Combining the HCT-CI, G8, and AML-Score for Fitness Evaluation of Elderly Patients with Acute Myeloid Leukemia: A Single Center Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
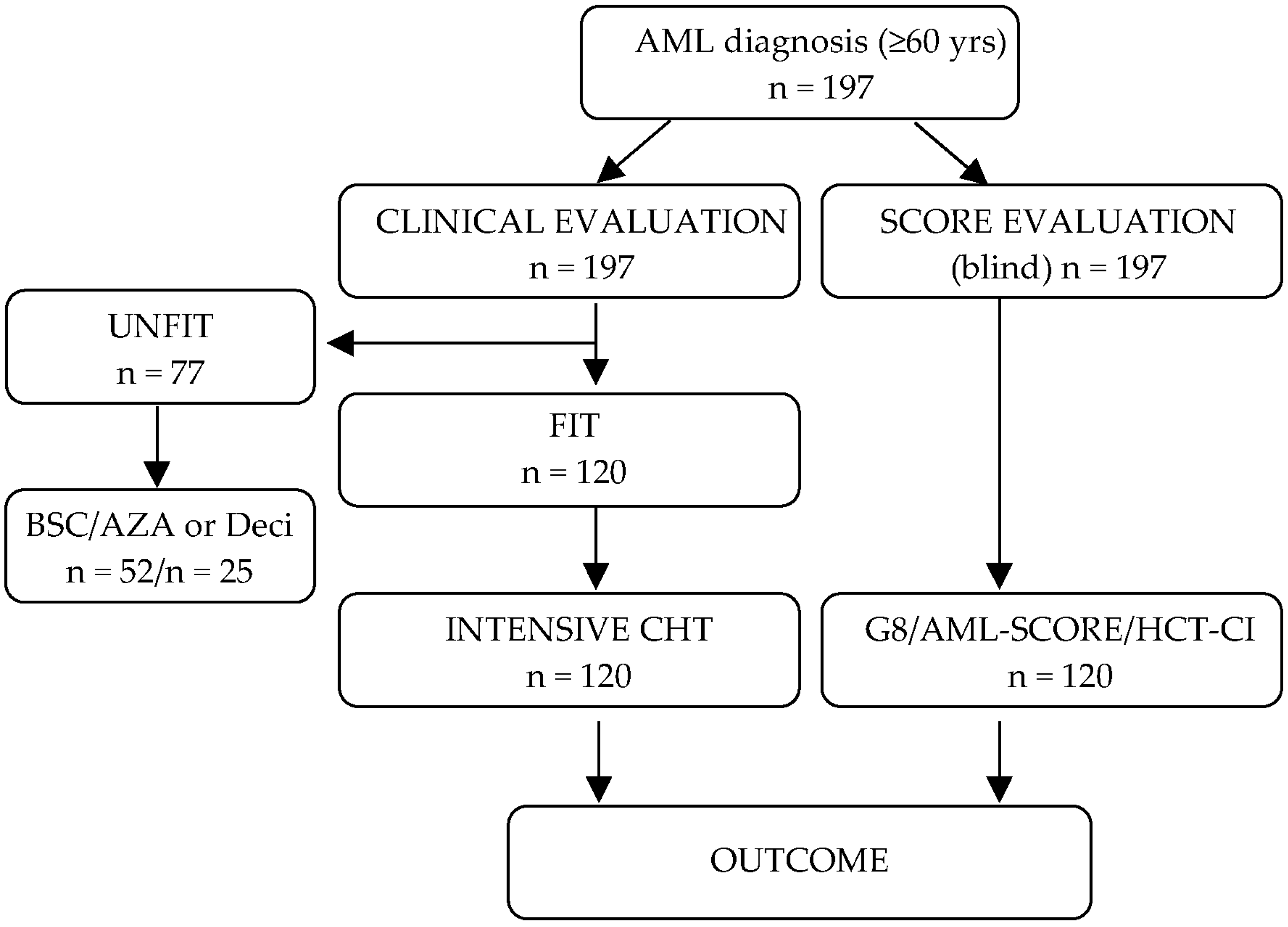
2.1. Patients and Treatments
2.2. Frailty Assessment Scores
2.3. Statistical Analysis
3. Results
3.1. Patients and Treatment Characteristics
3.2. Toxicity and Overall Response
3.3. Fitness Score Evaluation in the Intensively Treated Patient Subgroup
3.4. Fitness Score Evaluation in the Non-Intensively Treated Subgroup
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estey, E. AML in older patients: Are we making progress? Best Pract. Res. Clin. Haematol. 2009, 22, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; O’brien, S.; Cortes, J.; Giles, F.; Faderl, S.; Jabbour, E.; Garcia-Manero, G.; Wierda, W.; Pierce, S.; Shan, J.; et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 2006, 106, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Büchner, T.; Berdel, W.E.; Haferlach, C.; Haferlach, T.; Schnittger, S.; Müller-Tidow, C.; Braess, J.; Spiekermann, K.; Kienast, J.; Staib, P.; et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: A study by the German Acute Myeloid Leukemia Cooperative Group. J. Clin. Oncol. 2009, 27, 61–69. [Google Scholar] [CrossRef]
- Juliusson, G.; Antunovic, P.; Derolf, A.; Lehmann, S.; Möllgård, L.; Stockelber, D.; Tidefelt, U.; Wahlin, A.; Höglund, M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009, 113, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Estey, E.H. General approach to, and perspectives on clinical research in, older patients with newly diagnosed acute myeloid leukemia. Semin. Hematol. 2006, 43, 89–95. [Google Scholar] [CrossRef]
- Grimwade, D.; Hills, R.K. Independent prognostic factors for AML outcome. Hematol. Am. Soc. Hematol. Educ. Program 2009, 2009, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, R.; Othus, M.; Halpern, A.B.; Percival, M.M.; Godwin, C.D.; Becker, P.S.; Walter, R.B. Accuracy of SIE/SIES/GITMO Consensus Criteria for Unfitness to Predict Early Mortality after Intensive Chemotherapy in Adults with AML or Other High-Grade Myeloid Neoplasm. J. Clin. Oncol. 2020, 38, 4163–4174. [Google Scholar] [CrossRef]
- Abel, G.A.; Klepin, H.D. Frailty and the management of hematologic malignancies. Blood 2018, 131, 515–524. [Google Scholar] [CrossRef]
- Cortes, J.E.; Mehta, P. Determination of fitness and therapeutic options in older patients with acute myeloid leukemia. Am. J. Hematol. 2021, 96, 493–507. [Google Scholar] [CrossRef]
- Klepin, H.D.; Geiger, A.M.; Tooze, J.A.; Kritchevsky, S.B.; Williamson, J.D.; Pardee, T.S.; Ellis, L.R.; Powell, B.L. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 2013, 121, 4287–4294. [Google Scholar] [CrossRef]
- Timilshina, N.; Breunis, H.; Tomlinson, G.; Brandwein, J.; Alibhai, S.M. Do quality of life, physical function, or the Wheatley index at diagnosis predict 1-year mortality with intensive chemotherapy in older acute myeloid leukemia patients? Leuk. Res. 2016, 47, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Krug, U.; Röllig, C.; Koschmieder, A.; Heinecke, A.; Sauerland, M.C.; Schaich, M.; Thiede, C.; Kramer, M.; Braess, J.; Spiekermann, K.; et al. Study Alliance Leukemia Investigators. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: A web-based application for prediction of outcomes. Lancet 2010, 376, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Min, G.J.; Cho, B.S.; Park, S.S.; Park, S.; Jeon, Y.W.; Shin, S.H.; Yahng, S.A.; Yoon, J.H.; Lee, S.E.; Eom, K.S.; et al. Geriatric assessment predicts nonfatal toxicities and survival for intensively treated older adults with AML. Blood 2022, 139, 1646–1658. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef]
- Sorror, M.L.; Storer, B.; Storb, R.F. Validation of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in single and multiple institutions: Limitations and inferences. Biol. Blood Marrow Transpl. 2009, 15, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Sorror, M.L.; Logan, B.R.; Zhu, X.; Rizzo, J.D.; Cooke, K.R.; McCarthy, P.L.; Ho, V.T.; Horowitz, M.M.; Pasquini, M.C. Prospective Validation of the Predictive Power of the Hematopoietic Cell Transplantation Comorbidity Index: A Center for International Blood and Marrow Transplant Research Study. Biol. Blood Marrow Transpl. 2015, 21, 1479–1487. [Google Scholar] [CrossRef]
- Salit, R.B.; Oliver, D.C.; Delaney, C.; Sorror, M.L.; Milano, F. Prognostic Value of the Hematopoietic Cell Transplantation Comorbidity Index for Patients Undergoing Reduced-Intensity Conditioning Cord Blood Transplantation. Biol. Blood Marrow Transpl. 2017, 23, 654–658. [Google Scholar] [CrossRef]
- Busca, A.; Passera, R.; Maffini, E.; Festuccia, M.; Brunello, L.; Dellacasa, C.M.; Aydin, S.; Frairia, C.; Manetta, S.; Butera, S.; et al. Hematopoietic cell transplantation comorbidity index and risk of developing invasive fungal infections after allografting. Bone Marrow Transpl. 2018, 53, 1304–1310. [Google Scholar] [CrossRef]
- Giles, F.J.; Borthakur, G.; Ravandi, F.; Faderl, S.; Verstovsek, S.; Thomas, D.; Wierda, W.; Ferrajoli, A.; Kornblau, S.; Pierce, S.; et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br. J. Haematol. 2007, 136, 624–627. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Soubeyran, P.; Bellera, C.; Goyard, J.; Heitz, D.; Curé, H.; Rousselot, H.; Albrand, G.; Servent, V.; Jean, O.S.; van Praagh, I.; et al. Screening for vulnerability in older cancer patients: The Oncodage Prospective Multicenter Cohort Study. PLoS ONE 2014, 9, e115060. [Google Scholar] [CrossRef]
- Guigoz, Y. The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466–485. [Google Scholar] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Lausen, B.; Schumacher, M. Maximally Selected Rank Statistic. Biometrics 1992, 48, 73–85. [Google Scholar] [CrossRef]
- Lichtman, M.A. A historical perspective on the development of the cytarabine (7days) and daunorubicin (3days) treatment regimen for acute myelogenous leukemia: 2013 the 40th anniversary of 7 + 3. Blood Cells Mol. Dis. 2013, 50, 119–130. [Google Scholar] [CrossRef]
- Cerrano, M.; Candoni, A.; Crisà, E.; Dubbini, M.V.; D’Ardia, S.; Zannier, M.E.; Boccadoro, M.; Audisio, E.; Bruno, B.; Ferrero, D. FLAI induction regimen in elderly patients with acute myeloid leukemia. Leuk. Lymphoma 2019, 60, 3339–3340. [Google Scholar] [CrossRef]
- Etienne, A.; Esterni, B.; Charbonnier, A.; Mozziconacci, M.J.; Arnoulet, C.; Coso, D.; Puig, B.; Gastaut, J.A.; Maraninchi, D.; Vey, N. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer 2007, 109, 1376–1383. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Sorror, M.L.; Storer, B.E.; Fathi, A.T.; Gerds, A.T.; Medeiros, B.C.; Shami, P.; Brunner, A.M.; Sekeres, M.A.; Mukherjee, S.; Peña, E.; et al. Development and Validation of a Novel Acute Myeloid Leukemia-Composite Model to Estimate Risks of Mortality. JAMA Oncol. 2017, 3, 1675–1682. [Google Scholar] [CrossRef]
- Wheatley, K.; Brookes, C.L.; Howman, A.J.; Goldstone, A.H.; Milligan, D.W.; Prentice, A.G.; Moorman, A.V.; Burnett, A.K. United Kingdom National Cancer Research Institute Haematological Oncology Clinical Studies Group and Acute Myeloid Leukaemia Subgroup. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br. J. Haematol. 2009, 145, 598–605. [Google Scholar] [CrossRef]
- Prassek, V.V.; Rothenberg-Thurley, M.; Sauerland, M.C.; Herold, T.; Janke, H.; Ksienzyk, B.; Konstandin, N.P.; Goerlich, D.; Krug, U.; Faldum, A.; et al. Genetics of acute myeloid leukemia in the elderly: Mutation spectrum and clinical impact in intensively treated patients aged 75 years or older. Haematologica 2018, 103, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Gardin, C.; Pautas, C.; Fournier, E.; Itzykson, R.; Lemasle, E.; Bourhis, J.H.; Adès, L.; Marolleau, J.P.; Malfuson, J.V.; Gastaud, L.; et al. Added prognostic value of secondary AML-like gene mutations in ELN intermediate-risk older AML: ALFA-1200 study results. Blood Adv. 2020, 4, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R.; Gundacker, H.; Head, D.R.; Slovak, M.L.; Willman, C.L.; Godwin, J.E.; Anderson, J.E.; Petersdorf, S.H. Age and acute myeloid leukemia. Blood 2006, 107, 3481–3485. [Google Scholar] [CrossRef] [PubMed]
- Metzeler, K.H.; Herold, T.; Rothenberg-Thurley, M.; Amler, S.; Sauerland, M.C.; Görlich, D.; Schneider, S.; Konstandin, N.P.; Dufour, A.; Bräundl, K.; et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood 2016, 128, 686–698. [Google Scholar] [CrossRef]
- Itzykson, R.; Fournier, E.; Berthon, C.; Röllig, C.; Braun, T.; Marceau-Renaut, A.; Pautas, C.; Nibourel, O.; Lemasle, E.; Micol, J.B.; et al. Genetic identification of patients with AML older than 60 years achieving long-term survival with intensive chemotherapy. Blood 2021, 138, 507–519. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wie, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.P.; et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- Kadia, T.M.; Reville, P.K.; Wang, X.; Rausch, C.R.; Borthakur, G.; Pemmaraju, N.; Daver, N.G.; DiNardo, C.D.; Sasaki, K.; Issa, G.C.; et al. Phase II Study of Venetoclax Added to Cladribine Plus Low-Dose Cytarabine Alternating With 5-Azacitidine in Older Patients with Newly Diagnosed Acute Myeloid Leukemia. J. Clin. Oncol. 2022, 40, 3848–3857. [Google Scholar] [CrossRef]
- Maiti, A.; DiNardo, C.D.; Daver, N.G.; Rausch, C.R.; Ravandi, F.; Kadia, T.M.; Pemmaraju, N.; Borthakur, G.; Bose, P.; Issa, G.C.; et al. Triplet therapy with venetoclax, FLT3 inhibitor and decitabine for FLT3-mutated acute myeloid leukemia. Blood Cancer J. 2021, 11, 25. [Google Scholar] [CrossRef]
- Yilmaz, M.; Kantarjian, H.; Short, N.J.; Reville, P.; Konopleva, M.; Kadia, T.; DiNardo, C.; Borthakur, G.; Pemmaraju, N.; Maiti, A.; et al. Hypomethylating agent and venetoclax with FLT3 inhibitor “triplet” therapy in older/unfit patients with FLT3 mutated AML. Blood Cancer J. 2022, 12, 77. [Google Scholar] [CrossRef]
- Urbino, I.; Secreto, C.; Olivi, M.; Apolito, V.; D’Ardia, S.; Frairia, C.; Giai, V.; Aydin, S.; Freilone, R.; Dellacasa, C.; et al. Evolving Therapeutic Approaches for Older Patients with Acute Myeloid Leukemia in 2021. Cancers 2021, 13, 5075. [Google Scholar] [CrossRef] [PubMed]
- Ustun, C.; Le-Rademacher, J.; Wang, H.L.; Othus, M.; Sun, Z.; Major, B.; Zhang, M.J.; Storrick, E.; Lafky, J.M.; Chow, S.; et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60–75 years in first complete remission (CR1): An alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia 2019, 33, 2599–2609. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, S.; Bérard, E.; Huguet, F.; Huynh, A.; Tavitian, S.; Vergez, F.; Dobbelstein, S.; Dastugue, N.; Mansat-De Mas, V.; Delabesse, E.; et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood 2013, 121, 2618–2626. [Google Scholar] [CrossRef]
- Röllig, C.; Kramer, M.; Schliemann, C.; Mikesch, J.H.; Steffen, B.; Krämer, A.; Noppeney, R.; Schäfer-Eckart, K.; Krause, S.W.; Hänel, M.; et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood 2020, 136, 823–830. [Google Scholar] [CrossRef]
- Solh, M.M.; Solomon, S.R.; Morris, L.E.; Zhang, X.; Holland, H.K.; Bashey, A. Improved Post remission survival of non-favorable risk Acute Myelogenous Leukemia (AML) patients following initial remission induction therapy with FLAG+/-Idarubicin versus 3 + 7 (Anthracycline + Cytarabine). Leuk. Res. 2020, 93, 106318. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wie, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]


| Variable | n (%) |
|---|---|
| Patients | 120 |
| Median age, years (IQR) | 68 (60–77) |
| Sex (male) | 72 (60) |
| WBC > 100,000 × 106/µL | 12 (10) |
| WHO n = 120 | |
| de novo AML | 48 (40) |
| t-AML | 9 (7.5) |
| MDS related | 57 (47.5) |
| Myeloid sarcoma | 6 (5) |
| Molecular markers, n = 113 | |
| AML/ETO, CBF, inv16 or t(8; 21) | 2 (1.7) |
| NPM mut | 27 (22.5) |
| FLT3-ITD/FLT3-TKD mut | 24 (20) |
| MLL mut | 13 (10.8) |
| Cytogenetics, n = 97 | |
| Normal karyotype | 45 (37.2) |
| Complex karyotype | 20 (16.7) |
| Monosomy | 16 (13.3) |
| del(5q), abn(17p) | 19 (15.8) |
| Fitness scoring | |
| G8, fit (>14) | 65 (54.2) |
| Sorror (0–2) | 94 (78.3) |
| Treatment regimen | |
| 7+3 | 93 (77.5) |
| FLAI | 27 (22.5) |
| Allogeneic HSCT | 33 (27.4) |
| Grade 3/4 toxicity | 54 (45) |
| Infection | 27 (22.5) |
| Atrial fibrillation | 4 (3.3) |
| Bleeding | 5 (4.1) |
| Cerebral ischemia | 3 (2.5) |
| Treatment response | |
| Death in induction | 7 (5.8) |
| CR1 | 73 (60.8) |
| PIF | 40 (33.3) |
| CR2 | 13 (10.8) |
| Univariate Models | Multivariate Model | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| HCT-CI (unfit vs. fit) | 1.78 (1.29–2.46) | <0.001 | 1.20 (0.85–1.70) | 0.305 |
| G8 (unfit vs. fit) | 2.33 (1.68–3.25) | <0.001 | 2.03 (1.46–2.84) | <0.001 |
| AML (high vs. low risk) | 4.07 (1.99–8.32) | <0.001 | 3.27 (1.59–6.73) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, S.; Passera, R.; Cerrano, M.; Giai, V.; D’Ardia, S.; Iovino, G.; Dellacasa, C.M.; Audisio, E.; Busca, A. Combining the HCT-CI, G8, and AML-Score for Fitness Evaluation of Elderly Patients with Acute Myeloid Leukemia: A Single Center Analysis. Cancers 2023, 15, 1002. https://doi.org/10.3390/cancers15041002
Aydin S, Passera R, Cerrano M, Giai V, D’Ardia S, Iovino G, Dellacasa CM, Audisio E, Busca A. Combining the HCT-CI, G8, and AML-Score for Fitness Evaluation of Elderly Patients with Acute Myeloid Leukemia: A Single Center Analysis. Cancers. 2023; 15(4):1002. https://doi.org/10.3390/cancers15041002
Chicago/Turabian StyleAydin, Semra, Roberto Passera, Marco Cerrano, Valentina Giai, Stefano D’Ardia, Giorgia Iovino, Chiara Maria Dellacasa, Ernesta Audisio, and Alessandro Busca. 2023. "Combining the HCT-CI, G8, and AML-Score for Fitness Evaluation of Elderly Patients with Acute Myeloid Leukemia: A Single Center Analysis" Cancers 15, no. 4: 1002. https://doi.org/10.3390/cancers15041002
APA StyleAydin, S., Passera, R., Cerrano, M., Giai, V., D’Ardia, S., Iovino, G., Dellacasa, C. M., Audisio, E., & Busca, A. (2023). Combining the HCT-CI, G8, and AML-Score for Fitness Evaluation of Elderly Patients with Acute Myeloid Leukemia: A Single Center Analysis. Cancers, 15(4), 1002. https://doi.org/10.3390/cancers15041002







