Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Western Blot Analysis
2.3. Cell Viability Assays
2.4. Determination of Apoptosis by Muse Annexin V & Dead Cell Assay
2.5. TUNEL Assay
2.6. RNA Extraction, Reverse Transcription and Real-Time PCR
- ERRα:
- CYCLIN D1:
- HK1:
- HK2:
- PFKL:
- LDHA:
- ACO1:
- ATP5F1B:
- SUCLG2:
- IDH2:
- GPT:
- SLC1A5:
- GLS1:
- 18S:
2.7. Wound Healing Assay
2.8. Boyden Chamber Assay
2.9. Intracellular Cholesterol Extraction and Colorimetric Cholesterol Assay
2.10. Seahorse XFe96 Metabolic Flux Analysis
2.10.1. Mitochondrial Stress Analysis
2.10.2. Glycolytic Stress Analysis
2.11. Statistical Analysis
3. Results
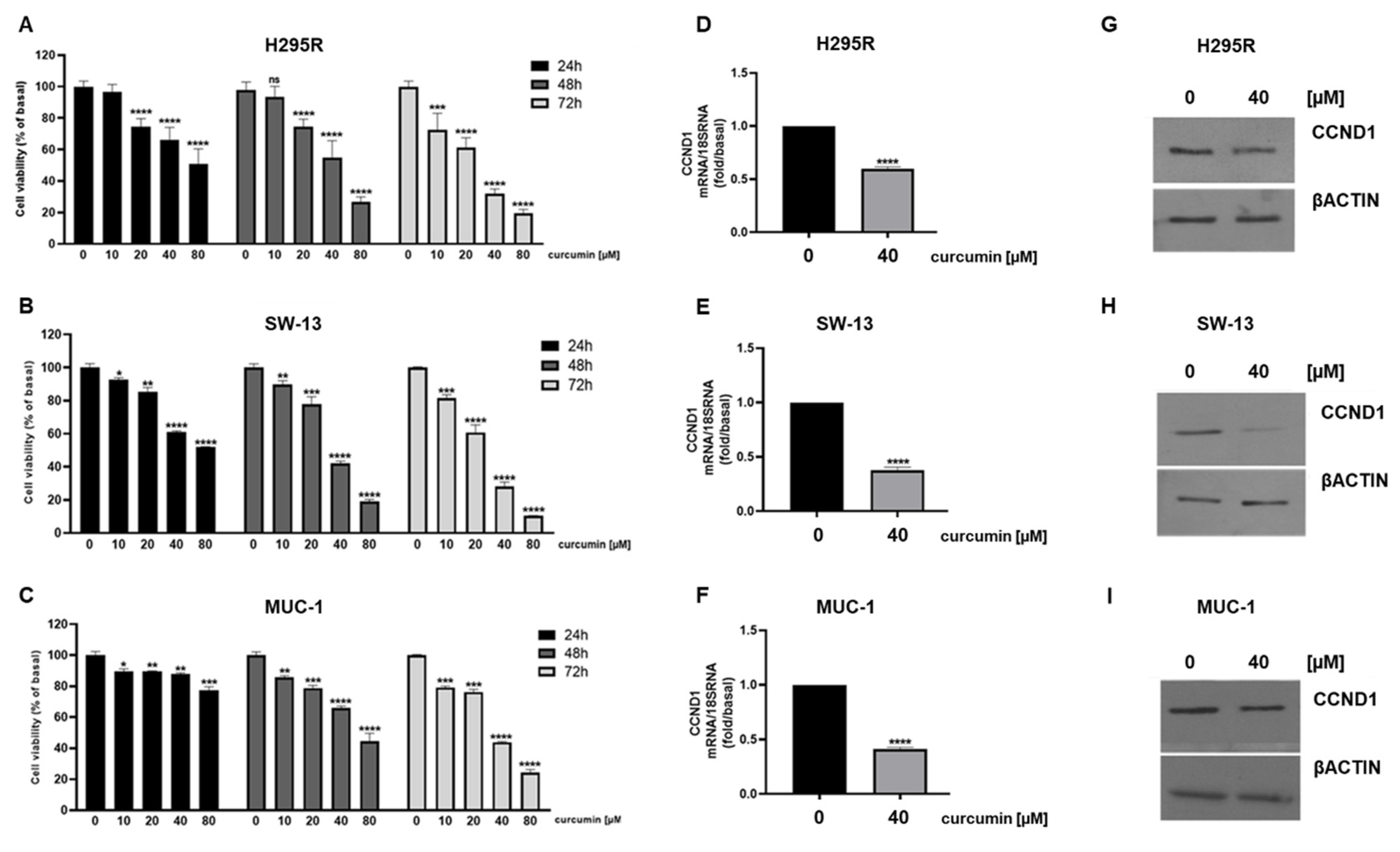
3.1. Curcumin Reduces Viability and Induces Apoptosis in ACC Cells
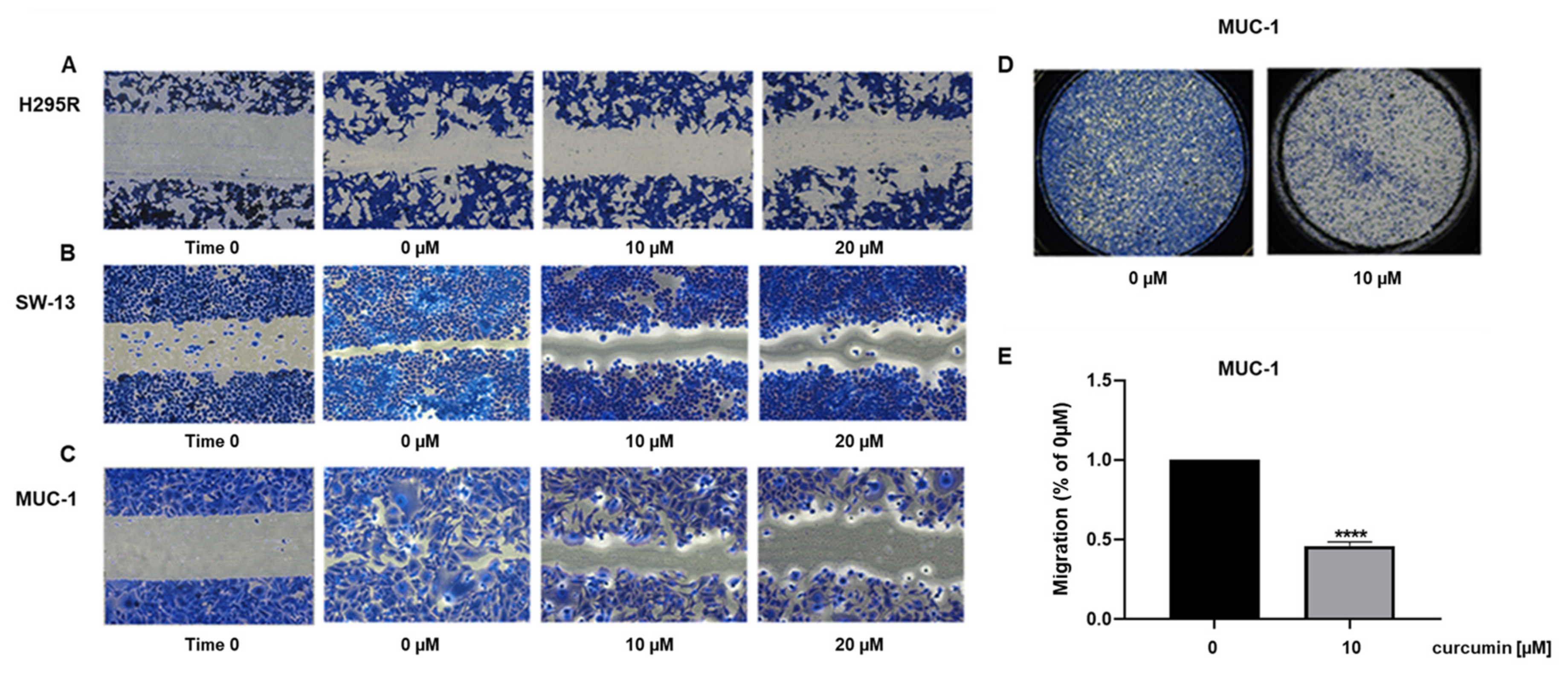
3.2. Curcumin Reduces ACC Cell Motility
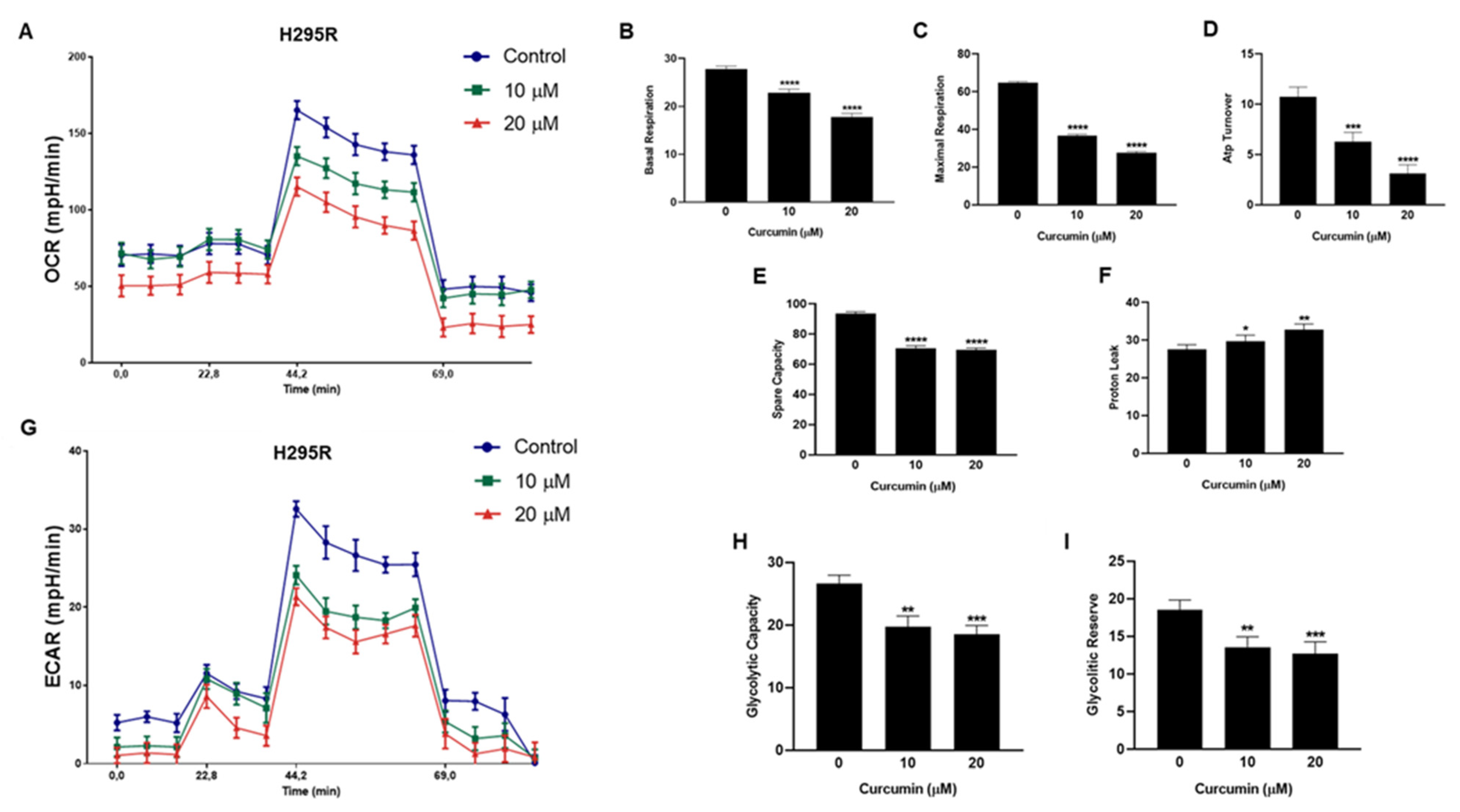
3.3. Metabolic Effects of Curcumin on H295R Cells
3.4. Curcumin Reduces ERRα Expression and Cholesterol Content in ACC Cells
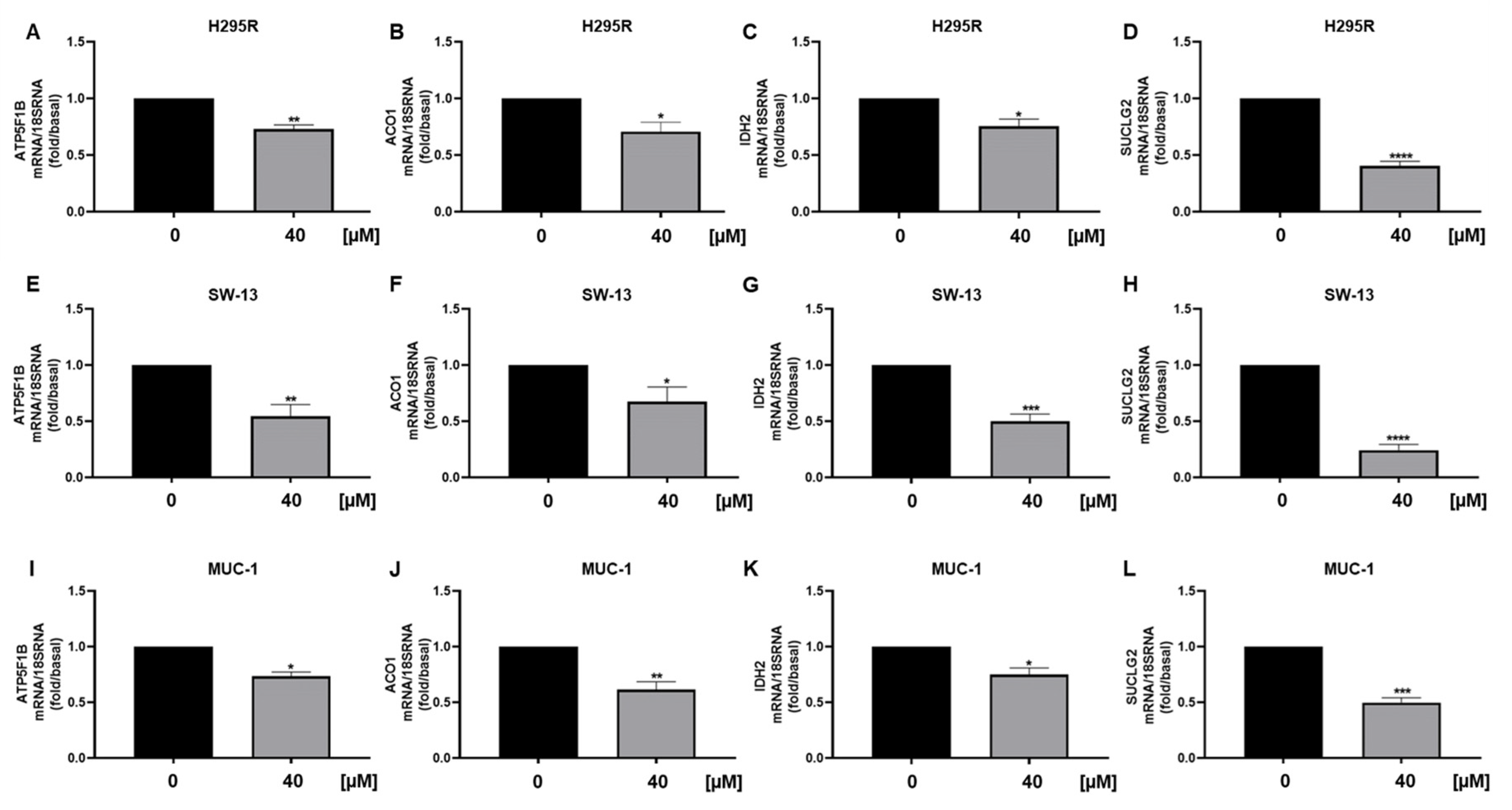
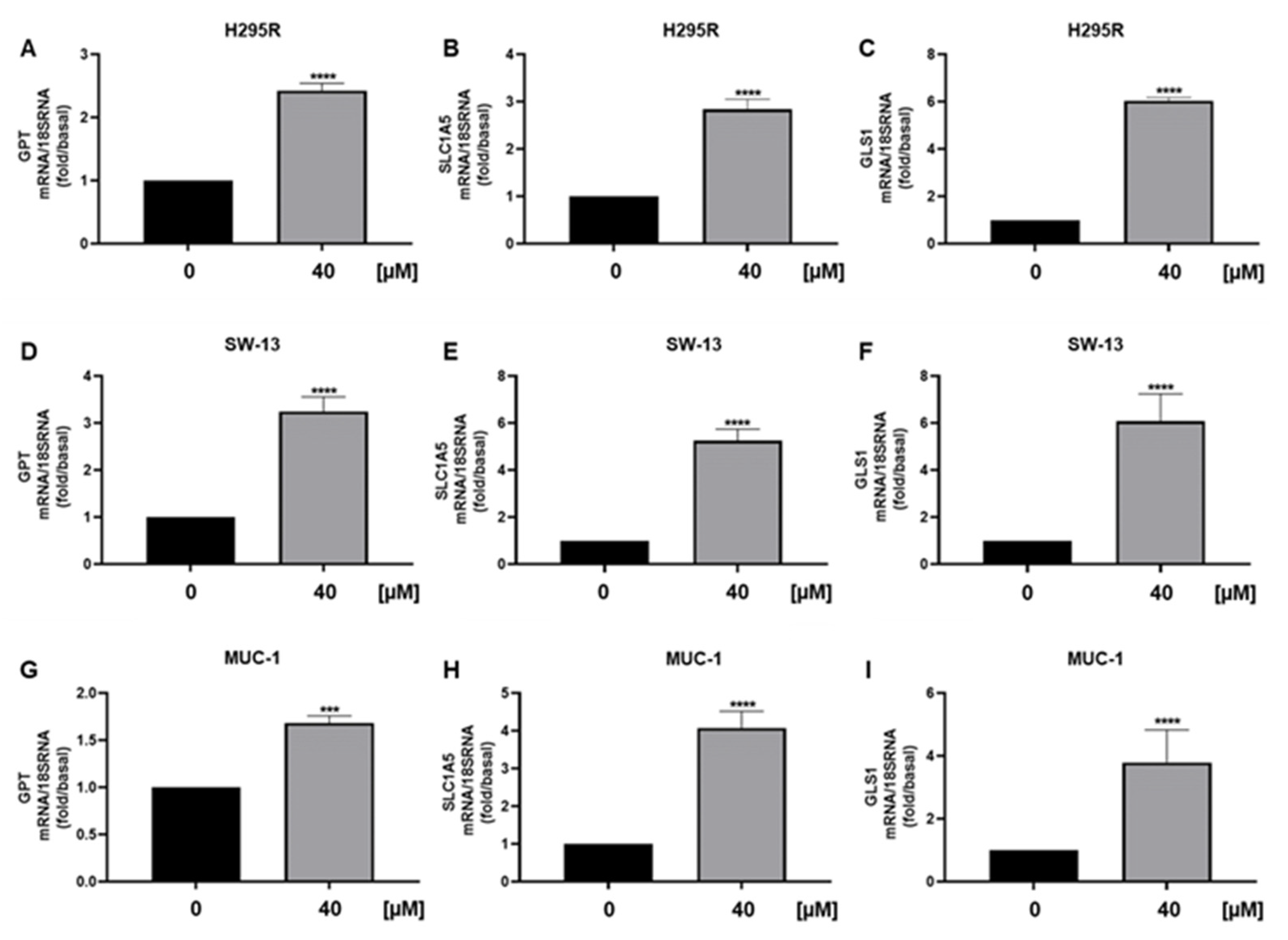
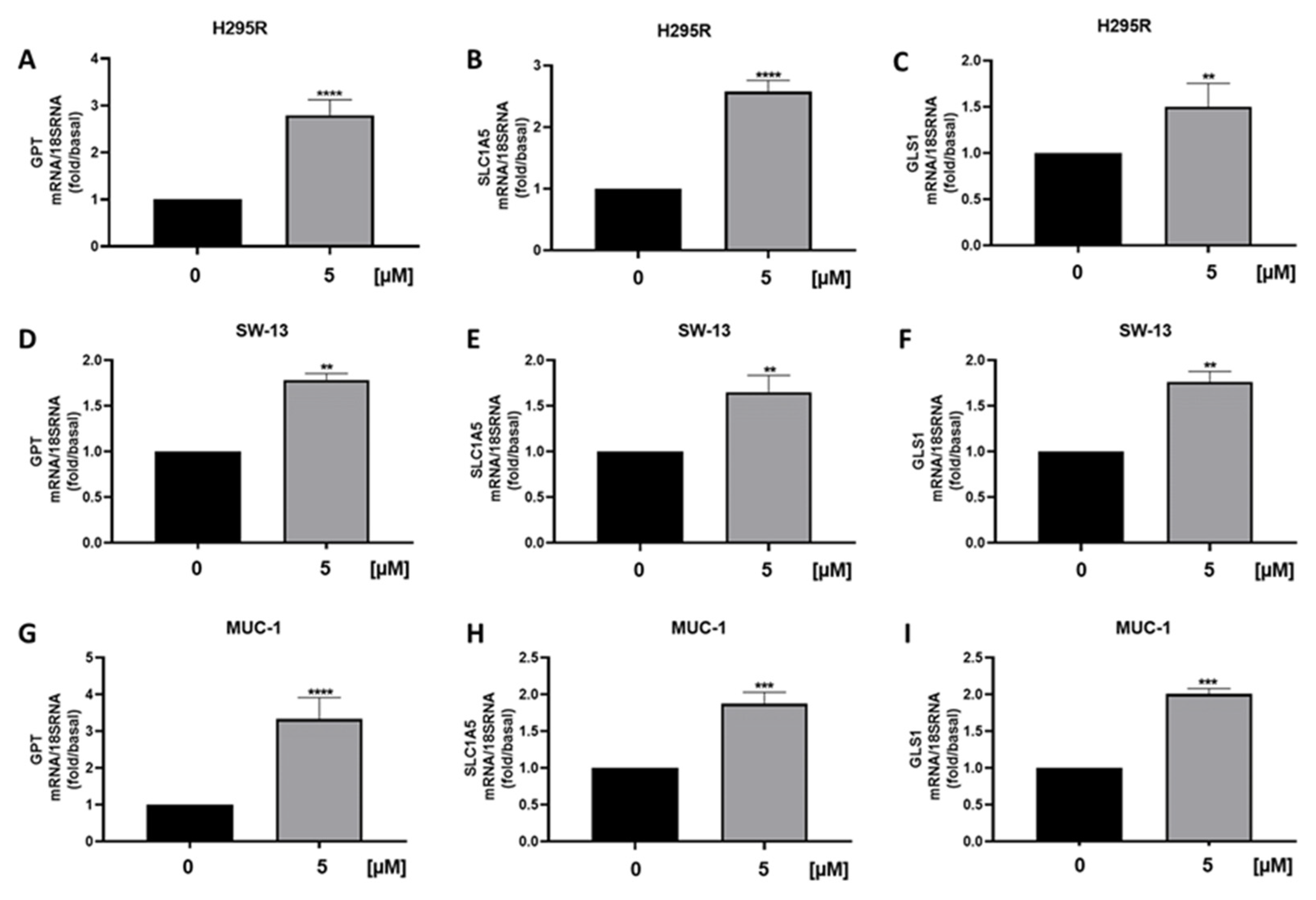
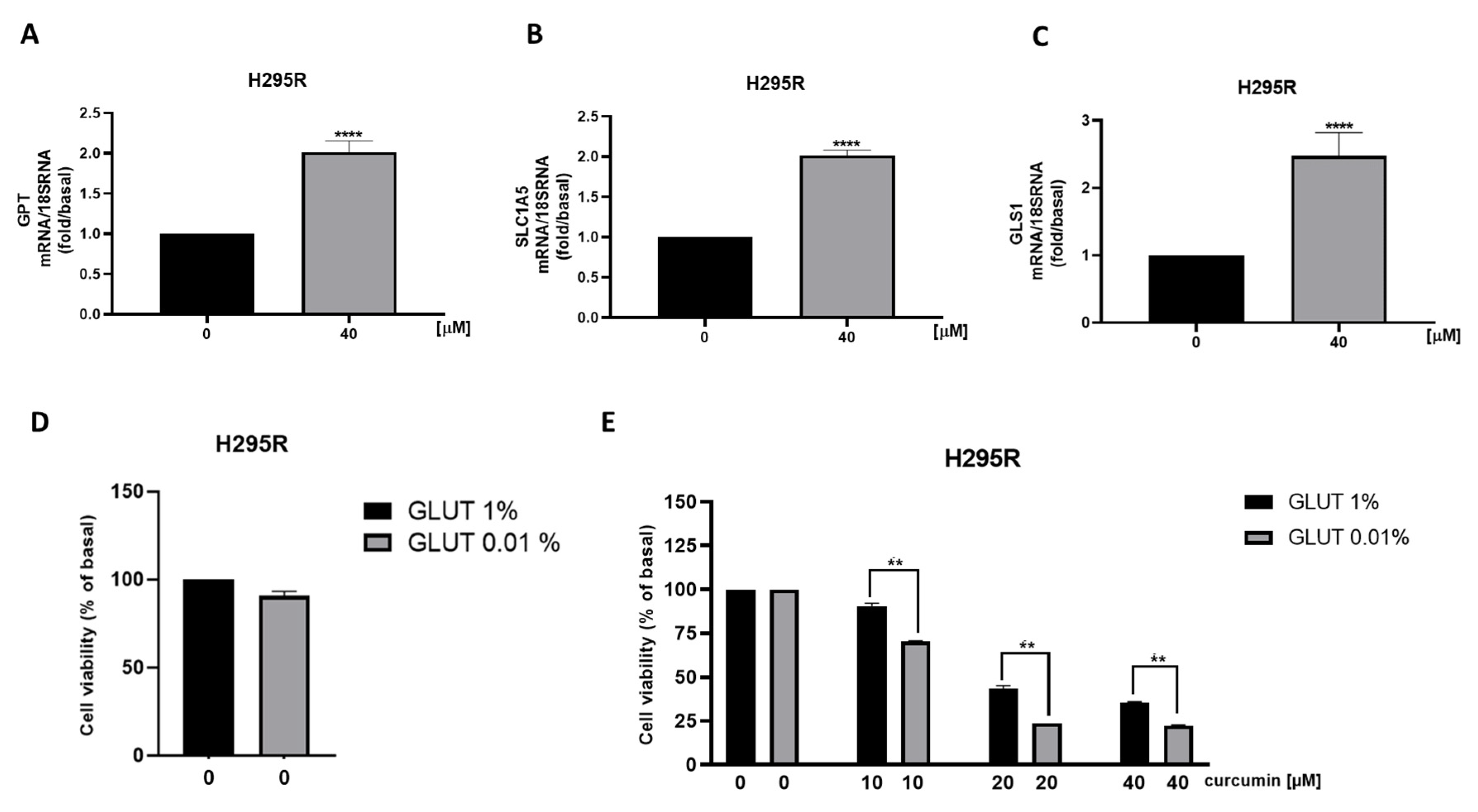
3.5. Metabolic Genes in ACC Cells Are Affected by Curcumin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical Carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef]
- Casaburi, I.; Avena, P.; de Luca, A.; Chimento, A.; Sirianni, R.; Malivindi, R.; Rago, V.; Fiorillo, M.; Domanico, F.; Campana, C.; et al. Estrogen Related Receptor α (ERRα) a Promising Target for the Therapy of Adrenocortical Carcinoma (ACC). Oncotarget 2015, 6, 25135–25148. [Google Scholar] [CrossRef] [PubMed]
- Avena, P.; de Luca, A.; Chimento, A.; Nocito, M.C.; Sculco, S.; la Padula, D.; Zavaglia, L.; Giulietti, M.; Hantel, C.; Sirianni, R.; et al. Estrogen Related Receptor Alpha (ERRα) a Bridge between Metabolism and Adrenocortical Cancer Progression. Cancers 2022, 14, 3885. [Google Scholar] [CrossRef]
- Sancho, P.; Barneda, D.; Heeschen, C. Hallmarks of Cancer Stem Cell Metabolism. Br. J. Cancer 2016, 114, 1305–1312. [Google Scholar] [CrossRef]
- Michalek, R.D.; Gerriets, V.A.; Nichols, A.G.; Inoue, M.; Kazmin, D.; Chang, C.Y.; Dwyer, M.A.; Nelson, E.R.; Pollizzi, K.N.; Ilkayeva, O.; et al. Estrogen-Related Receptor-α Is a Metabolic Regulator of Effector T-Cell Activation and Differentiation. Proc. Natl. Acad. Sci. USA 2011, 108, 18348–18353. [Google Scholar] [CrossRef]
- Chisamore, M.J.; Wilkinson, H.A.; Flores, O.; Chen, J.D. Estrogen-Related Receptor-Alpha Antagonist Inhibits Both Estrogen Receptor-Positive and Estrogen Receptor-Negative Breast Tumor Growth in Mouse Xenografts. Mol. Cancer 2009, 8, 672–681. [Google Scholar] [CrossRef]
- Kokabu, T.; Mori, T.; Matsushima, H.; Yoriki, K.; Kataoka, H.; Tarumi, Y.; Kitawaki, J. Antitumor Effect of XCT790, an ERRα Inverse Agonist, on ERα-Negative Endometrial Cancer Cells. Cell Oncol. 2019, 42, 223–235. [Google Scholar] [CrossRef]
- Nocito, M.C.; de Luca, A.; Prestia, F.; Avena, P.; la Padula, D.; Zavaglia, L.; Sirianni, R.; Casaburi, I.; Puoci, F.; Chimento, A.; et al. Antitumoral Activities of Curcumin and Recent Advances to Improve Its Oral Bioavailability. Biomedicines 2021, 9, 1476. [Google Scholar] [CrossRef]
- Trujillo, J.; Granados-Castro, L.F.; Zazueta, C.; Andérica-Romero, A.C.; Chirino, Y.I.; Pedraza-Chaverrí, J. Mitochondria as a Target in the Therapeutic Properties of Curcumin. Arch. Pharm. 2014, 347, 873–884. [Google Scholar] [CrossRef]
- Jung, K.H.; Lee, J.H.; Park, J.W.; Moon, S.H.; Cho, Y.S.; Choe, Y.S.; Lee, K.H. Effects of Curcumin on Cancer Cell Mitochondrial Function and Potential Monitoring with 18F-FDG Uptake. Oncol. Rep. 2016, 35, 861–868. [Google Scholar] [CrossRef]
- Chen, P.; Wang, H.; Yang, F.; Chen, H.; He, W.; Wang, J. Curcumin Promotes Osteosarcoma Cell Death by Activating MiR-125a/ERRα Signal Pathway. J. Cell Biochem. 2017, 118, 74–81. [Google Scholar] [CrossRef]
- Qin, S.; Huang, L.; Gong, J.; Shen, S.; Huang, J.; Ren, H.; Hu, H. Efficacy and Safety of Turmeric and Curcumin in Lowering Blood Lipid Levels in Patients with Cardiovascular Risk Factors: A Meta-Analysis of Randomized Controlled Trials. Nutr. J. 2017, 16, 68. [Google Scholar] [CrossRef]
- Yuan, H.-Y.; Kuang, S.-Y.; Zheng, X.; Ling, H.-Y.; Yang, Y.-B.; Yan, P.-K.; Li, K.; Liao, D.-F. Curcumin Inhibits Cellular Cholesterol Accumulation by Regulating SREBP-1/Caveolin-1 Signaling Pathway in Vascular Smooth Muscle Cells 1. Acta Pharm. Sin. 2008, 29, 555–563. [Google Scholar] [CrossRef]
- Wei, W.; Schwaid, A.G.; Wang, X.; Chu, Q.; Saghatelian, A.; Wan, Y.; Wang, X.; Chen, S. Ligand Activation of ERRα by Cholesterol Mediates Statin and Bisphosphonate Effects. Cell Metab. 2016, 23, 479–491. [Google Scholar] [CrossRef]
- Trotta, F.; Avena, P.; Chimento, A.; Rago, V.; de Luca, A.; Sculco, S.; Nocito, M.C.; Malivindi, R.; Fallo, F.; Pezzani, R.; et al. Statins Reduce Intratumor Cholesterol Affecting Adrenocortical Cancer Growth. Mol. Cancer 2020, 19, 1909–1921. [Google Scholar] [CrossRef]
- Park, S.; Chang, C.Y.; Safi, R.; Liu, X.; Baldi, R.; Jasper, J.S.; Anderson, G.R.; Liu, T.; Rathmell, J.C.; Dewhirst, M.W.; et al. ERRα Regulated Lactate Metabolism Contributes to Resistance to Targeted Therapies in Breast Cancer. Cell Rep. 2016, 15, 323. [Google Scholar] [CrossRef]
- Stein, R.A.; McDonnell, D.P. Estrogen-Related Receptor α as a Therapeutic Target in Cancer. Endocr. Relat. Cancer 2006, 13, S25–S32. [Google Scholar] [CrossRef]
- Castaño, P.R.; Parween, S.; Pandey, A.V. Bioactivity of Curcumin on the Cytochrome P450 Enzymes of the Steroidogenic Pathway. Int. J. Mol. Sci. 2019, 20, 4606. [Google Scholar] [CrossRef]
- Sirianni, R.; Zolea, F.; Chimento, A.; Ruggiero, C.; Cerquetti, L.; Fallo, F.; Pilon, C.; Arnaldi, G.; Carpinelli, G.; Stigliano, A.; et al. Targeting Estrogen Receptor-α Reduces Adrenocortical Cancer (ACC) Cell Growth in Vitro and in Vivo: Potential Therapeutic Role of Selective Estrogen Receptor Modulators (SERMs) for ACC Treatment. J. Clin. Endocrinol. Metab. 2012, 97, E2238–E2250. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, K.; Li, Y.; Guo, R.; Zhang, K.; Zhong, G.; He, Q. Oestrogen-Related Receptor Alpha Mediates Chemotherapy Resistance of Osteosarcoma Cells via Regulation of ABCB1. J. Cell. Mol. Med. 2019, 23, 2115–2124. [Google Scholar] [CrossRef]
- Wang, J.B.; Qi, L.L.; di Zheng, S.; Wu, T.X. Curcumin Induces Apoptosis through the Mitochondria-Mediated Apoptotic Pathway in HT-29 Cells. J. Zhejiang Univ. Sci. B 2009, 10, 93. [Google Scholar] [CrossRef]
- Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Khan, A.Q.; Ahmed, E.I.; Akhtar, S.; Ali, T.A.; Merhi, M.; Dermime, S.; Steinhoff, M.; et al. Curcumin Induces Apoptotic Cell Death via Inhibition of PI3-Kinase/Akt Pathway in B-Precursor Acute Lymphoblastic Leukemia. Front. Oncol. 2019, 9, 484. [Google Scholar] [CrossRef]
- Endo, H.; Inoue, I.; Masunaka, K.; Tanaka, M.; Yano, M. Curcumin Induces Apoptosis in Lung Cancer Cells by 14-3-3 Protein-Mediated Activation of Bad. Biosci. Biotechnol. Biochem. 2020, 84, 2440–2447. [Google Scholar] [CrossRef]
- Bothou, C.; Sharma, A.; Oo, A.; Kim, B.; Perge, P.; Igaz, P.; Ronchi, C.L.; Shapiro, I.; Hantel, C. Novel Insights into the Molecular Regulation of Ribonucleotide Reductase in Adrenocortical Carcinoma Treatment. Cancers 2021, 13, 4200. [Google Scholar] [CrossRef]
- Abate, A.; Tamburello, M.; Rossini, E.; Basnet, R.M.; Ribaudo, G.; Gianoncelli, A.; Hantel, C.; Cosentini, D.; Laganà, M.; Grisanti, S.; et al. Trabectedin Impairs Invasiveness and Metastasis in Adrenocortical Carcinoma Preclinical Models. Endocr. Relat. Cancer 2022, 30, e220273. [Google Scholar] [CrossRef]
- Ma, J.H.; Qi, J.; Lin, S.Q.; Zhang, C.Y.; Liu, F.Y.; Xie, W.D.; Li, X. STAT3 Targets ERR-α to Promote Epithelial-Mesenchymal Transition, Migration, and Invasion in Triple-Negative Breast Cancer Cells. Mol. Cancer Res. 2019, 17, 2184–2195. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Shang, J.; Zhaang, Z.; Cui, B.; Lin, Y.; Yang, Y.; Song, Y.; Yu, S.; Xia, J. Estrogen Related Receptor Alpha Triggers the Migration and Invasion of Endometrial Cancer Cells via up Regulation of TGFB1. Cell Adh. Migr. 2018, 12, 538–547. [Google Scholar] [CrossRef]
- Zhong, Y.; He, K.; Shi, L.; Chen, L.; Zhou, B.; Ma, R.; Yu, H.; Zhang, J.; Shuai, Y.; Fei, Y.; et al. Down-Regulation of Estrogen-Related Receptor Alpha (ERRα) Inhibits Gastric Cancer Cell Migration and Invasion in Vitro and in Vivo. Aging 2021, 13, 5845–5857. [Google Scholar] [CrossRef]
- Ye, X.; Guo, J.; Zhang, H.; Meng, Q.; Ma, Y.; Lin, R.; Yi, X.; Lu, H.; Bai, X.; Cheng, J. The Enhanced Expression of Estrogen-Related Receptor α in Human Bladder Cancer Tissues and the Effects of Estrogen-Related Receptor α Knockdown on Bladder Cancer Cells. J. Cell. Biochem. 2019, 120, 13841–13852. [Google Scholar] [CrossRef]
- Mukherjee, T.K.; Malik, P.; Hoidal, J.R. The Emerging Role of Estrogen Related Receptorα in Complications of Non-Small Cell Lung Cancers. Oncol. Lett. 2021, 21, 258. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Du, Z.; Li, G.; Chen, M.; Chen, X.; Liang, G.; Chen, T. Curcumin Suppresses Gastric Tumor Cell Growth via ROS-Mediated DNA Polymerase γ Depletion Disrupting Cellular Bioenergetics. J. Exp. Clin. Cancer Res. 2017, 36, 1–14. [Google Scholar] [CrossRef]
- Huang, M.; Xiong, H.; Luo, D.; Xu, B.; Liu, H. CSN5 Upregulates Glycolysis to Promote Hepatocellular Carcinoma Metastasis via Stabilizing the HK2 Protein. Exp. Cell Res. 2020, 388, 111876. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fan, H.; Chen, Q.; Ma, G.; Zhu, M.; Zhang, X.; Zhang, Y.; Yu, J. Curcumin Inhibits Aerobic Glycolysis and Induces Mitochondrial-Mediated Apoptosis through Hexokinase II in Human Colorectal Cancer Cells in Vitro. Anticancer Drugs 2015, 26, 15–24. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Garcia-Smith, R.; Dorsey, J.; Griffith, J.K.; Bisoffi, M.; Trujillo, K.A. Tumor Necrosis Factor Alpha Induces Warburg-like Metabolism and Is Reversed by Anti-Inflammatory Curcumin in Breast Epithelial Cells. Int. J. Cancer 2013, 133, 2504–2510. [Google Scholar] [CrossRef]
- Liao, H.; Wang, Z.; Deng, Z.; Ren, H.; Li, X. Curcumin Inhibits Lung Cancer Invasion and Metastasis by Attenuating GLUT1/MT1-MMP/MMP2 Pathway. Int. J. Clin. Exp. Med. 2015, 8, 8948. [Google Scholar] [PubMed]
- Monteleone, F.; Taverna, S.; Alessandro, R.; Fontana, S. SWATH-MS Based Quantitative Proteomics Analysis Reveals That Curcumin Alters the Metabolic Enzyme Profile of CML Cells by Affecting the Activity of MiR-22/IPO7/HIF-1α Axis. J. Exp. Clin. Cancer Res. 2018, 37, 170. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Wang, Y.; Yu, T.; Zhu, C.; Zhang, X.; Guan, J. Curcumin Suppresses Tumorigenesis by Ferroptosis in Breast Cancer. PLoS ONE 2022, 17, e0261370. [Google Scholar] [CrossRef]
- Zhu, L.; Ploessl, K.; Zhou, R.; Mankoff, D.; Kung, H.F. Metabolic Imaging of Glutamine in Cancer. J. Nucl. Med. 2017, 58, 533–537. [Google Scholar] [CrossRef]
- Ganji, S.K.; An, Z.; Tiwari, V.; McNeil, S.; Pinho, M.C.; Pan, E.; Mickey, B.E.; Maher, E.A.; Choi, C. In Vivo Detection of 2-Hydroxyglutarate in Brain Tumors by Optimized Point-Resolved Spectroscopy (PRESS) at 7T. Magn. Reson. Med. 2017, 77, 936–944. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocito, M.C.; Avena, P.; Zavaglia, L.; De Luca, A.; Chimento, A.; Hamad, T.; La Padula, D.; Stancati, D.; Hantel, C.; Sirianni, R.; et al. Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers 2023, 15, 1050. https://doi.org/10.3390/cancers15041050
Nocito MC, Avena P, Zavaglia L, De Luca A, Chimento A, Hamad T, La Padula D, Stancati D, Hantel C, Sirianni R, et al. Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers. 2023; 15(4):1050. https://doi.org/10.3390/cancers15041050
Chicago/Turabian StyleNocito, Marta Claudia, Paola Avena, Lucia Zavaglia, Arianna De Luca, Adele Chimento, Tarig Hamad, Davide La Padula, Davide Stancati, Constanze Hantel, Rosa Sirianni, and et al. 2023. "Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment" Cancers 15, no. 4: 1050. https://doi.org/10.3390/cancers15041050
APA StyleNocito, M. C., Avena, P., Zavaglia, L., De Luca, A., Chimento, A., Hamad, T., La Padula, D., Stancati, D., Hantel, C., Sirianni, R., Casaburi, I., & Pezzi, V. (2023). Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers, 15(4), 1050. https://doi.org/10.3390/cancers15041050






