Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Current Limitations of Breast Cancer Chemotherapy Regimens
1.2. The Phytotherapeutics: Benefits and Their Delivery Challenges
2. Advanced Phytochemical Delivery Strategies
2.1. Phytochemical-Loaded Nanocarriers
2.1.1. Polymeric Nanoparticles (PNs)
2.1.2. Cell-Derived Nanovesicles (CDNs)
2.1.3. Lipid Nanoparticles
2.1.4. Transferosomes
2.1.5. Ethosomes
2.1.6. Niosomes
2.2. Phytochemical-Assisted Nanocarriers
3. Evidence of the Role of Phytofabricated Nanocarriers against Breast Cancer
3.1. Anticancer Activity
3.1.1. Immunostimulation
3.1.2. Apoptosis
3.1.3. Metastasis
3.1.4. Angiogenesis
3.1.5. Inhibition of Cancer Stem Cells
3.1.6. Anti-Proliferative Activities
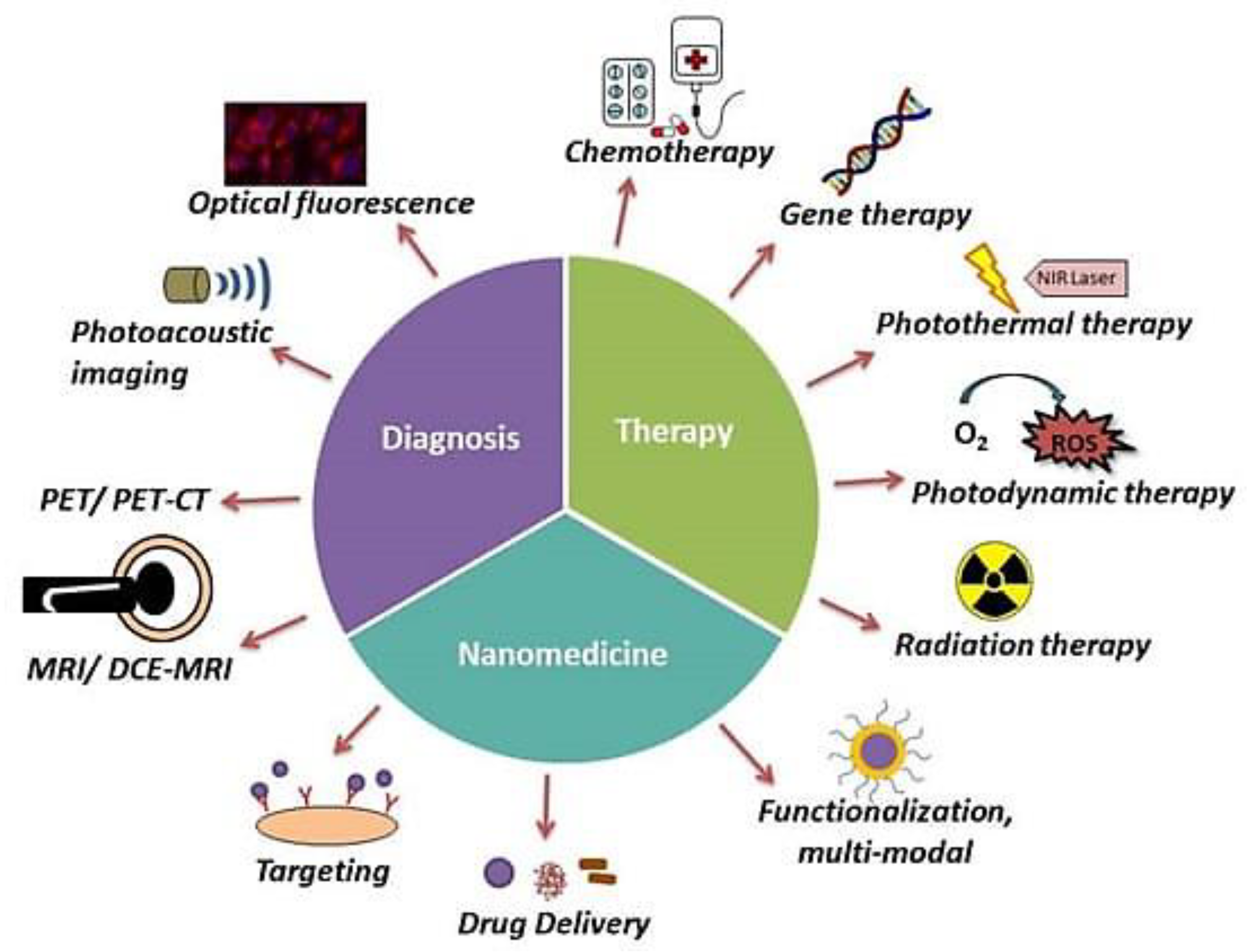
3.2. Theranostic Targeting
Theranostic-Related Patents
4. Phytonanomedicines Approved by the FDA or in Preclinical and Clinical Trials
5. Lacunas of Phytofabricated Nanocarriers
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, L.; Torre, A.; Ahmedin, D. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Glob. Cancer Stat. 2021, 73, 209–249. [Google Scholar]
- Greco, S.J. Breast cancer risk in a rapidly aging population: Advances and approaches to study the aging tissue microenvironment. Breast Cancer Targets Ther. 2019, 11, 111. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Qadumii, L.; Zihlif, M.; Al-Ameer, H.J.; Salou, R.A.; Jaber, A.Y.; Hamad, I. Green Synthesis of Gold, Iron and Selenium Nanoparticles Using Phytoconstituents: Preliminary Evaluation of Antioxidant and Biocompatibility Potential. Molecules 2022, 27, 1334. [Google Scholar] [CrossRef]
- Turashvili, G.; Brogi, E. Tumor heterogeneity in breast cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.; Killen, E.; Tesdale, A.; Sangster, K.; Thomson, M.; Steele, R.; Blackie, R. Oestrogen receptors, lactate dehydrogenase and cellularity in human breast cancer. Clin. Chim. Acta 1988, 175, 89–96. [Google Scholar] [CrossRef]
- WHO Classification of Tumours of the Breast. Available online: https://espace.library.uq.edu.au/view/UQ:8984059 (accessed on 24 January 2023).
- Dean-Colomb, W.; Esteva, F.J. Her2-positive breast cancer: Herceptin and beyond. Eur. J. Cancer 2008, 44, 2806–2812. [Google Scholar] [CrossRef] [PubMed]
- Bardou, V.-J.; Arpino, G.; Elledge, R.M.; Osborne, C.K.; Clark, G.M. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J. Clin. Oncol. 2003, 21, 1973–1979. [Google Scholar] [CrossRef]
- Harvey, J.M.; Clark, G.M.; Osborne, C.K.; Allred, D.C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999, 17, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Sørlie, T.; Borgan, E.; Myhre, S.; Vollan, H.K.; Russnes, H.; Zhao, X.; Nilsen, G.; Lingjærde, O.C.; Børresen-Dale, A.-L.; Rødland, E. The importance of gene-centring microarray data. Lancet Oncol. 2010, 11, 719–720. [Google Scholar] [CrossRef]
- Bhushan, A.; Gonsalves, A.; Menon, J.U. Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics 2021, 13, 723. [Google Scholar] [CrossRef]
- Galmarini, D.; Galmarini, C.M.; Galmarini, F.C. Cancer chemotherapy: A critical analysis of its 60 years of history. Crit. Rev. Oncol. Hematol. 2012, 84, 181–199. [Google Scholar] [CrossRef]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef]
- Sawanny, R.; Pramanik, S.; Agarwal, U. Role of Phytochemicals in the Treatment of Breast Cancer: Natural Swords Battling Cancer Cells. Curr. Cancer Ther. Rev. 2021, 17, 179–196. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed]
- DiMarco-Crook, C.; Xiao, H. Diet-based strategies for cancer chemoprevention: The role of combination regimens using dietary bioactive components. Annu. Rev. Food Sci. Technol. 2015, 6, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.B.; Hernández, P.S. Cancer chemoprevention by dietary phytochemicals: Epidemiological evidence. Maturitas 2016, 94, 13–19. [Google Scholar] [CrossRef]
- Bhattacharjee, H.; Balabathula, P.; Wood, G.C. Targeted nanoparticulate drug-delivery systems for treatment of solid tumors: A review. Ther. Deliv. 2010, 1, 713–734. [Google Scholar] [CrossRef]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Chemotherapy for Breast Cancer. Available online: https://www.mayoclinic.org/tests-procedures/chemotherapy-for-breast-cancer/about/pac-20384931 (accessed on 24 January 2023).
- Grobmyer, S.R.; Zhou, G.; Gutwein, L.G.; Iwakuma, N.; Sharma, P.; Hochwald, S.N. Nanoparticle delivery for metastatic breast cancer. Nanomed. Nanotechnol. Biol. Med. 2012, 8, S21–S30. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.; Hiscox, S. New therapeutic approaches in breast cancer. Maturitas 2011, 68, 121–128. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, S.; Lillard, J.W., Jr.; Singh, R. Drug delivery approaches for breast cancer. Int. J. Nanomed. 2017, 12, 6205. [Google Scholar] [CrossRef]
- Eroles, P.; Bosch, A.; Pérez-Fidalgo, J.A.; Lluch, A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat. Rev. 2012, 38, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, Z.; Yin, C.; Zhang, C.; Lin, G.; Li, Q. Controllable drug release and simultaneously carrier decomposition of SiO2-drug composite nanoparticles. J. Am. Chem. Soc. 2013, 135, 5709–5716. [Google Scholar] [CrossRef]
- Beloqui, A.; Alhouayek, M.; Carradori, D.; Vanvarenberg, K.; Muccioli, G.G.; Cani, P.D.; Preat, V. A mechanistic study on nanoparticle-mediated glucagon-like peptide-1 (GLP-1) secretion from enteroendocrine L cells. Mol. Pharm. 2016, 13, 4222–4230. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- India Regulatory Services > Phytopharmaceutical Drug—CliniExperts. Available online: https://cliniexperts.com/india-regulatory-services/phytopharmaceutical-drug/ (accessed on 24 December 2022).
- Singh, B. Herbal insecticides, Repellents Biomedicines: Effectiveness Commercialization; Springer: New Delhi, India, 2016; pp. 127–145. [Google Scholar]
- Boon, H.S.; Olatunde, F.; Zick, S.M. Trends in complementary/alternative medicine use by breast cancer survivors: Comparing survey data from 1998 and 2005. BMC Women’s Health 2007, 7, 4. [Google Scholar] [CrossRef]
- O’brien, K. Complementary and alternative medicine: The move into mainstream health care. Clin. Exp. Optom. 2004, 87, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Carpenter, C.L.; Sullivan-Halley, J.; Bernstein, L. The roles of herbal remedies in survival and quality of life among long-term breast cancer survivors-results of a prospective study. BMC Cancer 2011, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.W.; Leung, Y.; Chan, C. Herbal medicine in the treatment of cancer. Curr. Med. Chem.-Anti-Cancer Agents 2002, 2, 209–214. [Google Scholar] [CrossRef]
- Bahmani, M.; Shirzad, H.; Shahinfard, N.; Sheivandi, L.; Rafieian-Kopaei, M.J. Cancer phytotherapy: Recent views on the role of antioxidant and angiogenesis activities. J. Evid.-Based Complement. Altern. Med. 2017, 22, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Musthaba, S.M.; Baboota, S.; Ahmed, S.; Ahuja, A.; Ali, J. Status of novel drug delivery technology for phytotherapeutics. Expert Opin. Drug Deliv. 2009, 6, 625–637. [Google Scholar] [CrossRef]
- Feng-Lin, Y.; Tzu-Hui, W.; Liang-Tzung, L.; Thau-Ming, C.; Chun-Ching, L. Preparation and characterization of Cuscuta chinensis nanoparticles. Food Chem. Toxicol. 2008, 46, 1771–1777. [Google Scholar]
- Caiolfa, V.; Zamai, M.; Fiorino, A.; Frigerio, E.; Pellizzoni, C.; d’Argy, R.; Ghiglieri, A.; Castelli, M.; Farao, M.; Pesenti, E. Polymer-bound camptothecin: Initial biodistribution and antitumour activity studies. J. Control. Release 2000, 65, 105–119. [Google Scholar] [CrossRef]
- Min, K.H.; Park, K.; Kim, Y.-S.; Bae, S.M.; Lee, S.; Jo, H.G.; Park, R.-W.; Kim, I.-S.; Jeong, S.Y.; Kim, K. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J. Control. Release 2008, 127, 208–218. [Google Scholar] [CrossRef]
- Sánchez, A.; Mejía, S.P.; Orozco, J. Recent advances in polymeric nanoparticle-encapsulated drugs against intracellular infections. Molecules 2020, 25, 3760. [Google Scholar] [CrossRef]
- Dong, Y.; Fu, R.; Yang, J.; Ma, P.; Liang, L.; Mi, Y.; Fan, D. Folic acid-modified ginsenoside Rg5-loaded bovine serum albumin nanoparticles for targeted cancer therapy in vitro and in vivo. Int. J. Nanomed. 2019, 14, 6971. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Sharma, N.; Manchanda, R.; Gupta, N.; Syed, A.; Bahkali, A.H.; Nimesh, S. PGMD/curcumin nanoparticles for the treatment of breast cancer. Sci. Rep. 2021, 11, 3824. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yan, J.; Luo, L.; Ma, M.; Zhu, H. Preparation and characterization of (−)-Epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on Human breast cancer MCF-7 cells. Sci. Rep. 2017, 7, 45521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solanki, R.; Patel, K.; Patel, S.J.C.; Physicochemical, S.A.; Aspects, E. Bovine serum albumin nanoparticles for the efficient delivery of berberine: Preparation, characterization and in vitro biological studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125501. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Jabir, M.S.; Hameed, A.H. Nanoscale modification of chrysin for improved of therapeutic efficiency and cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 708–720. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, D.; Xue, G.; Yu, S.; Yuan, C.; Huang, M.; Jiang, L. Improved therapeutic efficacy of quercetin-loaded polymeric nanoparticles on triple-negative breast cancer by inhibiting uPA. RSC Adv. 2020, 10, 34517–34526. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials 2021, 269, 120467. [Google Scholar] [CrossRef]
- Ortega, A.; Martinez-Arroyo, O.; Forner, M.J.; Cortes, R. Exosomes as drug delivery systems: Endogenous nanovehicles for treatment of systemic lupus erythematosus. Pharmaceutics 2020, 13, 3. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Sun, M.; Li, J.; Wang, H.-M.D. Toward the next-generation phyto-nanomedicines: Cell-derived nanovesicles (CDNs) for natural product delivery. Biomed. Pharmacother. 2022, 145, 112416. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered extracellular vesicles for cancer therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Lu, Z.; Zhang, L.; Hu, Y.; Li, Q.; Du, W.; Feng, X.; Jia, H.; Liu, B.-F. The use of RGD-engineered exosomes for enhanced targeting ability and synergistic therapy toward angiogenesis. Nanoscale 2017, 9, 15598–15605. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ye, H.; Zhang, X.; Wang, X.; Yang, B.; Luo, C.; Zhao, Z.; Zhao, J.; Lu, Q.; Zhang, H. An exosome-like programmable-bioactivating paclitaxel prodrug nanoplatform for enhanced breast cancer metastasis inhibition. Biomaterials 2020, 257, 120224. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liang, N.; Wang, D.; Yan, P.; Kawashima, Y.; Cui, F.; Sun, S. Amphiphilic polymeric micelles based on deoxycholic acid and folic acid modified chitosan for the delivery of paclitaxel. Int. J. Mol. Sci. 2018, 19, 3132. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Quezada, J.; Guajardo-Flores, D.; González-Valdez, J. Enhanced exosome-mediated delivery of black bean phytochemicals (Phaseolus vulgaris L.) for cancer treatment applications. Biomed. Pharmacother. 2020, 131, 110771. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Mudd, A.M.; Kyakulaga, A.H.; Singh, I.P.; Vadhanam, M.V.; Gupta, R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017, 393, 94–102. [Google Scholar] [CrossRef]
- Kanchanapally, R.; Khan, M.A.; Deshmukh, S.K.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Exosomal formulation escalates cellular uptake of honokiol leading to the enhancement of its antitumor efficacy. ACS Omega 2020, 5, 23299–23307. [Google Scholar] [CrossRef]
- Antimisiaris, S.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.K.; Mutha, R.E.; Surana, S.J. Electrostatic deposition assisted preparation, characterization and evaluation of chrysin liposomes for breast cancer treatment. Drug Dev. Ind. Pharm. 2021, 47, 809–819. [Google Scholar] [CrossRef]
- Patel, G.; Thakur, N.S.; Kushwah, V.; Patil, M.D.; Nile, S.H.; Jain, S.; Banerjee, U.C.; Kai, G. Liposomal delivery of mycophenolic acid with quercetin for improved breast cancer therapy in SD rats. Front. Bioeng. Biotechnol. 2020, 8, 631. [Google Scholar] [CrossRef]
- Farcas, C.G.; Dehelean, C.; Pinzaru, I.A.; Mioc, M.; Socoliuc, V.; Moaca, E.-A.; Avram, S.; Ghiulai, R.; Coricovac, D.; Pavel, I. Thermosensitive betulinic acid-loaded magnetoliposomes: A promising antitumor potential for highly aggressive human breast adenocarcinoma cells under hyperthermic conditions. Int. J. Nanomed. 2020, 15, 8175. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, Y.; Sun, J.; Liang, Z.; Wu, Q.; Cui, S.; Zhi, D.; Guo, S.; Zhen, Y.; Zhang, S. Anti-breast cancer activity of resveratrol encapsulated in liposomes. J. Mater. Chem. B 2020, 8, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, P.; Machowska, M.; Wisniewski, K.; Grynkiewicz, G.; Hrynyk, R.; Rzepecki, R.; Gubernator, J. Novel pegylated liposomal formulation of docetaxel with 3-n-pentadecylphenol derivative for cancer therapy. Eur. J. Pharm. Sci. 2021, 163, 105838. [Google Scholar] [CrossRef] [PubMed]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS PharmSciTech 2014, 15, 834–844. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S. Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y. Anticancer effects of resveratrol-loaded solid lipid nanoparticles on human breast cancer cells. Molecules 2017, 22, 1814. [Google Scholar] [CrossRef] [PubMed]
- Niazvand, F.; Orazizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of quercetin-loaded nanoparticles on MCF-7 human breast cancer cells. Medicina 2019, 55, 114. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef] [PubMed]
- Sivannarayana, P.; Rani, A.P.; Saikishore, V.; VenuBabu, C.; SriRekha, V. Transfersomes: Ultra deformable vesicular carrier systems in transdermal drug delivery system. Res. J. Pharm. Dos. Technol. 2012, 4, 1. [Google Scholar]
- Cevc, G.; Schätzlein, A.; Richardsen, H. Ultradeformable lipid vesicles can penetrate the skin and other semi-permeable barriers unfragmented. Evidence from double label CLSM experiments and direct size measurements. Biochim. Biophys. Acta-Biomembr. 2002, 1564, 21–30. [Google Scholar] [CrossRef]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Chauhan, P.; Tyagi, B.K. Herbal novel drug delivery systems and transfersomes. J. Drug Deliv. Ther. 2018, 8, 162–168. [Google Scholar] [CrossRef]
- Modi, C.; Bharadia, P. Transfersomes: New dominants for transdermal drug delivery. Am. J. PharmTech Res. AJPTR 2012, 2, 71–91. [Google Scholar]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Moawad, F.A.; Ali, A.A.; Salem, H.F. Nanotransfersomes-loaded thermosensitive in situ gel as a rectal delivery system of tizanidine HCl: Preparation, in vitro and in vivo performance. Drug Deliv. 2017, 24, 252–260. [Google Scholar] [CrossRef]
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; O’Neill, F.; Roberts, M. Surfactant effects on lipid-based vesicles properties. J. Pharm. Sci. 2018, 107, 1237–1246. [Google Scholar] [CrossRef]
- Gadag, S.; Narayan, R.; Sabhahit, J.N.; Hari, G.; Nayak, Y.; Pai, K.S.R.; Garg, S.; Nayak, U.Y. Transpapillary iontophoretic delivery of resveratrol loaded transfersomes for localized delivery to breast cancer. Biomater. Adv. 2022, 140, 213085. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M.J. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Touitou, E. Ethosomes: New prospects in transdermal delivery. Crit. Rev. Ther. Drug Carr. Syst. 2003, 20, 63–102. [Google Scholar] [CrossRef]
- Ainbinder, D.; Touitou, E. Testosterone ethosomes for enhanced transdermal delivery. Drug Deliv. 2005, 12, 297–303. [Google Scholar] [CrossRef]
- Lopez-Pinto, J.; Gonzalez-Rodriguez, M.; Rabasco, A. Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int. J. Pharm. 2005, 298, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E. Compositions for Applying Active Substances to or through the Skin. U.S. Patent US5540934A, 30 July 1996. [Google Scholar]
- Touitou, E. Composition for Applying Active Substances to or through the Skin. U.S. Patent US5716638A, 10 February 1998. [Google Scholar]
- Nasri, S.; Ebrahimi-Hosseinzadeh, B.; Rahaie, M.; Hatamian-Zarmi, A.; Sahraeian, R. Thymoquinone-loaded ethosome with breast cancer potential: Optimization, in vitro and biological assessment. J. Nanostruct. Chem. 2020, 10, 19–31. [Google Scholar] [CrossRef]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Adeli-Sardou, M. Evaluation of carum-loaded niosomes on breast cancer cells: Physicochemical properties, in vitro cytotoxicity, flow cytometric, DNA fragmentation and cell migration assay. Sci. Rep. 2019, 9, 7139. [Google Scholar] [CrossRef]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Nematollahi, M.H. Lawsone-loaded Niosome and its antitumor activity in MCF-7 breast Cancer cell line: A Nano-herbal treatment for Cancer. DARU J. Pharm. Sci. 2018, 26, 11–17. [Google Scholar] [CrossRef]
- Honarvari, B.; Karimifard, S.; Akhtari, N.; Mehrarya, M.; Moghaddam, Z.S.; Ansari, M.J.; Jalil, A.T.; Matencio, A.; Trotta, F.; Yeganeh, F.E. Folate-targeted curcumin-loaded niosomes for site-specific delivery in breast cancer treatment: In silico and In vitro study. Molecules 2022, 27, 4634. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A. Plants as potential synthesiser of precious metal nanoparticles: Progress and prospects. IET Nanobiotechnol. 2013, 7, 117–124. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Parsons, J.; Gomez, E.; Peralta-Videa, J.; Troiani, H.; Santiago, P.; Yacaman, M.J. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett. 2002, 2, 397–401. [Google Scholar] [CrossRef]
- Haverkamp, R.G.; Marshall, A.T.; van Agterveld, D. Pick your carats: Nanoparticles of gold–silver–copper alloy produced in vivo. J. Nanopart. Res. 2007, 9, 697–700. [Google Scholar] [CrossRef]
- Harris, A.T.; Bali, R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanopart. Res. 2008, 10, 691–695. [Google Scholar] [CrossRef]
- Ankamwar, B. Biosynthesis of gold nanoparticles (green-gold) using leaf extract of Terminalia catappa. E-J. Chem. 2010, 7, 1334–1339. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Xu, C.; Merlin, D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers 2016, 4, e1134415. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.-G.; Guo, P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.-G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor SitesHijacked Leukocyte Pathway for Targeted Delivery. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef]
- Yi, S.; Wang, Y.; Huang, Y.; Xia, L.; Sun, L.; Lenaghan, S.C.; Zhang, M. Tea nanoparticles for immunostimulation and chemo-drug delivery in cancer treatment. J. Biomed. Nanotechnol. 2014, 10, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Minaei, A.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol. Biol. Rep. 2016, 43, 99–105. [Google Scholar] [CrossRef]
- Halder, A.; Jethwa, M.; Mukherjee, P.; Ghosh, S.; Das, S.; Helal Uddin, A.; Mukherjee, A.; Chatterji, U.; Roy, P. Lactoferrin-tethered betulinic acid nanoparticles promote rapid delivery and cell death in triple negative breast and laryngeal cancer cells. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1362–1371. [Google Scholar] [CrossRef]
- Tabassam, Q.; Mehmood, T.; Raza, A.R.; Ullah, A.; Saeed, F.; Anjum, F.M. Synthesis, characterization and anti-cancer therapeutic potential of withanolide-A with 20nm sAuNPs conjugates against SKBR3 breast cancer cell line. Int. J. Nanomed. 2020, 15, 6649. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, J.; Liu, P.; Chang, J.; Ali, D.; Tian, X. Gold nanoparticles synthesized from Curcuma wenyujin inhibits HER-2/neu transcription in breast cancer cells (MDA-MB-231/HER2). Arab. J. Chem. 2020, 13, 7264–7273. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, H.; Zhang, M. Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin. Drug Deliv. 2017, 14, 123–136. [Google Scholar] [CrossRef]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and triple-negative breast cancer: Challenges and treatment options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, G.; He, F.; Raghunathan, S.; Turan, O.; Peiris, P.M.; Schiemann, W.P.; Karathanasis, E. Effective treatment of cancer metastasis using a dual-ligand nanoparticle. PLoS ONE 2019, 14, e0220474. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yin, Q.; Shen, J.; Chen, L.; Yu, H.; Zhang, Z.; Li, Y. Synergistic inhibition of breast cancer metastasis by silibinin-loaded lipid nanoparticles containing TPGS. Int. J. Pharm. 2013, 454, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, V.; Lijnen, H.R. Angiogenesis and development of adipose tissue. Mol. Cell. Endocrinol. 2010, 318, 2–9. [Google Scholar] [CrossRef]
- Yoshida, S.; Ono, M.; Shono, T.; Izumi, H.; Ishibashi, T.; Suzuki, H.; Kuwano, M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol. Cell. Biol. 1997, 17, 4015–4023. [Google Scholar] [CrossRef] [Green Version]
- Rocha, T.G.R.; Lopes, S.C.D.A.; Cassali, G.D.; Ferreira, Ê.; Veloso, E.S.; Leite, E.A.; Braga, F.C.; Ferreira, L.A.M.; Balvay, D.; Garofalakis, A. Evaluation of antitumor activity of long-circulating and pH-sensitive liposomes containing ursolic acid in animal models of breast tumor and gliosarcoma. Integr. Cancer Ther. 2016, 15, 512–524. [Google Scholar] [CrossRef]
- Abraham, B.K.; Fritz, P.; McClellan, M.; Hauptvogel, P.; Athelogou, M.; Brauch, H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin. Cancer Res. 2005, 11, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Charafe-Jauffret, E.; Ginestier, C.; Iovino, F.; Tarpin, C.; Diebel, M.; Esterni, B.; Houvenaeghel, G.; Extra, J.-M.; Bertucci, F.; Jacquemier, J. Aldehyde dehydrogenase 1–Positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin. Cancer Res. 2010, 16, 45–55. [Google Scholar] [CrossRef]
- Singh, P.; Sahoo, S.K. Piperlongumine loaded PLGA nanoparticles inhibit cancer stem-like cells through modulation of STAT3 in mammosphere model of triple negative breast cancer. Int. J. Pharm. 2022, 616, 121526. [Google Scholar] [CrossRef]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin. Cancer Biol. 2022, 80, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Gonzalez, E.; Arumugam, A.; Nandy, S.; Gonzalez, V.; Medel, J.; Camacho, F.; Ortega, A.; Bonkoungou, S.; Narayan, M. Nimbolide inhibits pancreatic cancer growth and metastasis through ROS-mediated apoptosis and inhibition of epithelial-to-mesenchymal transition. Sci. Rep. 2016, 6, 19819. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Qiu, S.; Xie, L.; Liu, C.; Sun, S. Nimbolide suppresses non-small cell lung cancer cell invasion and migration via manipulation of DUSP4 expression and ERK1/2 signaling. Biomed. Pharmacother. 2017, 92, 340–346. [Google Scholar] [CrossRef]
- Sophia, J.; Kowshik, J.; Dwivedi, A.; Bhutia, S.K.; Manavathi, B.; Mishra, R.; Nagini, S. Nimbolide, a neem limonoid inhibits cytoprotective autophagy to activate apoptosis via modulation of the PI3K/Akt/GSK-3β signalling pathway in oral cancer. Cell Death Dis. 2018, 9, 1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, S.; Bhat, F.; Raja Singh, P.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.; Arunakaran, J. Gold nanoparticle–conjugated quercetin inhibits epithelial–mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Vinothkumar, B.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316. [Google Scholar] [CrossRef]
- Gulia, K.; James, A.; Pandey, S.; Dev, K.; Kumar, D.; Sourirajan, A. Bio-Inspired Smart Nanoparticles in Enhanced Cancer Theranostics and Targeted Drug Delivery. J. Funct. Biomater. 2022, 13, 207. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Gopal, K. Plant-mediated biosynthesis of silver and gold nanoparticles. J. Biomed. Nanotechnol. 2011, 7, 163–164. [Google Scholar] [CrossRef]
- Cavalcanti, I.D.L.; Ximenes, R.M.; Pessoa, O.D.L.; Magalhães, N.S.S.; de Britto Lira-Nogueira, M.C. Fucoidan-coated PIBCA nanoparticles containing oncocalyxone A: Activity against metastatic breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 65, 102698. [Google Scholar] [CrossRef]
- Awad, A.B.; Williams, H.; Fink, C.S. Phytosterols reduce in vitro metastatic ability of MDA-MB-231 human breast cancer cells. Nutr. Cancer 2001, 40, 157–164. [Google Scholar] [CrossRef]
- Hamad, F.B.; Mubofu, E.B. Potential biological applications of bio-based anacardic acids and their derivatives. Int. J. Mol. Sci. 2015, 16, 8569–8590. [Google Scholar] [CrossRef]
- Kushwah, V.; Katiyar, S.S.; Dora, C.P.; Agrawal, A.K.; Lamprou, D.A.; Gupta, R.C.; Jain, S. Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomater. 2018, 73, 424–436. [Google Scholar] [CrossRef]
- Iwanowycz, S.; Wang, J.; Hodge, J.; Wang, Y.; Yu, F.; Fan, D. Emodin Inhibits Breast Cancer Growth by Blocking the Tumor-Promoting Feedforward Loop between Cancer Cells and MacrophagesEmodin Blocks Cancer Cell–Macrophage Interaction. Mol. Cancer Ther. 2016, 15, 1931–1942. [Google Scholar] [CrossRef]
- Roopan, S.M.; Surendra, T.V.; Elango, G.; Kumar, S.H.S. Biosynthetic trends and future aspects of bimetallic nanoparticles and its medicinal applications. Appl. Microbiol. Biotechnol. 2014, 98, 5289–5300. [Google Scholar] [CrossRef]
- Wu, P.; Gao, Y.; Zhang, H.; Cai, C. Aptamer-guided silver–gold bimetallic nanostructures with highly active surface-enhanced raman scattering for specific detection and near-infrared photothermal therapy of human breast cancer cells. Anal. Chem. 2012, 84, 7692–7699. [Google Scholar] [CrossRef]
- Przystupski, D.; Niemczura, M.J.; Górska, A.; Supplitt, S.; Kotowski, K.; Wawryka, P.; Rozborska, P.; Woźniak, K.; Michel, O.; Kiełbik, A. In search of Panacea—Review of recent studies concerning nature-derived anticancer agents. Nutrients 2019, 11, 1426. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.-Y.; He, K.-M.; Chen, J.-L.; Xu, Y.-M.; Lau, A.T. Phytofabrication of nanoparticles as novel drugs for anticancer applications. Molecules 2019, 24, 4246. [Google Scholar] [CrossRef] [PubMed]
- Lohiya, G.; Katti, D.S. A synergistic combination of niclosamide and doxorubicin as an efficacious therapy for all clinical subtypes of breast cancer. Cancers 2021, 13, 3299. [Google Scholar] [CrossRef] [PubMed]
- Sartaj, A.; Baboota, S.; Ali, J. Assessment of Combination Approaches of Phytoconstituents with Chemotherapy for the Treatment of Breast Cancer: A Systematic Review. Curr. Pharm. Des. 2021, 27, 4630–4648. [Google Scholar] [CrossRef]
- Wala, K.; Szlasa, W.; Sauer, N.; Kasperkiewicz-Wasilewska, P.; Szewczyk, A.; Saczko, J.; Rembiałkowska, N.; Kulbacka, J.; Baczyńska, D. Anticancer Efficacy of 6-Gingerol with Paclitaxel against Wild Type of Human Breast Adenocarcinoma. Molecules 2022, 27, 2693. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, S.; Wang, Y.; Yu, Y.; Li, F.; Zhu, H.; Shen, Y.; Huang, S.; Guo, S. Near-infrared triggered co-delivery of doxorubicin and quercetin by using gold nanocages with tetradecanol to maximize anti-tumor effects on MCF-7/ADR cells. J. Colloid Interface Sci. 2018, 509, 47–57. [Google Scholar] [CrossRef] [PubMed]
- A Bioequivalence Study of Vinorelbine Tartrate Injectable Emulsion in Patients with Advanced Cancer. 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT00432562?term=NCT00432562&draw=2&rank=1 (accessed on 27 December 2022).
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef]
- Park, I.H.; Sohn, J.H.; Kim, S.B.; Lee, K.S.; Chung, J.S.; Lee, S.H.; Kim, T.Y.; Jung, K.H.; Cho, E.K.; Kim, Y.S. An open-label, randomized, parallel, phase III trial evaluating the efficacy and safety of polymeric micelle-formulated paclitaxel compared to conventional cremophor EL-based paclitaxel for recurrent or metastatic HER2-negative breast cancer. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2017, 49, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Prasanna, R.; Biswas, G.; Das Majumdar, S.K.; Joshi, N.; Bunger, D.; Khan, M.A.; Ahmad, I. Nanosomal docetaxel lipid suspension-based chemotherapy in breast cancer: Results from a multicenter retrospective study. Breast Cancer Targets Ther. 2020, 12, 77–85. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F.; Gouda, G.A.; Hassan, S.H. A review of green methods for phyto-fabrication of hematite (α-Fe2O3) nanoparticles and their characterization, properties, and applications. Heliyon 2021, 7, e05806. [Google Scholar] [CrossRef]
- Baghbani-Arani, F.; Movagharnia, R.; Sharifian, A.; Salehi, S.; Shandiz, S.A.S. Photo-catalytic, anti-bacterial, and anti-cancer properties of phyto-mediated synthesis of silver nanoparticles from Artemisia tournefortiana Rchb extract. J. Photochem. Photobiol. B Biol. 2017, 173, 640–649. [Google Scholar] [CrossRef]
- Singh, A.; Dar, M.Y.; Joshi, B.; Sharma, B.; Shrivastava, S.; Shukla, S. Phytofabrication of silver nanoparticles: Novel drug to overcome hepatocellular ailments. Toxicol. Rep. 2018, 5, 333–342. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Krishnamoorthy, V.; Jagadeesan, Y.; Vilwanathan, R.; Balaiah, A. Annona muricata assisted biogenic synthesis of silver nanoparticles regulates cell cycle arrest in NSCLC cell lines. Bioorg. Chem. 2020, 95, 103451. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Chaudhary, D.; Kumar, V.; Verma, A. Amelioration of diethylnitrosamine (DEN) induced renal oxidative stress and inflammation by Carissa carandas embedded silver nanoparticles in rodents. Toxicol. Rep. 2021, 8, 636–645. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Al-Megrin, W.A. Biological Potential of Silver Nanoparticles Mediated by Leucophyllum frutescens and Russelia equisetiformis Extracts. Nanomaterials 2021, 11, 2098. [Google Scholar] [CrossRef] [PubMed]
- Aabed, K.; Mohammed, A.E. Phytoproduct, Arabic Gum and Opophytum forsskalii Seeds for Bio-Fabrication of Silver Nanoparticles: Antimicrobial and Cytotoxic Capabilities. Nanomaterials 2021, 11, 2573. [Google Scholar] [CrossRef]
- Mirzaie, A.; Badmasti, F.; Dibah, H.; Hajrasouliha, S.; Yousefi, F.; Andalibi, R.; Kashtali, A.B.; Rezaei, A.H.; Bakhtiatri, R. Phyto-Fabrication of Silver Nanoparticles Using Typha azerbaijanensis Aerial Part and Root Extracts. Iran. J. Public Health 2022, 51, 1097. [Google Scholar] [CrossRef] [PubMed]
- Chandraker, S.K.; Lal, M.; Khanam, F.; Dhruve, P.; Singh, R.P.; Shukla, R. Therapeutic potential of biogenic and optimized silver nanoparticles using Rubia cordifolia L. leaf extract. Sci. Rep. 2022, 12, 1–15. [Google Scholar]
- Muhammad, W.; Khan, M.A.; Nazir, M.; Siddiquah, A.; Mushtaq, S.; Hashmi, S.S.; Abbasi, B.H. Papaver somniferum L. mediated novel bioinspired lead oxide (PbO) and iron oxide (Fe2O3) nanoparticles: In-vitro biological applications, biocompatibility and their potential towards HepG2 cell line. Mater. Sci. Eng. C 2019, 103, 109740. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Islam, M.; Tabassum, S.; Fernandes, N.F.; de Blanco, E.J.C.; Zia, M. Green synthesis of hematite (α-Fe2O3) nanoparticles using Rhus punjabensis extract and their biomedical prospect in pathogenic diseases and cancer. J. Mol. Struct. 2019, 1185, 1–7. [Google Scholar] [CrossRef]
- Rath, K.; Sen, S. Garlic extract based preparation of size controlled superparamagnetic hematite nanoparticles and their cytotoxic applications. Indian J. Biotechnol. 2019, 18, 108–118. [Google Scholar]
- Sharma, D.; Ledwani, L.; Mehrotra, T.; Kumar, N.; Pervaiz, N.; Kumar, R. Biosynthesis of hematite nanoparticles using Rheum emodi and their antimicrobial and anticancerous effects in vitro. J. Photochem. Photobiol. B Biol. 2020, 206, 111841. [Google Scholar] [CrossRef]
- Miri, A.; Khatami, M.; Sarani, M. Biosynthesis, magnetic and cytotoxic studies of hematite nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020, 30, 767–774. [Google Scholar] [CrossRef]
- Abbasi, B.A.; Iqbal, J.; Khan, Z.; Ahmad, R.; Uddin, S.; Shahbaz, A.; Zahra, S.A.; Shaukat, M.; Kiran, F.; Kanwal, S. Phytofabrication of cobalt oxide nanoparticles from Rhamnus virgata leaves extract and investigation of different bioactivities. Microsc. Res. Tech. 2021, 84, 192–201. [Google Scholar] [CrossRef]
- Hosny, M.; Eltaweil, A.S.; Mostafa, M.; El-Badry, Y.A.; Hussein, E.E.; Omer, A.M.; Fawzy, M. Facile synthesis of gold nanoparticles for anticancer, antioxidant applications, and photocatalytic degradation of toxic organic pollutants. ACS Omega 2022, 7, 3121–3133. [Google Scholar] [CrossRef]
- Vundela, S.R.; Kalagatur, N.K.; Nagaraj, A.; Kadirvelu, K.; Chandranayaka, S.; Kondapalli, K.; Hashem, A.; Abd Allah, E.F.; Poda, S. Multi-biofunctional properties of phytofabricated selenium nanoparticles from Carica papaya fruit extract: Antioxidant, antimicrobial, antimycotoxin, anticancer, and biocompatibility. Front. Microbiol. 2021, 12, 769891. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Al-Otaibi, W.A.; Saber, T.; AlMotwaa, S.M.; Alshallash, K.S.; Elhady, M.; Badr, N.F.; Abdel-Rahman, M.A. Antimicrobial, antiviral, and in-vitro cytotoxicity and mosquitocidal activities of Portulaca oleracea-based green synthesis of selenium nanoparticles. J. Funct. Biomater. 2022, 13, 157. [Google Scholar] [CrossRef] [PubMed]





| Cargo Loaded | CDNs Source | Preparation | Therapeutic Effect | References |
|---|---|---|---|---|
| Cucurbitacin B | MDA-MB-231 cells | Isolation, Bio fabrication | Metastasis inhibition | [57] |
| Paclitaxel | MDA-MB-231 cells | Isolation, Bio fabrication | Excellent antitumor activity | [58] |
| Withaferin A, anthocyanidins, and curcumin | Milk from Holstein and Jersey cows | Mixing | Inhibits inflammation | [59] |
| Black bean-derived phytoconstituents | Human mammary (MCF7), prostate (PC3), colon (Caco2), and liver (HepG2) cells | Electroporation | Induces cell death and cell cycle arrest | [60] |
| Berry-derived anthocyanidins | Raw milk from pasteurized Jersey cows | Simple mixing | Inhibits proliferation and inflammation | [61] |
| Honokiol (extracted from Magnolia plant) | Mesenchymal stem cells | Sonication | Inhibits cell cycle arrest and apoptosis | [62] |
| Sr. No. | Patent | Nanoparticle | Remarks | Inventor(s) |
|---|---|---|---|---|
| 1 | US10201622B2 | Magnetic core Gd-chelates | Target-Matrix metalloproteinases 14 (MMP-14) Imaging-MRI | Paul Loadman, Robert Falconer, Jason Gill, Jianghong Rao, Heike E. Daldrup-Link |
| 2 | WO2015014756A1 | Magnetic core Gd-chelates | Target-Matrix metalloproteinases 14 (MMP-14) Imaging-MRI | Paul Loadman, Robert Falconer, Jason Gill, Jianghong Rao, Heike E. Daldrup-Link |
| 3 | CN104225595A | Aptamer (Cell SELEX) | Target-MDA-MB-231 breast cancer cell Imaging-near Infrared | Ju Yu Xiantian Jiang Wei Ding Lin Yu Junsheng Shen Zhen |
| 4 | US20150160222A1 | Not clarified | Target-SET/KifC1 | Ritu Aneja, Padmashree C.G. Rida |
| 5 | US9675714B1 | Chitosan functionalized 2D graphene sheets Superparamagnetic iron oxide | Imaging-Nuclear magnetic resonance (NMR) | Subhra Mohapatra, Chunyan Wang |
| 6 | US20130323165A1 | Magnetic cationic liposomal nanoparticles | Imaging-PET, MRI | Robert B. Campbell, Srinivas Sridhar |
| Phytochemical Constituent | Anticancer Agent | Nanocarrier | Condition | Remarks | Reference |
|---|---|---|---|---|---|
| 6-Gingerol | Paclitaxel | PEGylated naniosome | In vivo | Increased the effectiveness of paclitaxel, and lower dose of paclitaxel is needed for the anti-neoplastic activity. | [146] |
| Quercetin | Doxorubicin | Lecithin | In vivo | Prevents doxorubicin resistance in tumor cells and increases drug absorption and toxicities in malignant cells. | [110] |
| Quercetin | Doxorubicin | Au nanocages | In vitro | Gives synergistic effect by retaining the drug for longer period of time in malignant cells. | [147] |
| Phytochemical Constituent-Based Drug | Nanocarrier | Phase of Clinical Trial | Condition | Remarks | References |
|---|---|---|---|---|---|
| Vinorelbine tartrate | Liposomal vinorelbine tartrate | Phase 1 | Breast cancer | Inhibits microtubule polymerization and promotes cell apoptosis. | [148] |
| Paclitaxel | Albumin-stabilized paclitaxel | Phase 3 | Metastatic breast cancer | Less exposure of toxic cremophor of the drug to non-cancerous cells thus enables higher dosing and improves paclitaxel penetration inside the cancer cells | [149] |
| Paclitaxel-loaded polymeric nanoparticles | Phase 4 | Breast cancer | Increased blood stability and tumor-specific action by releasing drug inside tumor cells via a PH-sensitive action | [150] | |
| Docetaxel | Nanosomal docetaxel lipid suspension | Phase 3 | Breast cancer | Better stability, lower cytotoxicity to normal cells and easily pass-through leaky vasculature of tumor blood vessels | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavda, V.P.; Nalla, L.V.; Balar, P.; Bezbaruah, R.; Apostolopoulos, V.; Singla, R.K.; Khadela, A.; Vora, L.; Uversky, V.N. Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer. Cancers 2023, 15, 1023. https://doi.org/10.3390/cancers15041023
Chavda VP, Nalla LV, Balar P, Bezbaruah R, Apostolopoulos V, Singla RK, Khadela A, Vora L, Uversky VN. Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer. Cancers. 2023; 15(4):1023. https://doi.org/10.3390/cancers15041023
Chicago/Turabian StyleChavda, Vivek P., Lakshmi Vineela Nalla, Pankti Balar, Rajashri Bezbaruah, Vasso Apostolopoulos, Rajeev K. Singla, Avinash Khadela, Lalitkumar Vora, and Vladimir N. Uversky. 2023. "Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer" Cancers 15, no. 4: 1023. https://doi.org/10.3390/cancers15041023
APA StyleChavda, V. P., Nalla, L. V., Balar, P., Bezbaruah, R., Apostolopoulos, V., Singla, R. K., Khadela, A., Vora, L., & Uversky, V. N. (2023). Advanced Phytochemical-Based Nanocarrier Systems for the Treatment of Breast Cancer. Cancers, 15(4), 1023. https://doi.org/10.3390/cancers15041023











