Carfilzomib Therapy on Left Ventricular Function in Multiple Myeloma Patients. Front. Cardiovasc. Med. 2021, 8, 645678. [Google Scholar]
- Iannaccone, A.; Bruno, G.; Ravera, A.; Gay, F.; Salvini, M.; Bringhen, S.; Sabia, L.; Avenatti, E.; Veglio, F.; Milan, A. Evaluation of Cardiovascular Toxicity Associated with Treatments Containing Proteasome Inhibitors in Multiple Myeloma Therapy. High Blood Press. Cardiovasc. Prev. 2018, 25, 209–218. [Google Scholar] [PubMed]
- Liu, Z.; Zhang, L.; Liu, M.; Wang, F.; Xiong, Y.; Tang, Z.; Li, Q.; Lu, Q.; Liang, S.; Niu, T.; et al. Myocardial Injury in Multiple Myeloma Patients With Preserved Left Ventricular Ejection Fraction: Noninvasive Left Ventricular Pressure-Strain Myocardial Work. Front. Cardiovasc. Med. 2022, 8, 782580. [Google Scholar] [CrossRef]
- Chen, L.; Ta, S.; Wu, W.; Wang, C.; Zhang, Q. Prognostic and Added Value of Echocardiographic Strain for Prediction of Adverse Outcomes in Patients with Locally Advanced Non-Small Cell Lung Cancer after Radiotherapy. Ultrasound Med. Biol. 2019, 45, 98–107. [Google Scholar] [CrossRef]
- Lee Chuy, K.; Drill, E.; Yang, J.C.; Landau, H.; Hassoun, H.; Nahhas, O.; Chen, C.L.; Yu, A.F.; Steingart, R.M.; Liu, J.E. Incremental Value of Global Longitudinal Strain for Predicting Survival in Patients With Advanced AL Amyloidosis. JACC CardioOncol. 2020, 2, 223–231. [Google Scholar] [CrossRef]
- Awadalla, M.; Mahmood, S.S.; Groarke, J.D.; Hassan, M.Z.; Nohria, A.; Rokicki, A.; Murphy, S.P.; Mercaldo, N.D.; Zhang, L.; Zlotoff, D.A.; et al. Global Longitudinal Strain and Cardiac Events in Patients With Immune Checkpoint Inhibitor-Related Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 467–478. [Google Scholar] [CrossRef]
- Mincu, R.I.; Pohl, J.; Mrotzek, S.; Michel, L.; Hinrichs, L.; Lampe, L.; Rassaf, T.; Totzeck, M. Left ventricular global longitudinal strain reduction in patients with melanoma and extra-cardiac immune-related adverse events during immune checkpoint inhibitor therapy. Eur. Heart J. 2020, 41, ehaa946.3261. [Google Scholar] [CrossRef]
- Tamura, Y.; Tamura, Y.; Takemura, R.; Yamada, K.; Taniguchi, H.; Iwasawa, J.; Yada, H.; Kawamura, A. Longitudinal Strain and Troponin I Elevation in Patients Undergoing Immune Checkpoint Inhibitor Therapy. JACC CardioOncol. 2022, 4, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Fourati, N.; Charfeddine, S.; Chaffai, I.; Dhouib, F.; Farhat, L.; Boukhris, M.; Abid, L.; Kammoun, S.; Mnejja, W.; Daoud, J. Subclinical left ventricle impairment following breast cancer radiotherapy: Is there an association between segmental doses and segmental strain dysfunction? Int. J. Cardiol. 2021, 345, 130–136. [Google Scholar] [PubMed]
- Walker, V.; Lairez, O.; Fondard, O.; Pathak, A.; Pinel, B.; Chevelle, C.; Franck, D.; Jimenez, G.; Camilleri, J.; Panh, L.; et al. Early detection of subclinical left ventricular dysfunction after breast cancer radiation therapy using speckle-tracking echocardiography: Association between cardiac exposure and longitudinal strain reduction (BACCARAT study). Radiat. Ther. 2019, 14, 204. [Google Scholar]
- Xu, X.; Wang, D.; Yin, Y.; Dai, H.; Chen, L.; Xue, J.; Li, Q. Role of global longitudinal strain in evaluating radiotherapy-induced early cardiotoxicity in breast cancer: A meta-analysis. Kardiol. Pol. 2022; ahead of print. [Google Scholar]
- Keramida, K.; Farmakis, D.; Bingcang, J.; Sulemane, S.; Sutherland, S.; Bingcang, R.A.; Ramachandran, K.; Tzavara, C.; Charalampopoulos, G.; Filippiadis, D.; et al. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur. J. Heart Fail. 2019, 21, 529–535. [Google Scholar] [PubMed]
- Laufer-Perl, M.; Perelman-Gvili, M.; Sirota Dorfman, S.; Sulemane, S.; Sutherland, S.; Bingcang, R.A.; Ramachandran, K.; Tzavara, C.; Charalampopoulos, G.; Filippiadis, D.; et al. Prevalence of Right Ventricle Strain Changes following Anthracycline Therapy. Life 2022, 12, 291. [Google Scholar]
- Khairat, I.; Khalfallah, M.; Shaban, A.; Farag, I.A.; Elkady, A. Right ventricular 2D speckle-tracking echocardiography in children with osteosarcoma under chemotherapy. Egypt. Heart J. 2019, 71, 23. [Google Scholar]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar]
- Čelutkienė, J.; Pudil, R.; Lopez-Fernandez, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; von Haehling, S.; et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: A position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC) [published correction appears in Eur. J. Heart Fail. 2021, 23, 345]. Eur. J. Heart Fail. 2020, 22, 1504–1524. [Google Scholar]
- Virani, S.A.; Dent, S.; Brezden-Masley, C.; Clarke, B.; Davis, M.K.; Jassal, D.S.; Johnson, C.; Lemieux, J.; Paterson, I.; Sebag, I.A.; et al. Canadian Cardiovascular Society Guidelines for Evaluation and Management of Cardiovascular Complications of Cancer Therapy. Can. J. Cardiol. 2016, 32, 831–841. [Google Scholar]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) [published correction appears in Eur. Heart J. 24 December 2016]. Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar]
- Di Lisi, D.; Madaudo, C.; Di Fazio, L.; Gulotta, A.; Triolo, O.F.; Galassi, A.R.; Incorvaia, L.; Russo, A.; Novo, G. Higher Incidence of Cancer Therapy-Related Cardiac Dysfunction in the COVID-19 Era: A Single Cardio-Oncology Center Experience. J. Cardiovasc. Dev. Dis. 2023, 10, 23. [Google Scholar] [PubMed]
- Piveta, R.B.; Rodrigues, A.C.T.; Vieira, M.L.C.; Fischer, C.H.; Afonso, T.R.; Daminello, E.; Cruz, F.M.; Galvão, T.F.G.; Filho, E.B.L.; Katz, M.; et al. Early Change in Area Strain Detected by 3D Speckle Tracking Is Associated With Subsequent Cardiotoxicity in Patients Treated With Low Doses of Anthracyclines. Front. Cardiovasc. Med. 2022, 9, 842532. [Google Scholar]
- Chen, H.; Ouyang, D.; Baykaner, T.; Jamal, F.; Cheng, P.; Rhee, J.W. Artificial intelligence applications in cardio-oncology: Leveraging high dimensional cardiovascular data. Front. Cardiovasc. Med. 2022, 9, 941148. [Google Scholar] [CrossRef] [PubMed]
- Kar, J.; Cohen, M.V.; McQuiston, S.A.; Malozzi, C.M. Can global longitudinal strain (GLS) with magnetic resonance prognosticate early cancer therapy-related cardiac dysfunction (CTRCD) in breast cancer patients, a prospective study? [published online ahead of print, 2022 Dec 26]. Magn. Reson. Imaging 2022, 97, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, W.; Negishi, T.; Thavendiranathan, P.; Penicka, M.; De Blois, J.; Murbræch, K.; Miyazaki, S.; Shirazi, M.; Santoro, C.; Vinereanu, D.; et al. Incremental Value of Myocardial Work over Global Longitudinal Strain in the Surveillance for Cancer-Treatment-Related Cardiac Dysfunction: A Case-Control Study. J. Clin. Med. 2022, 11, 912. [Google Scholar] [CrossRef]
- Iida, N.; Tajiri, K.; Ishizu, T.; Sasamura-Koshizuka, R.; Nakajima, H.; Kawamatsu, N.; Sato, K.; Yamamoto, M.; Machino-Ohtsuka, T.; Bando, H.; et al. Echocardiography image quality of global longitudinal strain in cardio-oncology: A prospective real-world investigation. J. Echocardiogr. 2022, 20, 159–165. [Google Scholar] [PubMed]
- Marwick, T.H. Global Longitudinal Strain Monitoring to Guide Cardioprotective Medications During Anthracycline Treatment. Curr. Oncol. Rep. 2022, 24, 687–694. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Witteles, R.M. Global Longitudinal Strain in Cardio-Oncology. J. Am. Coll. Cardiol. 2021, 77, 402–404. [Google Scholar] [CrossRef]
- Negishi, T.; Thavendiranathan, P.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; Cho, G.Y.; Popescu, B.A.; et al. Cardioprotection Using Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy: 3-Year Results of the SUCCOUR Trial [published online ahead of print, 16 November 2022]. JACC Cardiovasc. Imaging 2022, S1936-878X(22)00616-7. [Google Scholar]
| Guidelines, Year | Anthracyclines | HER2-Targeted Therapies |
|---|---|---|
| 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO), and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC) [10] | Low baseline cardiotoxicity risk: at baseline and 12 months post-treatment (may be also considered after the fourth cycle) Moderate risk: at baseline and 12 months posttreatment (should be also considered after the fourth cycle). High and very high risk: at baseline, after 2nd, 4th, and 6th cycles and also 3 and 12 months after treatment | Low and moderate risk: at baseline; after 3, 6, 9, and 12 months; and then 12 months post-treatment High and very high risk: at baseline; after 3, 6, 9, and 12 months; and then 3 and 12 months post-treatment |
| 2021 British Society for Echocardiography and British Cardio-Oncology Society guideline for transthoracic echocardiographic assessment of adult cancer patients receiving anthracyclines and/or trastuzumab [8]. | Every 3 months during chemotherapy, and 3–12 months after its termination | Every 3 months during the therapy and 3–12 months after the end of therapy |
| 2020 Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations [48]. | After a cumulative dose of 250 mg/m2 doxorubicin or equivalent. Next after each additional 100 mg/m2 | Every 3 months |
| 2020 Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI), and the Cardio-Oncology Council of the European Society of Cardiology (ESC) [49]. | Depending on the risk calculated according to the planned therapy and patient-related risk factors, including age, comorbidities, and cardiovascular (CV) risk factors | Risk is calculated according to the planned therapy and patient-related factors, including age, comorbidities, and CV risk factors (range from 6 to 12 weeks) |
| 2016 Canadian Cardiovascular Society Guidelines for Evaluation and Management of Cardiovascular Complications of Cancer Therapy [50]. | No recommendations | Every 3 months |
| 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) [51]. | After a cumulative dose of 200 mg/m2 doxorubicin or equivalent | Every 4 cycles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławiński, G.; Hawryszko, M.; Liżewska-Springer, A.; Nabiałek-Trojanowska, I.; Lewicka, E. Global Longitudinal Strain in Cardio-Oncology: A Review. Cancers 2023, 15, 986. https://doi.org/10.3390/cancers15030986
Sławiński G, Hawryszko M, Liżewska-Springer A, Nabiałek-Trojanowska I, Lewicka E. Global Longitudinal Strain in Cardio-Oncology: A Review. Cancers. 2023; 15(3):986. https://doi.org/10.3390/cancers15030986
Chicago/Turabian StyleSławiński, Grzegorz, Maja Hawryszko, Aleksandra Liżewska-Springer, Izabela Nabiałek-Trojanowska, and Ewa Lewicka. 2023. "Global Longitudinal Strain in Cardio-Oncology: A Review" Cancers 15, no. 3: 986. https://doi.org/10.3390/cancers15030986
APA StyleSławiński, G., Hawryszko, M., Liżewska-Springer, A., Nabiałek-Trojanowska, I., & Lewicka, E. (2023). Global Longitudinal Strain in Cardio-Oncology: A Review. Cancers, 15(3), 986. https://doi.org/10.3390/cancers15030986






