The Role of Peritumoral Depapillation and Its Impact on Narrow-Band Imaging in Oral Tongue Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
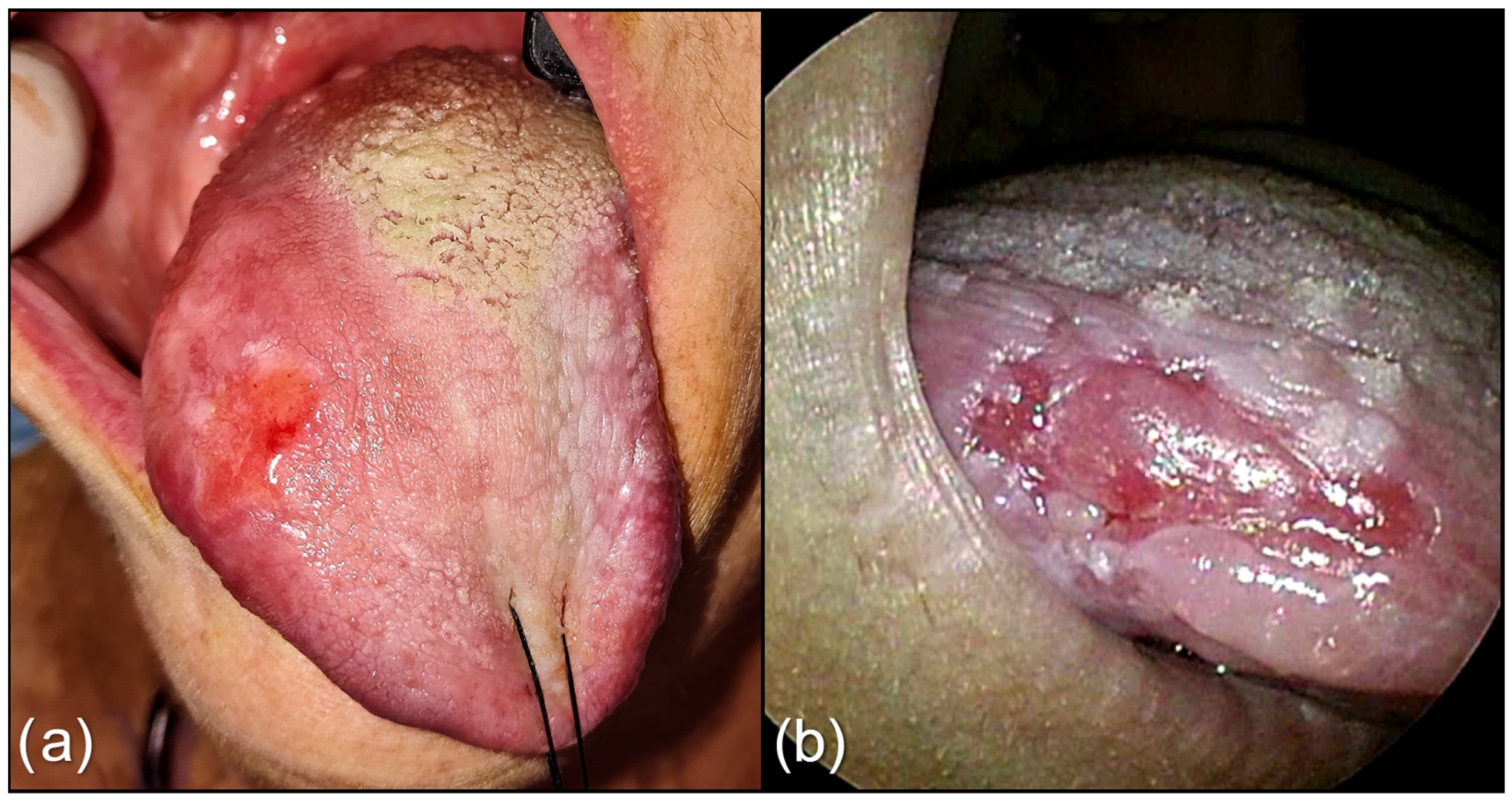
2.1. Study Design
2.2. Treatment Protocol
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Firth, A.U.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef]
- Xu, B.; Salama, A.M.; Valero, C.; Yuan, A.; Khimraj, A.; Saliba, M.; Zanoni, D.K.; Ganly, I.; Patel, S.G.; Katabi, N.; et al. The prognostic role of histologic grade, worst pattern of invasion, and tumor budding in early oral tongue squamous cell carcinoma: A comparative study. Virchows Arch. 2021, 479, 597–606. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Iandelli, A.; Marchi, F.; Huang, Y.; Tai, S.F.; Hung, S.Y.; Kao, H.; Chang, K. The Prognostic Value of Lymph Node Burden in Oral Cavity Cancer: Systematic Review and Meta-Analysis. Laryngoscope 2022, 132, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Thankappan, K.; Battoo, A.J.; Rajapurkar, M.; Kuriakose, M.A.; Iyer, S. Isolated skip nodal metastasis is rare in T1 and T2 oral tongue squamous cell carcinoma. Otolaryngol. –Head Neck Surg. 2012, 147, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Riemann, M.; Knipfer, C.; Rohde, M.; Adler, W.; Schuster, M.; Noeth, E.; Oetter, N.; Shams, N.; Neukam, F.-W.; Stelzle, F. Oral squamous cell carcinoma of the tongue: Prospective and objective speech evaluation of patients undergoing surgical therapy. Head Neck 2016, 38, 993–1001. [Google Scholar] [CrossRef]
- Rogers, S.N.; Brown, J.S.; Woolgar, J.A.; Lowe, D.; Magennis, P.; Shaw, R.J.; Sutton, D.; Errington, D.; Vaughan, D. Survival following primary surgery for oral cancer. Oral Oncol. 2009, 45, 201–211. [Google Scholar] [CrossRef]
- Brierley, J.; Gospodarowicz, M.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Union for International Cancer Control: Geneva, Switzerland, 2016. [Google Scholar]
- Iandelli, A.; Marchi, F.; Chen, A.-C.; Young, C.-K.; Liao, C.-T.; Tsao, C.-K.; Kang, C.-J.; Wang, H.-M.; Chang, T.-C.J.; Huang, S.-F. Adequacy of Disease Control by Supraomohyoid Neck Dissection in cT1/T2 Tongue Cancer. J. Pers. Med. 2022, 12, 1535. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.M.V.O.; Dedivitis, R.A.; Kowalski, L.P. Prognostic impact of perineural invasion in oral cancer: A systematic review. Acta Otorhinolaryngol. Ital. 2022, 42, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.S. Pathology of Perineural Spread. J. Neurol.Surg. Part B Skull Base 2016, 77, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.-K.; Li, W.-Y.; Chu, P.-Y.; Chang, S.-Y.; Tsai, T.-L.; Wang, Y.-F.; Huang, J.-L. Risks and clinical implications of perineural invasion in T1-2 oral tongue squamous cell carcinoma. Head Neck 2012, 34, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Erriu, M.; Pili, F.M.G.; Cadoni, S.; Garau, V. Diagnosis of Lingual Atrophic Conditions: Associations with Local and Systemic Factors. A Descriptive Review. Open Dent. J. 2016, 10, 619–635. [Google Scholar]
- Rahima, B.; Shingaki, S.; Nagata, M.; Saito, C. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2004, 97, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Mair, M.; Singhvi, H.; Mishra, A.; Nair, S.; Agrawal, J.; Chaturvedi, P. Perineural invasion: Independent prognostic factor in oral cancer that warrants adjuvant treatment. Head Neck 2018, 40, 1780–1787. [Google Scholar] [CrossRef]
- Singh, A.; Singhavi, H.; Sathe, P.; Mair, M.; Qayyumi, B.; Shetty, R.; Bal, M.; Joshi, P.; Nair, S.V.; Chaturvedi, P. The impact of peritumoral depapillation in cancers of the tongue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.H.; Yakushiji, T.; Kamiyama, I.; Nomura, T.; Katakura, A.; Takano, N.; Shibahara, T. Detecting early oral cancer: Narrowband imaging system observation of the oral mucosa microvasculature. Int. J. Oral Maxillofac. Surg. 2010, 39, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.; Farah, C.S. Narrow band imaging: Clinical applications in oral and oropharyngeal cancer. Oral Dis. 2016, 22, 383–390. [Google Scholar] [CrossRef]
- Piazza, C.; Cocco, D.; del Bon, F.; Mangili, S.; Nicolai, P.; Majorana, A.; Villaret, A.B.; Peretti, G. Narrow band imaging and high definition television in evaluation of oral and oropharyngeal squamous cell cancer: A prospective study. Oral Oncol. 2010, 46, 307–310. [Google Scholar] [CrossRef]
- Farah, C.S.; Dalley, A.J.; Nguyen, P.; Batstone, M.; Kordbacheh, F.; Perry-Keene, J.; Fielding, D. Improved surgical margin definition by narrow band imaging for resection of oral squamous cell carcinoma: A prospective gene expression profiling study. Head Neck 2016, 38, 832–839. [Google Scholar] [CrossRef]
- Tirelli, G.; Piovesana, M.; Gatto, A.; Tofanelli, M.; Biasotto, M.; Boscolo Nata, F. Narrow band imaging in the intra-operative defini-tion of resection margins in oral cavity and oropharyngeal cancer. Oral Oncol. 2015, 51, 908–913. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wang, W.H.; Lee, K.F.; Tsai, W.C.; Weng, H.H. Value of narrow band imaging endoscopy in early mucosal head and neck cancer. Head Neck 2012, 34, 1574–1579. [Google Scholar] [CrossRef]
- Piazza, C.; del Bon, F.; Paderno, A.; Grazioli, P.; Perotti, P.; Barbieri, D.; Majorana, A.; Bardellini, E.; Peretti, G.; Nicolai, P. The diagnostic value of narrow band imaging in different oral and oropharyngeal subsites. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3347–3353. [Google Scholar] [CrossRef]
- Picciani, B.L.; Domingos, T.A.; Teixeira-Souza, T.; Santos Vde, C.; Gonzaga, H.F.; Cardoso-Oliveira, J.; Gripp, A.C.; Dias, E.P.; Carneiro, S. Geographic tongue and psoriasis: Clinical, histopathological, immunohistochemical and genetic correlation—A literature review. An. Bras. Dermatol. 2016, 91, 410–421. [Google Scholar] [CrossRef]
- Piazza, C.; Del Bon, F.; Peretti, G.; Nicolai, P. “Biologic endoscopy”: Optimization of upper aerodigestive tract cancer evaluation. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 67–76. [Google Scholar] [CrossRef]
- Watanabe, A.; Taniguchi, M.; Tsujie, H.; Hosokawa, M.; Fujita, M.; Sasaki, S. The value of narrow band imaging endoscope for early head and neck cancers. Otolaryngol. Head Neck Surg. 2018, 138, 446–451. [Google Scholar] [CrossRef]
- Muto, M.; Katada, C.; Sano, Y.; Yoshida, S. Narrow band imaging: A new diagnostic approach to visualize angiogenesis in superficial neoplasia. Clin. Gastroenterol. Hepatol. 2005, 3 (Suppl. S1), 16–20. [Google Scholar] [CrossRef] [PubMed]
- Fuji, S.; Yamazaki, M.; Muto, M.; Ochiai, A. Microvascular irregularities are associated with composition of squamous epithelial lesions and correlate with subepithelial invasion of superficial-type pharyngeal squamous cell carcinoma. Histopathology 2010, 56, 510–522. [Google Scholar] [CrossRef]
- Chatterjee, D.; Bansal, V.; Malik, V.; Bhagat, R.; Punia, R.S.; Handa, U.; Gupta, A.; Dass, A. Tumor Budding and Worse Pattern of Invasion Can Predict Nodal Metastasis in Oral Cancers and Associated with Poor Survival in Early-Stage Tumors. Ear Nose Throat J. 2019, 98, E112–E119. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Ding, L.; Wang, Y.; Wang, Y.; Chen, S.; Huang, X.; He, Z.; Ni, Y.; Hu, Q. Biopsy pattern of invasion type to determine the surgical approach in early-stage oral squamous cell carcinoma. Virchows Arch. 2021, 479, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Available online: https://screening.iarc.fr/atlasoralglossdef.php?key=depapillation&img (accessed on 7 November 2022).
- Subramaniam, N.; Balasubramanian, D.; Hubert Low, T.-H.; Murthy, S.; Anand, A.; Prasad, C.; Vijayan, S.N.; Thankappan, K.; Iyer, S. Role of adverse pathological features in surgically treated early oral cavity carcinomas with adequate margins and the development of a scoring system to predict local control. Head Neck 2018, 40, 2329–2333. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, D.; Subramaniam, N.; Missale, F.; Marchi, F.; Dokhe, Y.; Vijayan, S.; Nambiar, A.; Mattavelli, D.; Calza, S.; Bresciani, L.; et al. Predictive nomograms for oral tongue squamous cell carcinoma applying the American Joint Committee on Cancer/Union Internationale Contre le Cancer 8th edition staging system. Head Neck 2021, 43, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-T.; Chang, J.T.-C.; Wang, H.-M.; Ng, S.-H.; Hsueh, C.; Lee, L.-Y.; Lin, C.-H.; Chen, I.-H.; Huang, S.-F.; Cheng, A.-J.; et al. Salvage therapy in relapsed squamous cell carcinoma of the oral cavity: How and when? Cancer 2008, 112, 94–103. [Google Scholar] [CrossRef]
- Missale, F.; Marchi, F.; Iandelli, A.; Subramaniam, N.; Dokhe, Y.; Sampieri, C.; Mattavelli, D.; Bresciani, L.; Carobbio, A.L.C.; Grammatica, A.; et al. Oncological outcomes of compartmental surgery and wide local excision in oral tongue and floor of the mouth cancer. Oral Oncol. 2022, 135, 106210. [Google Scholar] [CrossRef] [PubMed]
- Marchi, F.; Filauro, M.; Iandelli, A.; Luigi, A.; Carobbio, C.; Mazzola, F.; Santori, G.; Parrinello, G.; Canevari, F.R.M.; Piazza, C.; et al. Magnetic Resonance vs. Intraoral Ultrasonography in the Preoperative Assessment of Oral Squamous Cell Carcinoma : A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, S.; Casaleggio, A.; Tagliafico, A.S.; Conforti, C.; Borda, F.; Fiannacca, M.; Filauro, M.; Iandelli, A.; Marchi, F.; Parrinello, G.; et al. High-Frequency Intraoral Ultrasound for Preoperative Assessment of Depth of Invasion for Early Tongue Squamous Cell Carcinoma : Radiological–Pathological Correlations. Int. J. Environ. Res. Public Health 2022, 19, 14900. [Google Scholar] [CrossRef] [PubMed]
- Filauro, M.; Missale, F.; Marchi, F.; Iandelli, A.; Carobbio, A.L.C.; Mazzola, F.; Parrinello, G.; Barabino, E.; Cittadini, G.; Farina, D.; et al. Intraoral ultrasonography in the assessment of DOI in oral cavity squamous cell carcinoma: A comparison with magnetic resonance and histopathology. Eur. Arch. Oto-Rhino-Laryngol. 2020, 278, 2943–2952. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Gil, Z.; Amit, M.; Yen, T.-C.; Liao, C.-t.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Köhler, H.F.; Kreppel, M.; et al. Depth of invasion alone as an indication for postoperative radiotherapy in small oral squamous cell carcinomas: An International Collaborative Study. Head Neck 2019, 41, 1935–1942. [Google Scholar] [CrossRef]
- Larson, A.R.; Kemmer, J.; Formeister, E.; El-sayed, I.; Ha, P.; George, J.; Ryan, W.; Chan, E.; Heaton, C. Beyond Depth of Invasion: Adverse Pathologic Tumor Features in Early Oral Tongue Squamous Cell Carcinoma. Laryngoscope 2020, 130, 1715–1720. [Google Scholar] [CrossRef]
- Subramaniam, N.; Balasubramanian, D.; Murthy, S.; Thankappan, K.; Iyer, S. Predictors of locoregional control in stage I/II oral squamous cell carcinoma classi fi ed by AJCC 8th edition. Eur. J. Surg. Oncol. 2019, 45, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Brockhoff, H.C.; Kim, R.Y.; Braun, T.M.; Skouteris, C.; Helman, J.I.; Ward, B.B. Correlating the depth of invasion at specific anatomic locations with the risk for regional metastatic disease to lymph nodes in the neck for oral squamous cell carcinoma Hans. Head Neck 2017, 39, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Farhood, Z.; Simpson, M.; Ward, G.M.; Walker, R.J.; Osazuwa-Peters, N. Does anatomic subsite influence oral cavity cancer mortality? A SEER database analysis. Laryngoscope 2019, 129, 1400–1406. [Google Scholar] [CrossRef]
- Calabrese, L.; Bizzoca, M.E.; Grigolato, R.; Maffini, F.A.; Tagliabue, M.; Negro, R.; Leuci, S.; Mignogna, M.D.; Muzio, L.L. From bench to bedside in tongue muscle cancer invasion and back again: Gross anatomy, microanatomy, surgical treatments and basic research. Life 2020, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Deganello, A.; Rampinelli, V.; Gualtieri, T.; Paderno, A.; Bonomo, P.; di Monale e Bastia, M.B. Letter to “Medial lingual lymph node metastasis in carcinoma of the tongue”. Auris Nasus Larynx 2020, 47, 1091–1092. [Google Scholar] [CrossRef]
- Gvetadze, S.R.; Ilkaev, K.D. Lingual lymph nodes: Anatomy, clinical considerations, and oncological significance. World J. Clin. Oncol. 2020, 11, 337–347. [Google Scholar] [CrossRef]
- Kim, R.Y.; Helman, J.I.; Braun, T.M.; Ward, B.B. Increased Presence of Perineural Invasion in the Tongue and Floor of the Mouth: Could It Represent a More Aggressive Oral Squamous Cell Carcinoma, or Do Larger Aggressive Tumors Cause Perineural Invasion? J. Oral Maxillofac. Surg. 2019, 77, 852–858. [Google Scholar] [CrossRef]
- Liu, S.A.; Wang, C.C.; Jiang, R.S.; Lee, F.Y.; Lin, W.J.; Lin, J.C. Pathological features and their prognostic impacts on oral cavity cancer patients among different subsites—A singe institute’s experience in Taiwan. Sci. Rep. 2017, 7, 7451. [Google Scholar] [CrossRef]
- Tai, S.K.; Li, W.Y.; Yang, M.H.; Chu, P.Y.; Wang, Y.F. Perineural invasion in T1 oral squamous cell carcinoma indicates the need for aggressive elective neck dissection. Am. J. Surg. Pathol. 2013, 37, 1164–1172. [Google Scholar] [CrossRef]
- Bakst, R.L.; Glastonbury, C.M.; Parvathaneni, U.; Katabi, N.; Hu, K.S.; Yom, S.S. Perineural Invasion and Perineural Tumor Spread in Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1109–1124. [Google Scholar] [CrossRef]
- Li, Y.; Bai, S.; Carroll, W.; Dayan, D.; Dort, J.C.; Heller, K.; Jour, G.; Lau, H.; Penner, C.; Prystowsky, M.; et al. Validation of the Risk Model: High-Risk Classification and Tumor Pattern of Invasion Predict Outcome for Patients with Low-Stage Oral Cavity Squamous Cell Carcinoma. Head Neck Pathol. 2013, 7, 211–223. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Head and Neck Cancers. In NCCN Guidelines Version 3; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2019. [Google Scholar] [CrossRef]
- Nair, D.; Qayyumi, B.; Sharin, F.; Mair, M.; Bal, M.; Pimple, S.; Mishra, G.; Nair, S.; Chaturvedi, P. Narrow band imaging observed oral mucosa microvasculature as a tool to detect early oral cancer: An Indian experience. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 3965–3971. [Google Scholar] [CrossRef] [PubMed]
- Deganello, A.; Paderno, A.; Morello, R.; Fior, M.; Berretti, G.; Del Bon, F.; Alparone, M.; Bardellini, E.; Majorana, A.; Nicolai, P. Diagnostic Accuracy of Narrow Band Imaging in Patients with Oral Lichen Planus: A Prospective Study. Laryngoscope 2021, 131, E1156–E1161. [Google Scholar] [CrossRef] [PubMed]
- Guida, A.; Maglione, M.; Crispo, A.; Perri, F.; Villano, S.; Pavone, E.; Aversa, C.; Longo, F.; Feroce, F.; Botti, G.; et al. Oral lichen planus and other confounding factors in narrow band imaging (NBI) during routine inspection of oral cavity for early detection of oral squamous cell carcinoma: A retrospective pilot study. BMC Oral Health 2019, 19, 70. [Google Scholar] [CrossRef]
- Azam, M.A.; Sampieri, C.; Ioppi, A.; Benzi, P.; Giordano, G.G.; De Vecchi, M.; Campagnari, V.; Li, S.; Guastini, L.; Paderno, A.; et al. Videomics of the Upper Aero-Digestive Tract Cancer: Deep Learning Applied to White Light and Narrow Band Imaging for Automatic Segmentation of Endoscopic Images. Front. Oncol. 2022, 12, 900451. [Google Scholar] [CrossRef]
- Ling, Z.; Cheng, B.; Tao, X. Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities. Int. J. Cancer 2021, 148, 1548–1561. [Google Scholar] [CrossRef]
- Dik, E.A.; Ipenburg, N.A.; Kessler, P.A.; van Es, R.J.J.; Willems, S.M. The value of histological grading of biopsy and resection specimens in early stage oral squamous cell carcinomas. J. Cranio-Maxillofac. Surg. 2018, 46, 1001–1006. [Google Scholar] [CrossRef]
- Dik, E.A.; Ipenburg, N.A.; Adriaansens, S.O.; Kessler, P.A.; Van Es, R.J.; Willems, S.M. Poor correlation of histologic parameters between biopsy and resection specimen in early stage oral squamous cell carcinoma. Am. J. Clin. Pathol. 2015, 144, 659–666. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | N = 76 | |
|---|---|---|
| Age | Median (IQR) | 70.00 (56.00, 80.00) |
| Range | 22.00–100.00 | |
| Gender | F | 40 (53%) |
| M | 36 (47%) | |
| Smoking | No | 44 (64%) |
| yes | 25 (36%) | |
| Alcohol | No | 54 (81%) |
| yes | 13 (19%) | |
| cT | 1 | 37 (49%) |
| 2 | 26 (34%) | |
| 3 | 12 (16%) | |
| 4a | 1 (1.3%) | |
| cN | 0 | 68 (89%) |
| 1 | 1 (1.3%) | |
| 2c | 4 (5.3%) | |
| 2b | 2 (2.6%) | |
| 3b | 1 (1.3%) | |
| Overall Stage | 1 | 36 (47%) |
| 2 | 24 (32%) | |
| 3 | 8 (11%) | |
| 4a | 6 (7.9%) | |
| 4b | 2 (2.6%) | |
| pT | 1 | 35 (47%) |
| 2 | 22 (29%) | |
| 3 | 18 (24%) | |
| pN | 0 | 33 (75%) |
| 1 | 6 (14%) | |
| 2b | 2 (4.5%) | |
| 2c | 1 (2.3%) | |
| 3b | 2 (4.5%) | |
| Margins | Free | 37 (49%) |
| Close | 4 (5.3%) | |
| Positive | 35 (46%) | |
| Grading | 1 | 13 (18%) |
| 2 | 51 (71%) | |
| 3 | 8 (11%) | |
| LVI * | No | 55 (73%) |
| Yes | 20 (27%) | |
| PNI * | No | 44 (59%) |
| Yes | 31 (41%) | |
| Pathological DOI * | Median (IQR) | 6.00 (2.30, 10.00) |
| Range | 0.50–40.00 | |
| TILs * | No | 19 (34%) |
| Yes | 37 (66%) | |
| Budding | No | 38 (62%) |
| Yes | 23 (38%) | |
| WPOI * | 1 | 1 (5.0%) |
| 2 | 2 (10%) | |
| 3 | 8 (40%) | |
| 4 | 7 (35%) | |
| 5 | 2 (10%) | |
| Depapillation | No | 36 (47%) |
| Yes | 40 (53%) | |
| NBI pattern | I–II | 17 (22%) |
| III–IV | 59 (78%) | |
| Aspect | Exophytic | 30 (39%) |
| Plane | 23 (30%) | |
| Ulcerated | 23 (30%) | |
| Variable | Overall, N = 76 | Depapillation | p-Value | |
|---|---|---|---|---|
| No, N = 36 | Yes, N = 40 | |||
| cT | 0.90 | |||
| 1 | 37 (49%) | 19 (53%) | 18 (45%) | |
| 2 | 26 (34%) | 12 (33%) | 14 (35%) | |
| 3 | 12 (16%) | 5 (14%) | 7 (18%) | |
| 4a | 1 (1.3%) | 0 (0%) | 1 (2.5%) | |
| cN | 0.053 | |||
| 0 | 68 (89%) | 33 (92%) | 35 (88%) | |
| 1 | 1 (1.3%) | 0 (0%) | 1 (2.5%) | |
| 2b | 4 (5.3%) | 0 (0%) | 4 (10%) | |
| 2c | 2 (2.6%) | 2 (5.6%) | 0 (0%) | |
| 3b | 1 (1.3%) | 1 (2.8%) | 0 (0%) | |
| Clinical stage | 0.95 | |||
| 1 | 36 (47%) | 18 (50%) | 18 (45%) | |
| 2 | 24 (32%) | 11 (31%) | 13 (32%) | |
| 3 | 8 (11%) | 4 (11%) | 4 (10%) | |
| 4a | 6 (7.9%) | 2 (5.6%) | 4 (10%) | |
| 4b | 2 (2.6%) | 1 (2.8%) | 1 (2.5%) | |
| pT | 0.94 | |||
| 1 | 35 (47%) | 17 (47%) | 18 (46%) | |
| 2 | 22 (29%) | 11 (31%) | 11 (28%) | |
| 3 | 18 (24%) | 8 (22%) | 10 (26%) | |
| pN | 0.91 | |||
| 0 | 33 (75%) | 14 (82%) | 19 (70%) | |
| 1 | 6 (14%) | 2 (12%) | 4 (15%) | |
| 2b | 2 (4.5%) | 0 (0%) | 2 (7.4%) | |
| 2c | 1 (2.3%) | 0 (0%) | 1 (3.7%) | |
| 3b | 2 (4.5%) | 1 (5.9%) | 1 (3.7%) | |
| Pathological stage | 0.59 | |||
| 1 | 32 (43%) | 15 (43%) | 17 (44%) | |
| 2 | 18 (24%) | 10 (29%) | 8 (21%) | |
| 3 | 19 (26%) | 9 (26%) | 10 (26%) | |
| 4a | 3 (4.1%) | 0 (0%) | 3 (7.7%) | |
| 4b | 2 (2.7%) | 1 (2.9%) | 1 (2.6%) | |
| Margins | 0.49 | |||
| free | 37 (49%) | 15 (42%) | 22 (55%) | |
| close | 4 (5.3%) | 2 (5.6%) | 2 (5.0%) | |
| positive | 35 (46%) | 19 (53%) | 16 (40%) | |
| Grading | 0.49 | |||
| 1 | 13 (18%) | 8 (24%) | 5 (13%) | |
| 2 | 51 (71%) | 22 (65%) | 29 (76%) | |
| 3 | 8 (11%) | 4 (12%) | 4 (11%) | |
| LVI * | 0.060 | |||
| no | 55 (73%) | 30 (83%) | 25 (64%) | |
| yes | 20 (27%) | 6 (17%) | 14 (36%) | |
| PNI * | 0.022 | |||
| no | 44 (59%) | 26 (72%) | 18 (46%) | |
| yes | 31 (41%) | 10 (28%) | 21 (54%) | |
| Pathological DOI * | 0.73 | |||
| Median (IQR) | 6.00 (2.30, 10.00) | 5.50 (2.25, 9.75) | 6.00 (3.00, 10.50) | |
| TILs * | 0.64 | |||
| no | 19 (34%) | 10 (37%) | 9 (31%) | |
| yes | 37 (66%) | 17 (63%) | 20 (69%) | |
| Budding activity | 0.67 | |||
| no | 38 (62%) | 17 (65%) | 21 (60%) | |
| yes | 23 (38%) | 9 (35%) | 14 (40%) | |
| WPOI * | 0.25 | |||
| 1 | 1 (5.0%) | 0 (0%) | 1 (12%) | |
| 2 | 2 (10%) | 2 (17%) | 0 (0%) | |
| 3 | 8 (40%) | 3 (25%) | 5 (62%) | |
| 4 | 7 (35%) | 5 (42%) | 2 (25%) | |
| 5 | 2 (10%) | 2 (17%) | 0 (0%) | |
| NBI pattern | 0.56 | |||
| I–II | 17 (22%) | 7 (19%) | 10 (25%) | |
| III–IV | 59 (78%) | 29 (81%) | 30 (75%) | |
| Aspect | >0.99 | |||
| exophytic | 30 (39%) | 14 (39%) | 16 (40%) | |
| plane | 23 (30%) | 11 (31%) | 12 (30%) | |
| ulcerated | 23 (30%) | 11 (31%) | 12 (30%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iandelli, A.; Sampieri, C.; Marchi, F.; Pennacchi, A.; Carobbio, A.L.C.; Lovino Camerino, P.; Filauro, M.; Parrinello, G.; Peretti, G. The Role of Peritumoral Depapillation and Its Impact on Narrow-Band Imaging in Oral Tongue Squamous Cell Carcinoma. Cancers 2023, 15, 1196. https://doi.org/10.3390/cancers15041196
Iandelli A, Sampieri C, Marchi F, Pennacchi A, Carobbio ALC, Lovino Camerino P, Filauro M, Parrinello G, Peretti G. The Role of Peritumoral Depapillation and Its Impact on Narrow-Band Imaging in Oral Tongue Squamous Cell Carcinoma. Cancers. 2023; 15(4):1196. https://doi.org/10.3390/cancers15041196
Chicago/Turabian StyleIandelli, Andrea, Claudio Sampieri, Filippo Marchi, Alessia Pennacchi, Andrea Luigi Camillo Carobbio, Paola Lovino Camerino, Marta Filauro, Giampiero Parrinello, and Giorgio Peretti. 2023. "The Role of Peritumoral Depapillation and Its Impact on Narrow-Band Imaging in Oral Tongue Squamous Cell Carcinoma" Cancers 15, no. 4: 1196. https://doi.org/10.3390/cancers15041196
APA StyleIandelli, A., Sampieri, C., Marchi, F., Pennacchi, A., Carobbio, A. L. C., Lovino Camerino, P., Filauro, M., Parrinello, G., & Peretti, G. (2023). The Role of Peritumoral Depapillation and Its Impact on Narrow-Band Imaging in Oral Tongue Squamous Cell Carcinoma. Cancers, 15(4), 1196. https://doi.org/10.3390/cancers15041196







