ONEST (Observers Needed to Evaluate Subjective Tests) Analysis of Stromal Tumour-Infiltrating Lymphocytes (sTILs) in Breast Cancer and Its Limitations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
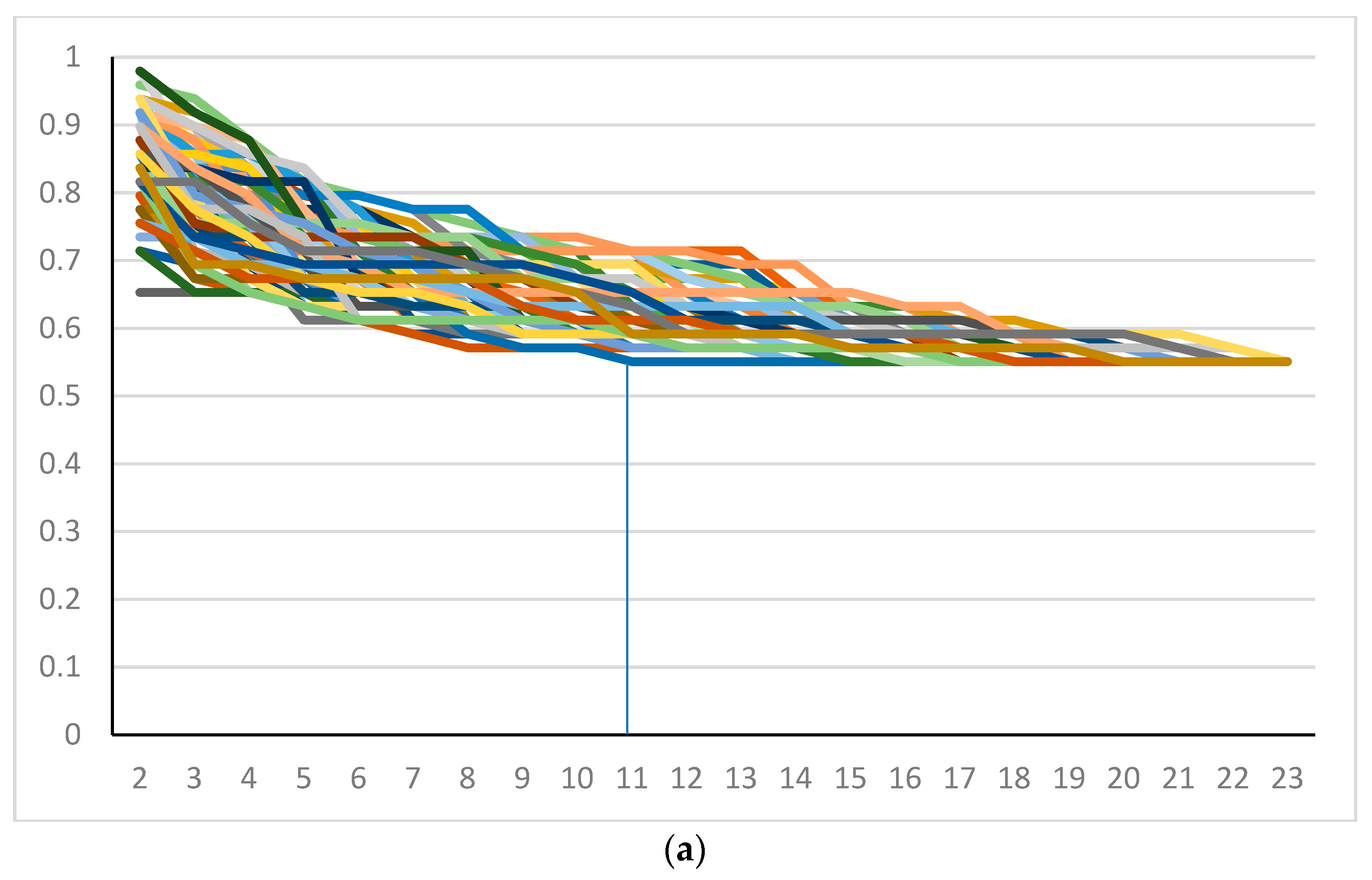
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Bairi, K.; Haynes, H.R.; Blackley, E.; Fineberg, S.; Shear, J.; Turner, S.; de Freitas, J.R.; Sur, D.; Amendola, L.C.; Gharib, M.; et al. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. N.P.J. Breast Cancer. 2021, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early-stage triple-negative breast cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G.; Serfőző, O.; Ambrózay, É.; Markó, L.; Krenács, L. Spontaneous pathological complete regression of a high grade triple negative breast cancer with axillary metastasis–report of a case. Pol. J. Pathol. 2019, 70, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Laenkholm, A.V.; Callagy, G.; Balancin, M.; Bartlett, J.M.S.; Sotiriou, C.; Marchio, C.; Kok, M.; Dos Anjos, C.H.; Salgado, R. Incorporation of TILs in daily breast cancer care: How much evidence can we bear? Virchows Arch. 2022, 480, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The Evaluation of Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer, Including Recommendations to Assess TILs in Residual Disease After Neoadjuvant Therapy and in Carcinoma In Situ: A Report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar]
- Denkert, C.; Wienert, S.; Poterie, A.; Loibl, S.; Budczies, J.; Badve, S.; Bago-Horvath, Z.; Bane, A.; Bedri, S.; Brock, J.; et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: Results of the ring studies of the international immuno-oncology biomarker working group. Mod. Pathol. 2016, 29, 1155–1164. [Google Scholar] [CrossRef]
- Kilmartin, D.; O’Loughlin, M.; Andreu, X.; Bagó-Horváth, Z.; Bianchi, S.; Chmielik, E.; Cserni, G.; Figueiredo, P.; Floris, G.; Foschini, M.P.; et al. Intra-Tumour Heterogeneity Is One of the Main Sources of Inter-Observer Variation in Scoring Stromal Tumour Infiltrating Lymphocytes in Triple Negative Breast Cancer. Cancers 2021, 13, 4410. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Reisenbichler, E.S.; Han, G.; Bellizzi, A.; Bossuyt, V.; Brock, J.; Cole, K.; Fadare, O.; Hameed, O.; Hanley, K.; Harrison, B.T.; et al. Prospective multi-institutional evaluation of pathologist assessment of PD-L1 assays for patient selection in triple negative breast cancer. Mod. Pathol. 2020, 33, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Cserni, B.; Bori, R.; Csörgő, E.; Oláh-Németh, O.; Pancsa, T.; Sejben, A.; Sejben, I.; Vörös, A.; Zombori, T.; Nyári, T.; et al. The additional value of ONEST (Observers Needed to Evaluate Subjective Tests) in assessing reproducibility of oestrogen receptor, progesterone receptor and Ki67 classification in breast cancer. Virchows Arch. 2021, 479, 1101–1109. [Google Scholar] [CrossRef]
- Cserni, B.; Bori, R.; Csörgő, E.; Oláh-Németh, O.; Pancsa, T.; Sejben, A.; Sejben, I.; Vörös, A.; Zombori, T.; Nyári, T.; et al. ONEST (Observers Needed to Evaluate Subjective Tests) suggests four or more observers for a reliable assessment of the consistency of histological grading of invasive breast carcinoma—A reproducibility study with a retrospective view on previous studies. Pathol. Res. Pract. 2021, 229, 153718. [Google Scholar] [CrossRef]
- Stanton, S.E.; Disis, M.L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother. Cancer 2016, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C.; et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef]
- Sasaki, R.; Horimoto, Y.; Yanai, Y.; Kurisaki-Arakawa, A.; Arakawa, A.; Nakai, K.; Saito, M.; Saito, T. Molecular Characteristics of Lymphocyte-predominant Triple-negative Breast Cancer. Anticancer Res. 2021, 41, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G.; Francz, M.; Járay, B.; Kálmán, E.; Kovács, I.; Krenács, T.; Udvarhelyi, N.; Tóth, E.; Vass, L.; Vörös, A.; et al. Pathological diagnosis, work-up and reporting of breast cancer. Recommendations from the 4th Breast Cancer Consensus Conference. Magy. Onkol. 2020, 64, 301–328. (In Hungarian) [Google Scholar] [PubMed]
- Cserni, G.; Francz, M.; Járay, B.; Kálmán, E.; Kovács, I.; Krenács, T.; Udvarhelyi, N.; Tóth, E.; Vass, L.; Vörös, A.; et al. Pathological Diagnosis, Work-Up and Reporting of Breast Cancer 1st Central-Eastern European Professional Consensus Statement on Breast. Cancer. Pathol. Oncol. Res. 2022, 28, 1610373. [Google Scholar] [CrossRef]
- Cserni, B. ONEST Calculator. Available online: https://github.com/csernib/onest (accessed on 12 November 2022).
- Zaiontz, C. Real Statistics Resource Pack|Real Statistics Using Excel. Available online: https://real-statistics.com (accessed on 22 September 2022).
- Tramm, T.; Di Caterino, T.; Jylling, A.-M.B.; Lelkaitis, G.; Lænkholm, A.-V.; Ragó, P.; Tabor, T.P.; Talman, M.-L.M.; Vouza, E.; Scientific Committee of Pathology, Danish Breast Cancer Group (DBCG). Standardized assessment of tumor-infiltrating lymphocytes in breast cancer: An evaluation of inter-observer agreement between pathologists. Acta. Oncol. 2018, 57, 90–94. [Google Scholar] [CrossRef]
- Kos, Z.; Roblin, E.; Kim, R.S.; Michiels, S.; Gallas, B.D.; Chen, W.; van de Vijver, K.K.; Goel, S.; Adams, S.; Demaria, S.; et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. N.P.J. Breast Cancer 2020, 6, 17. [Google Scholar] [CrossRef]
- Adams, S.; Gray, R.J.; Demaria, S.; Goldstein, L.; Perez, E.A.; Shulman, L.N.; Martino, S.; Wang, M.; Jones, V.E.; Saphner, T.J.; et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 2014, 32, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.A.; Wang, X.; Sun, H.; Compton, F.; Covinsky, M.; Zhang, S. Reproducible evaluation of tumor-infiltrating lymphocytes (TILs) using the recommendations of International TILs Working Group 2014. Ann. Diagn. Pathol. 2018, 35, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.S.; Song, N.; Gavin, P.G.; Salgado, R.; Bandos, H.; Kos, Z.; Floris, G.; Eynden, G.G.G.M.V.D.; Badve, S.; Demaria, S.; et al. Stromal Tumor-infiltrating Lymphocytes in NRG Oncology/NSABP B-31 Adjuvant Trial for Early-Stage HER2-Positive Breast Cancer. J. Natl. Cancer Inst. 2019, 111, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Buisseret, L.; Desmedt, C.; Garaud, S.; Fornili, M.; Wang, X.; Van den Eyden, G.; de Wind, A.; Duquenne, S.; Boisson, A.; Naveaux, C.; et al. Reliability of tumor-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod. Pathol. 2017, 30, 1204–1212. [Google Scholar] [CrossRef]
- Swisher, S.K.; Wu, Y.; Castaneda, C.A.; Lyons, G.R.; Yang, F.; Tapia, C.; Wang, X.; Casavilca, S.A.; Bassett, R.; Castillo, M.; et al. Interobserver Agreement Between Pathologists Assessing Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer Using Methodology Proposed by the International TILs Working Group. Ann. Surg. Oncol. 2016, 23, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Cabuk, F.K.; Aktepe, F.; Kapucuoglu, F.N.; Coban, I.; Sarsenov, D.; Ozmen, V. Interobserver reproducibility of tumor-infiltrating lymphocyte evaluations in breast cancer. Indian J. Pathol. Microbiol. 2018, 61, 181–186. [Google Scholar] [CrossRef]
- Khoury, T.; Peng, X.; Yan, L.; Wang, D.; Nagrale, V. Tumor-Infiltrating Lymphocytes in Breast Cancer: Evaluating Interobserver Variability, Heterogeneity, and Fidelity of Scoring Core Biopsies. Am. J. Clin. Pathol. 2018, 150, 441–450. [Google Scholar] [CrossRef]
- O’Loughlin, M.; Andreu, X.; Bianchi, S.; Chemielik, E.; Cordoba, A.; Cserni, G.; Figueiredo, P.; Floris, G.; Foschini, M.P.; Heikkilä, P.; et al. Reproducibility and predictive value of scoring stromal tumour infiltrating lymphocytes in triple-negative breast cancer: A multi-institutional study. Breast Cancer Res. Treat. 2018, 171, 1–9. [Google Scholar] [CrossRef]
- Van Bockstal, M.R.; François, A.; Altinay, S.; Arnould, L.; Balkenhol, M.; Broeckx, G.; Burguès, O.; Colpaert, C.; Dedeurwaerdere, F.; Dessauvagie, B.; et al. Interobserver variability in the assessment of stromal tumor-infiltrating lymphocytes (sTILs) in triple-negative invasive breast carcinoma influences the association with pathological complete response: The IVITA study. Mod. Pathol. 2021, 34, 2130–2140. [Google Scholar] [CrossRef]



| <50% vs. ≥50% | C1 | C1 without Divergent Raters 7 and 20 | C1s | C2 | C2 without Divergent Raters 4 and 13 | C3 |
|---|---|---|---|---|---|---|
| n | 23 | 21 | 14 | 14 | 12 | 9 |
| OPA(n) | 0.551 | 0.612 | 0.571 | 0.776 | 0.816 | 0.89 |
| Bandwidth | 0.327 | 0.245 | 0.265 | 0.184 | 0.143 | 0.07 |
| ONEST | 11 | 7 | 8 | 6 | 3 | 6 |
| <60% vs. ≥60% | ||||||
| n | 23 | 21 | 14 | 14 | 12 | 9 |
| OPA(n) | 0.612 | 0.796 | 0.612 | 0.796 | 0.837 | 0.91 |
| Bandwidth | 0.327 | 0.286 | 0.612 | 0.163 | 0.163 | 0.07 |
| ONEST | 9 | 7 | 4 | 6 | 2 | 2 |
| <30% vs. ≥30% | ||||||
| n | 23 | 21 | 14 | 14 | 12 | 9 |
| OPA(n) | 0.306 | 0.347 | 0.327 | 0.551 | 0.592 | 0.81 |
| Bandwidth | 0.408 | 0.306 | 0.306 | 0.306 | 0.204 | 0.09 |
| ONEST | 11 | 8 | 9 | 8 | 7 | 6 |
| ≤20%, 21–49%, ≥50% | ||||||
| n | 23 | 21 | 14 | 14 | 12 | 9 |
| OPA(n) | 0.163 | 0.204 | 0.408 | 0.408 | 0.449 | 0.74 |
| Bandwidth | 0.469 | 0.388 | 0.143 | 0.265 | 0.245 | 0.12 |
| ONEST | 8 | 7 | 7 | 5 | 6 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cserni, B.; Kilmartin, D.; O’Loughlin, M.; Andreu, X.; Bagó-Horváth, Z.; Bianchi, S.; Chmielik, E.; Figueiredo, P.; Floris, G.; Foschini, M.P.; et al. ONEST (Observers Needed to Evaluate Subjective Tests) Analysis of Stromal Tumour-Infiltrating Lymphocytes (sTILs) in Breast Cancer and Its Limitations. Cancers 2023, 15, 1199. https://doi.org/10.3390/cancers15041199
Cserni B, Kilmartin D, O’Loughlin M, Andreu X, Bagó-Horváth Z, Bianchi S, Chmielik E, Figueiredo P, Floris G, Foschini MP, et al. ONEST (Observers Needed to Evaluate Subjective Tests) Analysis of Stromal Tumour-Infiltrating Lymphocytes (sTILs) in Breast Cancer and Its Limitations. Cancers. 2023; 15(4):1199. https://doi.org/10.3390/cancers15041199
Chicago/Turabian StyleCserni, Bálint, Darren Kilmartin, Mark O’Loughlin, Xavier Andreu, Zsuzsanna Bagó-Horváth, Simonetta Bianchi, Ewa Chmielik, Paulo Figueiredo, Giuseppe Floris, Maria Pia Foschini, and et al. 2023. "ONEST (Observers Needed to Evaluate Subjective Tests) Analysis of Stromal Tumour-Infiltrating Lymphocytes (sTILs) in Breast Cancer and Its Limitations" Cancers 15, no. 4: 1199. https://doi.org/10.3390/cancers15041199
APA StyleCserni, B., Kilmartin, D., O’Loughlin, M., Andreu, X., Bagó-Horváth, Z., Bianchi, S., Chmielik, E., Figueiredo, P., Floris, G., Foschini, M. P., Kovács, A., Heikkilä, P., Kulka, J., Laenkholm, A.-V., Liepniece-Karele, I., Marchiò, C., Provenzano, E., Regitnig, P., Reiner, A., ... Cserni, G. (2023). ONEST (Observers Needed to Evaluate Subjective Tests) Analysis of Stromal Tumour-Infiltrating Lymphocytes (sTILs) in Breast Cancer and Its Limitations. Cancers, 15(4), 1199. https://doi.org/10.3390/cancers15041199










