Nanoscale Prognosis of Colorectal Cancer Metastasis from AFM Image Processing of Histological Sections
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Histological Tissue Preparation
2.2. AFM Image Analysis
2.3. Histological Tissue Optical Analysis
2.4. Gaussian Filtering Residuals RMS Deviation
2.5. Moments of Gaussian Filtering Residual Variograms
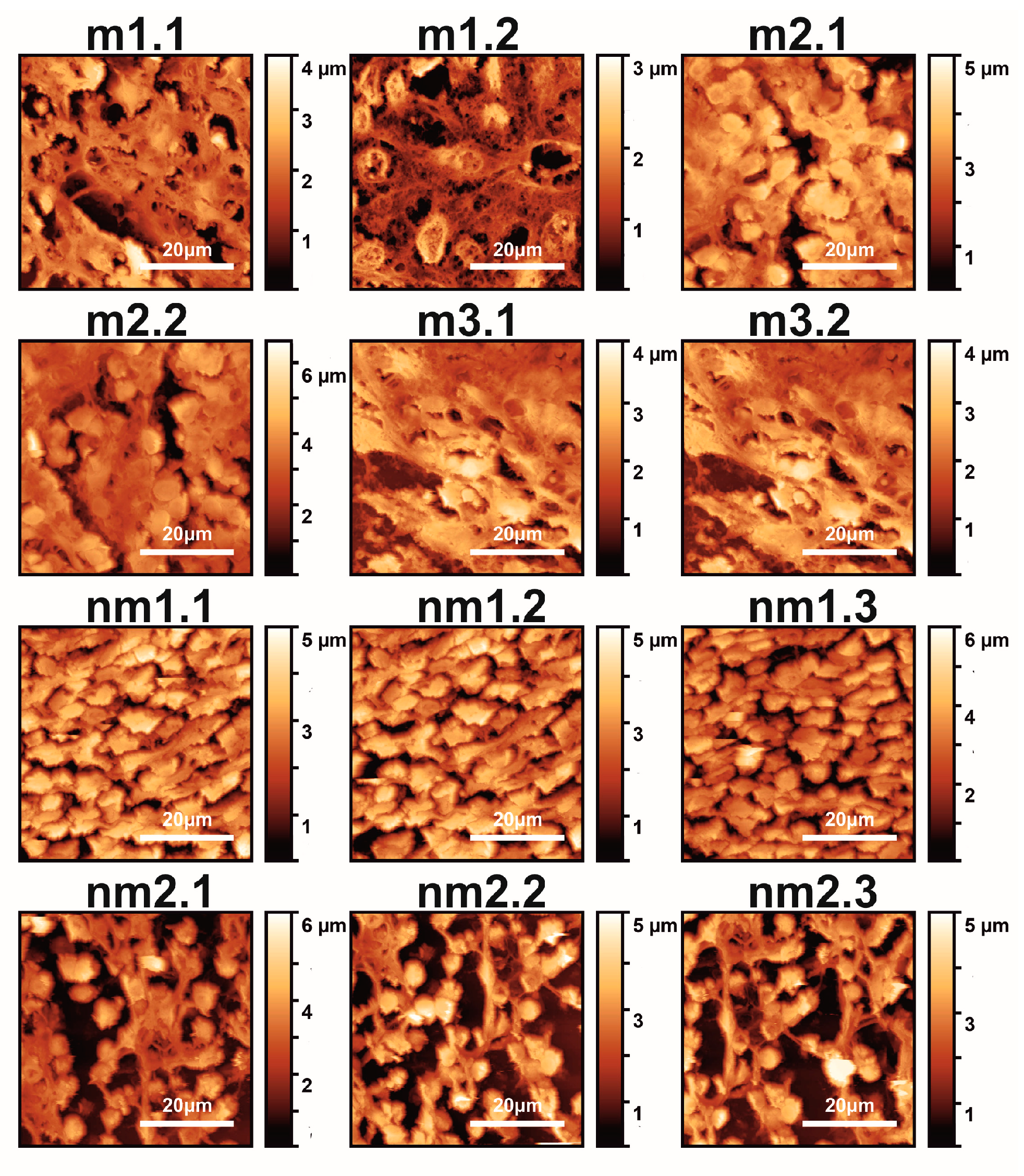
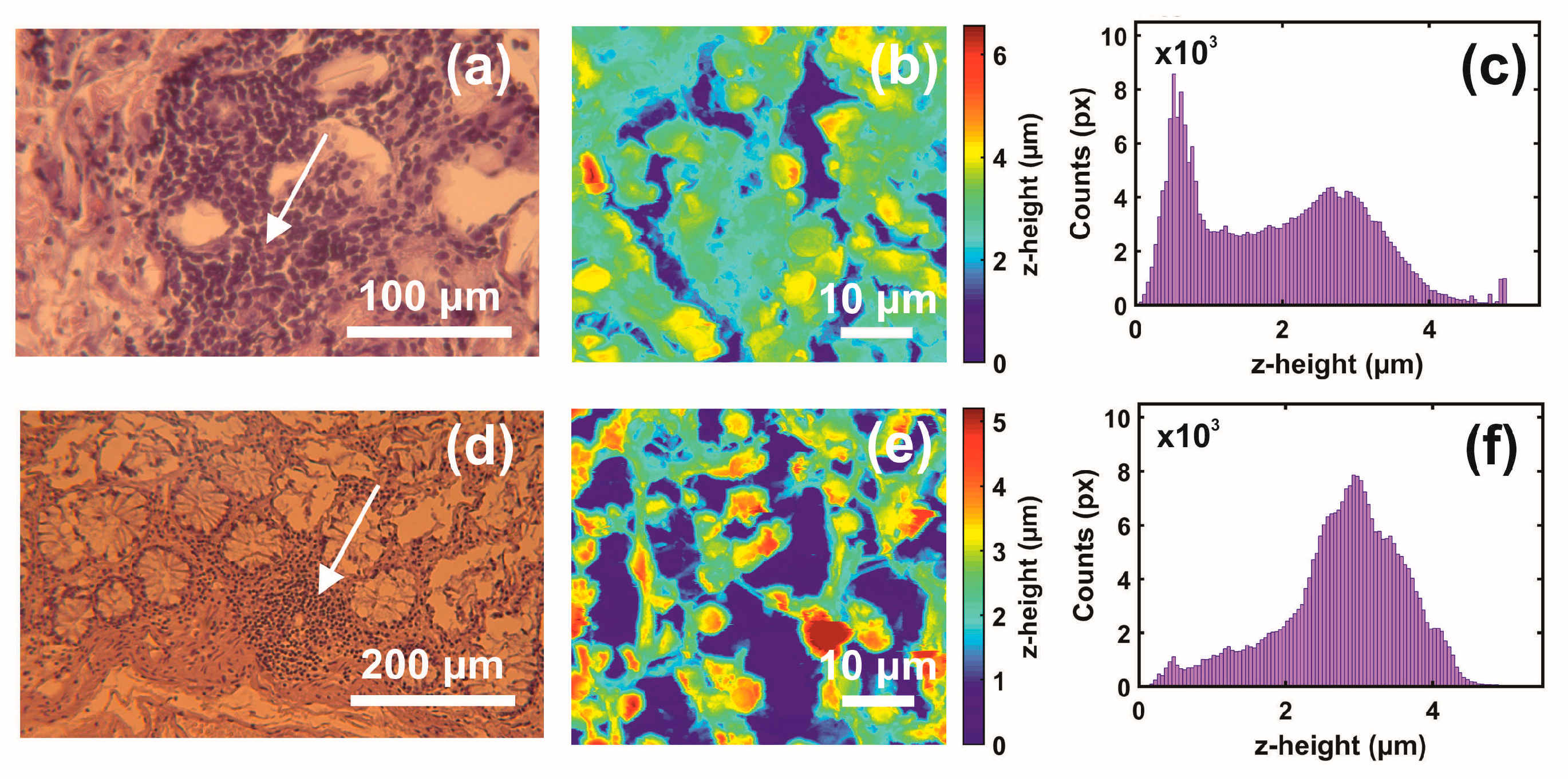
3. Results
3.1. Optical and AFM Microscopy of CRC Histological Sections
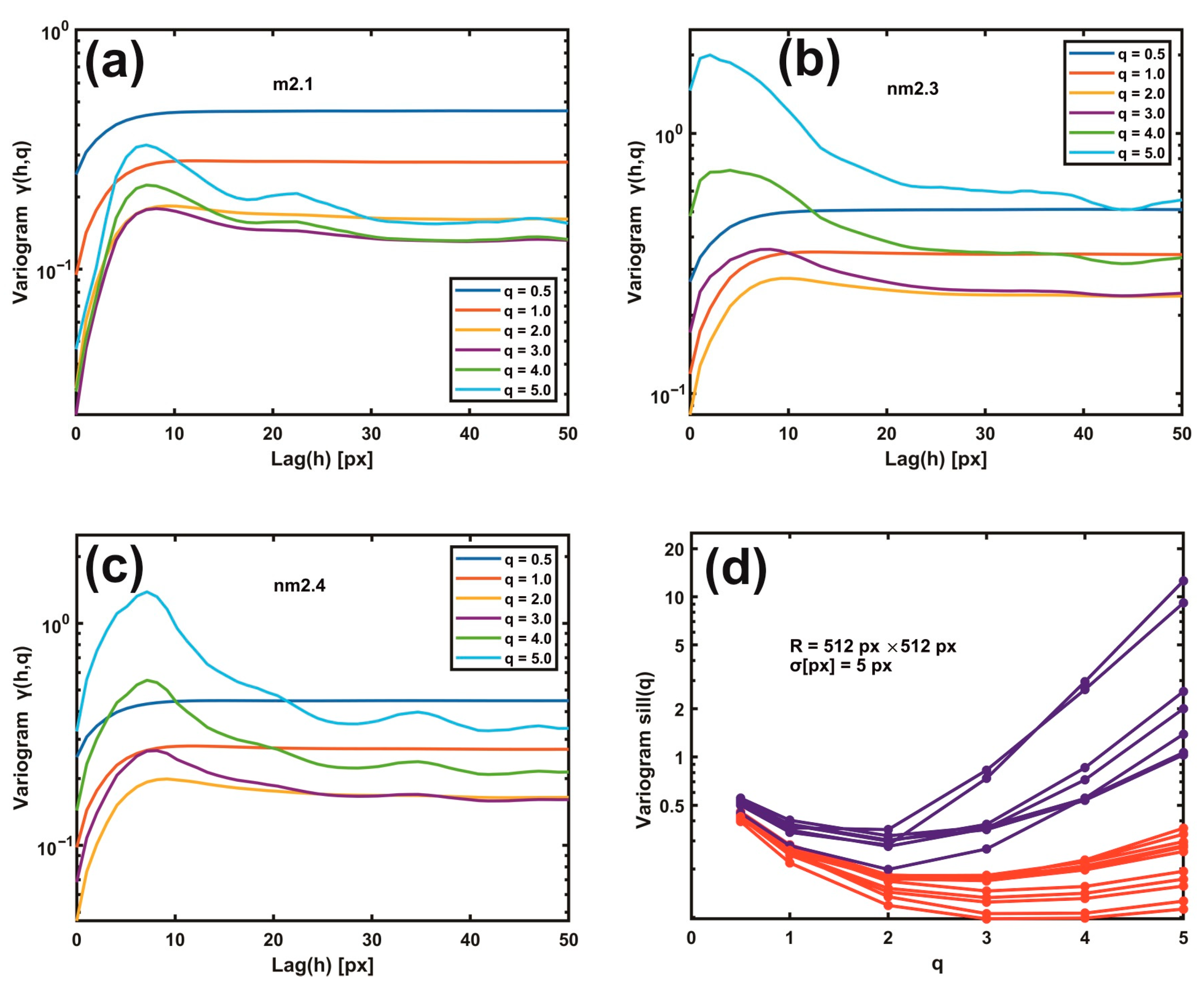
3.2. Variograms of Gaussian Filtering Residuals
3.3. Moments of Gaussian Filtering Residual Variograms
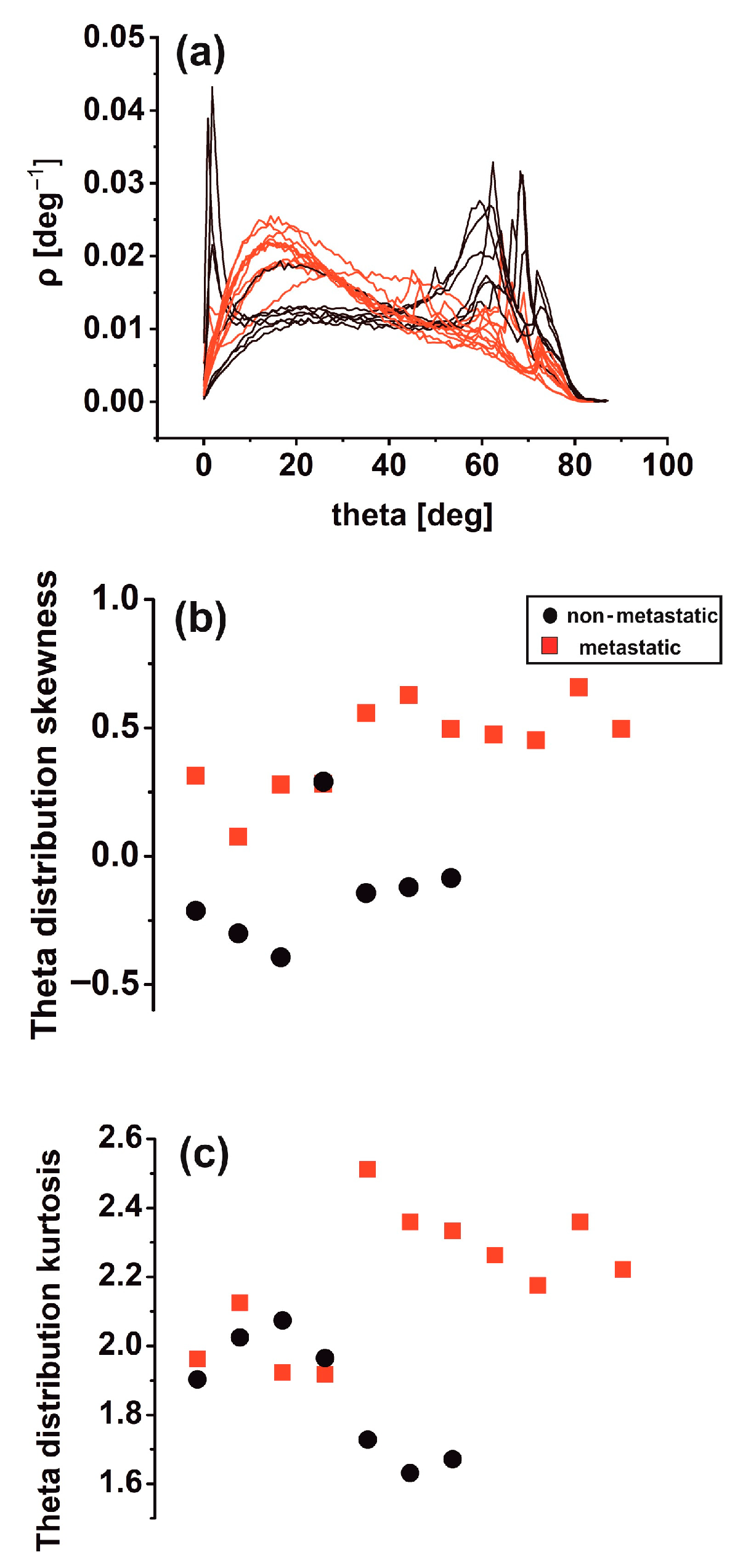
3.4. Theta Statistics
3.5. Surface Analysis
3.6. Rescaled Range Analysis (Hurst Exponent)
3.7. Phase Analysis
3.8. Monofractal Image Analysis
4. Discussion
4.1. The Intricacy of the Cancer Problem
4.2. Variograms and Theta Statistics: Diagnostic Tools for Early Cancer Metastasis
4.3. Cancer as a Dynamical Hierarchical Issue and Problem
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Agus, D.B.; Alexander, J.F.; Arap, W.; Ashili, S.; Aslan, J.E.; Austin, R.H.; Backman, V.; Bethel, K.J.; Bonneau, R.; Chen, W.-C.; et al. A physical sciences network characterisation of non-tumorigenic and metastatic cells. Sci. Rep. 2013, 3, 1449. [Google Scholar] [CrossRef] [PubMed]
- Runel, G.; Lopez-Ramirez, N.; Chlasta, J.; Masse, I. Biomechanical Properties of Cancer Cells. Cells 2021, 10, 887. [Google Scholar] [CrossRef]
- Chu, H.-Y.; Chen, Y.-J.; Hsu, C.-J.; Liu, Y.-W.; Chiou, J.-F.; Lu, L.-S.; Tseng, F.-G. Physical Cues in the Microenvironment Regulate Stemness-Dependent Homing of Breast Cancer Cells. Cancers 2020, 12, 2176. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, Q.; Li, X.; Feng, J.; Ao, Z.; Li, X.; Wang, J. Substrate Stiffness Modulates the Growth, Phenotype, and Chemoresistance of Ovarian Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 718834. [Google Scholar] [CrossRef]
- Semashko, V.V.; Pudovkin, M.S.; Cefalas, A.-C.; Zelenikhin, P.V.; Gavriil, V.E.; Nizamutdinov, A.S.; Kollia, Z.; Ferraro, A.; Sarantopoulou, E. Tiny Rare-Earth Fluoride Nanoparticles Activate Tumour Cell Growth via Electrical Polar Interactions. Nanoscale Res. Lett. 2018, 13, 370. [Google Scholar] [CrossRef]
- Ayad, N.M.E.; Kaushik, S.; Weaver, V.M. Tissue mechanics, an important regulator of development and disease. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180215. [Google Scholar] [CrossRef]
- Vicente-Munuera, P.; Burgos-Panadero, R.; Noguera, I.; Navarro, S.; Noguera, R.; Escudero, L.M. The topology of vitronectin: A complementary feature for neuroblastoma risk classification based on computer-aided detection. Int. J. Cancer 2020, 146, 553–565. [Google Scholar] [CrossRef]
- Blank, A.; Roberts D.E., 2nd; Dawson, H.; Zlobec, I.; Lugli, A. Tumor Heterogeneity in Primary Colorectal Cancer and Corresponding Metastases. Does the Apple Fall Far From the Tree? Front. Med. 2018, 5, 234. [Google Scholar] [CrossRef]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef]
- He, L.; Long, L.R.; Antani, S.; Thoma, G.R. Histology image analysis for carcinoma detection and grading. Comput. Methods Programs Biomed. 2012, 107, 538–556. [Google Scholar] [CrossRef]
- Kawasaki, H.; Itoh, T.; Takaku, Y.; Suzuki, H.; Kosugi, I.; Meguro, S.; Iwashita, T.; Hariyama, T. The NanoSuit method: A novel histological approach for examining paraffin sections in a nondestructive manner by correlative light and electron microscopy. Lab. Investig. 2020, 100, 161–173. [Google Scholar] [CrossRef]
- Li, M.; Xi, N.; Wang, Y.; Liu, L. Atomic force microscopy for revealing micro/nanoscale mechanics in tumor metastasis: From single cells to microenvironmental cues. Acta Pharmacol. Sin. 2021, 42, 323–339. [Google Scholar] [CrossRef]
- Deng, X.; Xiong, F.; Li, X.; Xiang, B.; Li, Z.; Wu, X.; Guo, C.; Li, X.; Li, Y.; Li, G.; et al. Application of atomic force microscopy in cancer research. J. Nanobiotechnology 2018, 16, 102. [Google Scholar] [CrossRef]
- Gensbittel, V.; Kräter, M.; Harlepp, S.; Busnelli, I.; Guck, J.; Goetz, J.G. Mechanical Adaptability of Tumor Cells in Metastasis. Dev. Cell 2021, 56, 164–179. [Google Scholar] [CrossRef]
- Lekka, M. Discrimination Between Normal and Cancerous Cells Using AFM. Bionanoscience 2016, 6, 65–80. [Google Scholar] [CrossRef]
- Plekhanov, A.A.; Sirotkina, M.A.; Sovetsky, A.A.; Gubarkova, E.V.; Kuznetsov, S.S.; Matveyev, A.L.; Matveev, L.A.; Zagaynova, E.V.; Gladkova, N.D.; Zaitsev, V.Y. Histological validation of in vivo assessment of cancer tissue inhomogeneity and automated morphological segmentation enabled by Optical Coherence Elastography. Sci. Rep. 2020, 10, 11781. [Google Scholar] [CrossRef]
- Hoffmann, S.C.; Wabnitz, G.H.; Samstag, Y.; Moldenhauer, G.; Ludwig, T. Functional analysis of bispecific antibody (EpCAMxCD3)-mediated T-lymphocyte and cancer cell interaction by single-cell force spectroscopy. Int. J. Cancer 2011, 128, 2096–2104. [Google Scholar] [CrossRef]
- Lopez, J.I.; Kang, I.; You, W.-K.; McDonald, D.M.; Weaver, V.M. In situ force mapping of mammary gland transformation. Integr. Biol. 2011, 3, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Wulczyn, E.; Steiner, D.F.; Xu, Z.; Sadhwani, A.; Wang, H.; Flament-Auvigne, I.; Mermel, C.H.; Chen, P.-H.C.; Liu, Y.; Stumpe, M.C. Deep learning-based survival prediction for multiple cancer types using histopathology images. PLoS ONE 2020, 15, e0233678. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, N.; Jiang, L.; Gao, F.; Shao, J.; Wang, T.; Zhang, E.; Yu, H.; Wang, X.; Zheng, J. Clinical use of a machine learning histopathological image signature in diagnosis and survival prediction of clear cell renal cell carcinoma. Int. J. Cancer 2021, 148, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jia, Z.; Wang, L.-B.; Ai, Y.; Zhang, F.; Lai, M.; Chang, E.I.-C. Large scale tissue histopathology image classification, segmentation, and visualisation via deep convolutional activation features. BMC Bioinformatics 2017, 18, 281. [Google Scholar] [CrossRef]
- Franssen, L.C.; Lorenzi, T.; Burgess, A.E.F.; Chaplain, M.A.J. A Mathematical Framework for Modelling the Metastatic Spread of Cancer. Bull. Math. Biol. 2019, 81, 1965–2010. [Google Scholar] [CrossRef]
- Sfakianakis, N.; Chaplain, M.A.J. Mathematical Modelling of Cancer Invasion: A Review. In Springer Proceedings in Mathematics and Statistics; Suzuki, T., Poignard, C., Chaplain, M., Quaranta, V., Eds.; Springer: Singapore, 2021; Volume 370, pp. 153–172. [Google Scholar]
- Gao, W. Fourier spectrum analysis of full-field optical coherence tomography for tissue imaging. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150099. [Google Scholar] [CrossRef]
- Da Silva, L.G.; da Silva Monteiro, W.R.S.; de Aguiar Moreira, T.M.; Rabelo, M.A.E.; de Assis, E.A.C.P.; de Souza, G.T. Fractal dimension analysis as an easy computational approach to improve breast cancer histopathological diagnosis. Appl. Microsc. 2021, 51, 6. [Google Scholar] [CrossRef]
- Stehlík, M.; Hermann, P.; Nicolis, O. Fractal based cancer modelling. Revstat -Stat. J. 2016, 14, 139–155. [Google Scholar]
- Fan, K.A.; Neish, C.D.; Zanetti, M.; Kukko, A. An Improved Methodology for the 3-Dimensional Characterisation of Surface Roughness as Applied to Lava Flows. In Proceedings of the 49th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 19–23 March 2018; Lunar and Planetary Institute: Houston, TX, USA, 2018; p. 2526. [Google Scholar]
- Da Silva, V.R.; de Paiva, A.C.; Silva, A.C.; de Oliveira, A.C.M. Semivariogram Applied for Classification of Benign and Malignant Tissues in Mammography BT—Image Analysis and Recognition; Campilho, A., Kamel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 570–579. [Google Scholar]
- Muniandy, S.V.; Stanslas, J. Modelling of chromatin morphologies in breast cancer cells undergoing apoptosis using generalised Cauchy field. Comput. Med. Imaging Graph. 2008, 32, 631–637. [Google Scholar] [CrossRef]
- Mastrolonardo, M.; Conte, E.; Zbilut, J.P. A fractal analysis of skin pigmented lesions using the novel tool of the variogram technique. Chaos Solitons Fractals 2006, 28, 1119–1135. [Google Scholar] [CrossRef]
- Gringarten, E.; Deutsch, C.V. Teacher’s aide: Variogram interpretation and modeling. Math. Geol. 2001, 33, 507–534. [Google Scholar] [CrossRef]
- Adhikari, P.; Hasan, M.; Sridhar, V.; Roy, D.; Pradhan, P. Studying nanoscale structural alterations in cancer cells to evaluate ovarian cancer drug treatment, using transmission electron microscopy imaging. Phys. Biol. 2020, 17, 36005. [Google Scholar] [CrossRef]
- Das, N.; Alexandrov, S.; Gilligan, K.E.; Dwyer, R.M.; Saager, R.B.; Ghosh, N.; Leahy, M. Characterization of nanosensitive multifractality in submicron scale tissue morphology and its alteration in tumor progression. J. Biomed. Opt. 2021, 26, 16003. [Google Scholar] [CrossRef]
- Stylianou, A.; Stylianopoulos, T. Atomic Force Microscopy Probing of Cancer Cells and Tumor Microenvironment Components. Bionanoscience 2016, 6, 33–46. [Google Scholar] [CrossRef]
- Marcuello, C.; Frempong, G.A.; Balsera, M.; Medina, M.; Lostao, A. Atomic force microscopy to elicit conformational transitions of ferredoxin-dependent flavin thioredoxin reductases. Antioxidants 2021, 10, 1437. [Google Scholar] [CrossRef]
- Azzalini, E.; Abdurakhmanova, N.; Parisse, P.; Bartoletti, M.; Canzonieri, V.; Stanta, G.; Casalis, L.; Bonin, S. Cell-stiffness and morphological architectural patterns in clinical samples of high grade serous ovarian cancers. Nanomed. Nanotechnol. Biol. Med. 2021, 37, 102452. [Google Scholar] [CrossRef]
- Peñuela, L.; Villaggio, B.; Raiteri, R.; Fiocca, R.; Vellone, V.G. Kidney Ultrastructure by Atomic Force Microscopy Imaging Directly From Formalin Fixed-Paraffin Embedded Biopsy: Is This a Dream Come True? Int. J. Surg. Pathol. 2017, 26, 532–533. [Google Scholar] [CrossRef]
- Lennon, F.E.; Cianci, G.C.; Cipriani, N.A.; Hensing, T.A.; Zhang, H.J.; Chen, C.-T.; Murgu, S.D.; Vokes, E.E.; Vannier, M.W.; Salgia, R. Lung cancer-a fractal viewpoint. Nat. Rev. Clin. Oncol. 2015, 12, 664–675. [Google Scholar] [CrossRef]
- Starodubtseva, M.N.; Starodubtsev, I.E.; Starodubtsev, E.G. Novel fractal characteristic of atomic force microscopy images. Micron 2017, 96, 96–102. [Google Scholar] [CrossRef]
- Guz, N.V.; Dokukin, M.E.; Woodworth, C.D.; Cardin, A.; Sokolov, I. Towards early detection of cervical cancer: Fractal dimension of AFM images of human cervical epithelial cells at different stages of progression to cancer. Nanomedicine 2015, 11, 1667–1675. [Google Scholar] [CrossRef]
- Sokolov, I. Fractals: A possible new path to diagnose and cure cancer? Futur. Oncol. 2015, 11, 3049–3051. [Google Scholar] [CrossRef] [PubMed]
- John, S. Nicolis Dynamics of Hierarchical Systems. An Evolutionary Approach, 1st ed.; Department of Electrical EngineeringUniversity of PatrasPatrasGreece; Springer: Berlin/Heidelberg, Germany, 1986; ISBN 978-3-540-13323-0. [Google Scholar]
- Velentzas, A.D.; Velentzas, P.D.; Katarachia, S.A.; Anagnostopoulos, A.K.; Sagioglou, N.E.; Thanou, E.V.; Tsioka, M.M.; Mpakou, V.E.; Kollia, Z.; Gavriil, V.E.; et al. The indispensable contribution of s38 protein to ovarian-eggshell morphogenesis in Drosophila melanogaster. Sci. Rep. 2018, 8, 16103. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Rigot, V.; Thimonier, J.; Montixi, C.; Parat, F.; Pommier, G.; Marvaldi, J.; Luis, J. Integrins and E-cadherin cooperate with IGF-I to induce migration of epithelial colonic cells. Int. J. Cancer 1999, 83, 497–505. [Google Scholar] [CrossRef]
- La Porta, C.; Zapperi, S. The Physics of Cancer; Cambridge University Press: Cambridge, UK, 2017; ISBN 978-1107109599. [Google Scholar]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, K.; Okazawa, Y.; Haeno, H.; Koyama, Y.; Sulidan, K.; Komiyama, H.; Saeki, H.; Ohtsuji, N.; Ito, Y.; Kojima, Y.; et al. Metastatic seeding of human colon cancer cell clusters expressing the hybrid epithelial/mesenchymal state. Int. J. Cancer 2020, 146, 2547–2562. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yang, J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Deshmukh, A.P.; den Hollander, P.; Addanki, S.; Kuburich, N.A.; Kudaravalli, S.; Joseph, R.; Chang, J.T.; Soundararajan, R.; Mani, S.A. EMTome: A resource for pan-cancer analysis of epithelial-mesenchymal transition genes and signatures. Br. J. Cancer 2021, 124, 259–269. [Google Scholar] [CrossRef]
- Dokukin, M.E.; Guz, N.V.; Woodworth, C.D.; Sokolov, I. Emerging of fractal geometry on surface of human cervical epithelial cells during progression towards cancer. New J. Phys. 2015, 17, 33019. [Google Scholar] [CrossRef]
- Bakalis, E.; Ferraro, A.; Gavriil, V.; Pepe, F.; Kollia, Z.; Cefalas, A.C.; Malapelle, U.; Sarantopoulou, E.; Troncone, G.; Zerbetto, F. Universal Markers Unveil Metastatic Cancerous Cross-Sections at Nanoscale. Cancers 2022, 14, 3728. [Google Scholar] [CrossRef]
- Bakalis, E.; Gavriil, V.; Cefalas, A.-C.; Kollia, Z.; Zerbetto, F.; Sarantopoulou, E. Viscoelasticity and Noise Properties Reveal the Formation of Biomemory in Cells. J. Phys. Chem. B 2021, 125, 10883–10892. [Google Scholar] [CrossRef]
- Pham, K.; Frieboes, H.B.; Cristini, V.; Lowengrub, J. Predictions of tumour morphological stability and evaluation against experimental observations. J. R. Soc. Interface 2011, 8, 16–29. [Google Scholar] [CrossRef]
- Marcuello, C.; Chambel, L.; Rodrigues, M.S.; Ferreira, L.P.; Cruz, M.M. Magnetotactic Bacteria: Magnetism beyond Magnetosomes. IEEE Trans. Nanobioscience 2018, 17, 555–559. [Google Scholar] [CrossRef]
- Popescu, R.C.; Andronescu, E.; Vasile, B.S. Recent advances in magnetite nanoparticle functionalisation for nanomedicine. Nanomaterials 2019, 9, 1791. [Google Scholar] [CrossRef]
- Darroudi, M.; Gholami, M.; Rezayi, M.; Khazaei, M. An overview and bibliometric analysis on the colorectal cancer therapy by magnetic functionalised nanoparticles for the responsive and targeted drug delivery. J. Nanobiotechnology 2021, 19, 399. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Hurst, H.E. Long-Term Storage Capacity of Reservoirs. Trans. Am. Soc. Civ. Eng. 1951, 116, 770–808. [Google Scholar] [CrossRef]
- HURST: MATLAB function to compute the Hurst exponent using R/S Analysis. Available online: https://ideas.repec.org/c/wuu/hscode/m11003.html (accessed on 30 September 2011).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavriil, V.; Ferraro, A.; Cefalas, A.-C.; Kollia, Z.; Pepe, F.; Malapelle, U.; De Luca, C.; Troncone, G.; Sarantopoulou, E. Nanoscale Prognosis of Colorectal Cancer Metastasis from AFM Image Processing of Histological Sections. Cancers 2023, 15, 1220. https://doi.org/10.3390/cancers15041220
Gavriil V, Ferraro A, Cefalas A-C, Kollia Z, Pepe F, Malapelle U, De Luca C, Troncone G, Sarantopoulou E. Nanoscale Prognosis of Colorectal Cancer Metastasis from AFM Image Processing of Histological Sections. Cancers. 2023; 15(4):1220. https://doi.org/10.3390/cancers15041220
Chicago/Turabian StyleGavriil, Vassilios, Angelo Ferraro, Alkiviadis-Constantinos Cefalas, Zoe Kollia, Francesco Pepe, Umberto Malapelle, Caterina De Luca, Giancarlo Troncone, and Evangelia Sarantopoulou. 2023. "Nanoscale Prognosis of Colorectal Cancer Metastasis from AFM Image Processing of Histological Sections" Cancers 15, no. 4: 1220. https://doi.org/10.3390/cancers15041220
APA StyleGavriil, V., Ferraro, A., Cefalas, A.-C., Kollia, Z., Pepe, F., Malapelle, U., De Luca, C., Troncone, G., & Sarantopoulou, E. (2023). Nanoscale Prognosis of Colorectal Cancer Metastasis from AFM Image Processing of Histological Sections. Cancers, 15(4), 1220. https://doi.org/10.3390/cancers15041220








