Targeting Ubiquitin-like Protein, ISG15, as a Novel Tumor Associated Antigen in Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Lines
2.3. Listeria Monocytogenes Strains
2.4. ISG15 Expression in Normal and Tumor Mouse Colon Tissue
2.5. Western Blot Analysis
2.6. In Vitro Cytotoxicity and Infectivity Assays
2.7. Tumor Immunotherapy with Lm-LLO-ISG15
2.8. Lymphocyte Depletion Experiments
2.9. Multi-Color Flow Cytometry
2.10. Statistical Analyses
3. Results
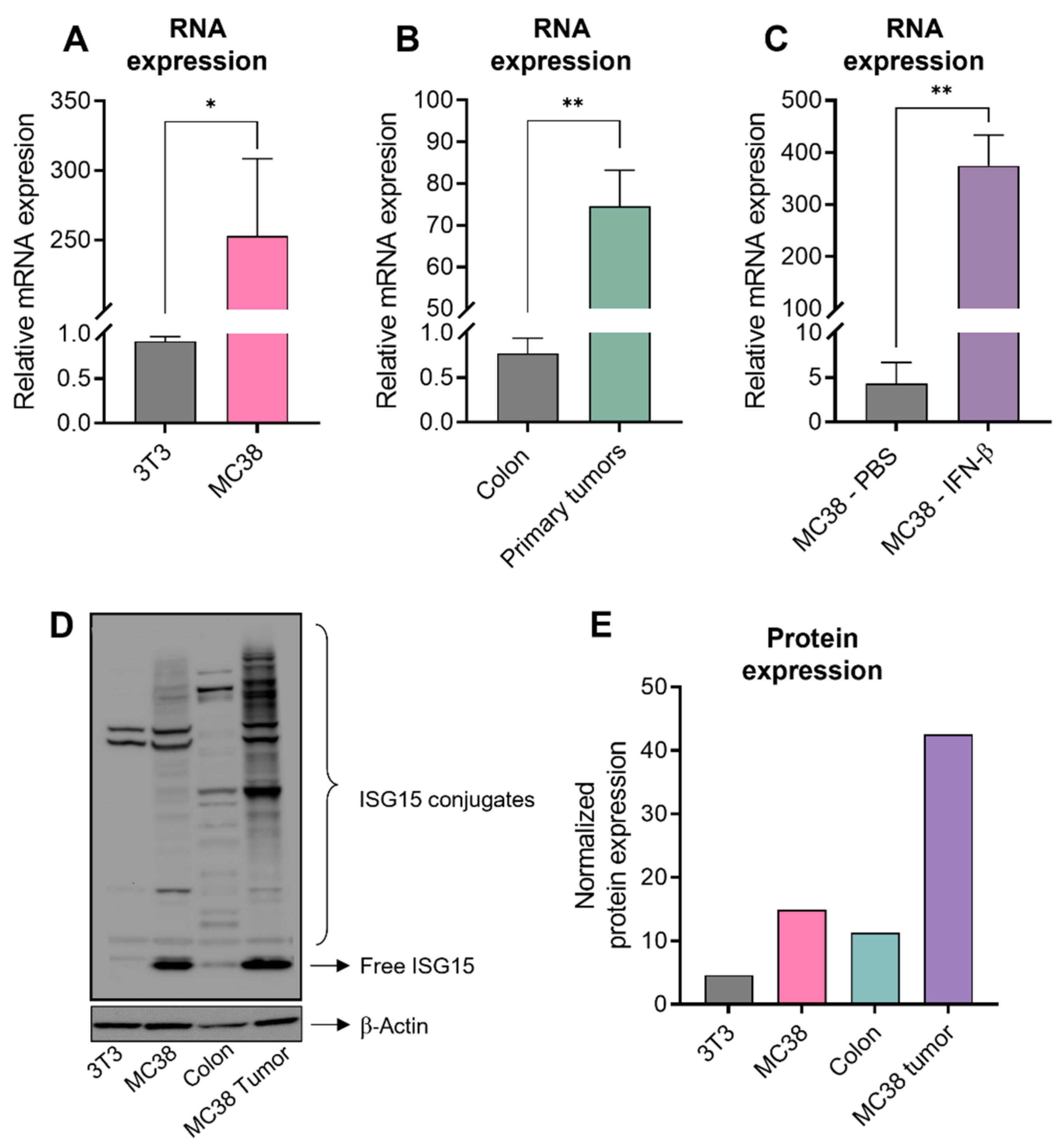
3.1. Elevation of ISG15 Expression in CRC Tumors Is Correlated with an Unfavorable Prognosis
3.2. High Level of ISG15 Expression in Human CRC Is Also Conserved in Murine CRC
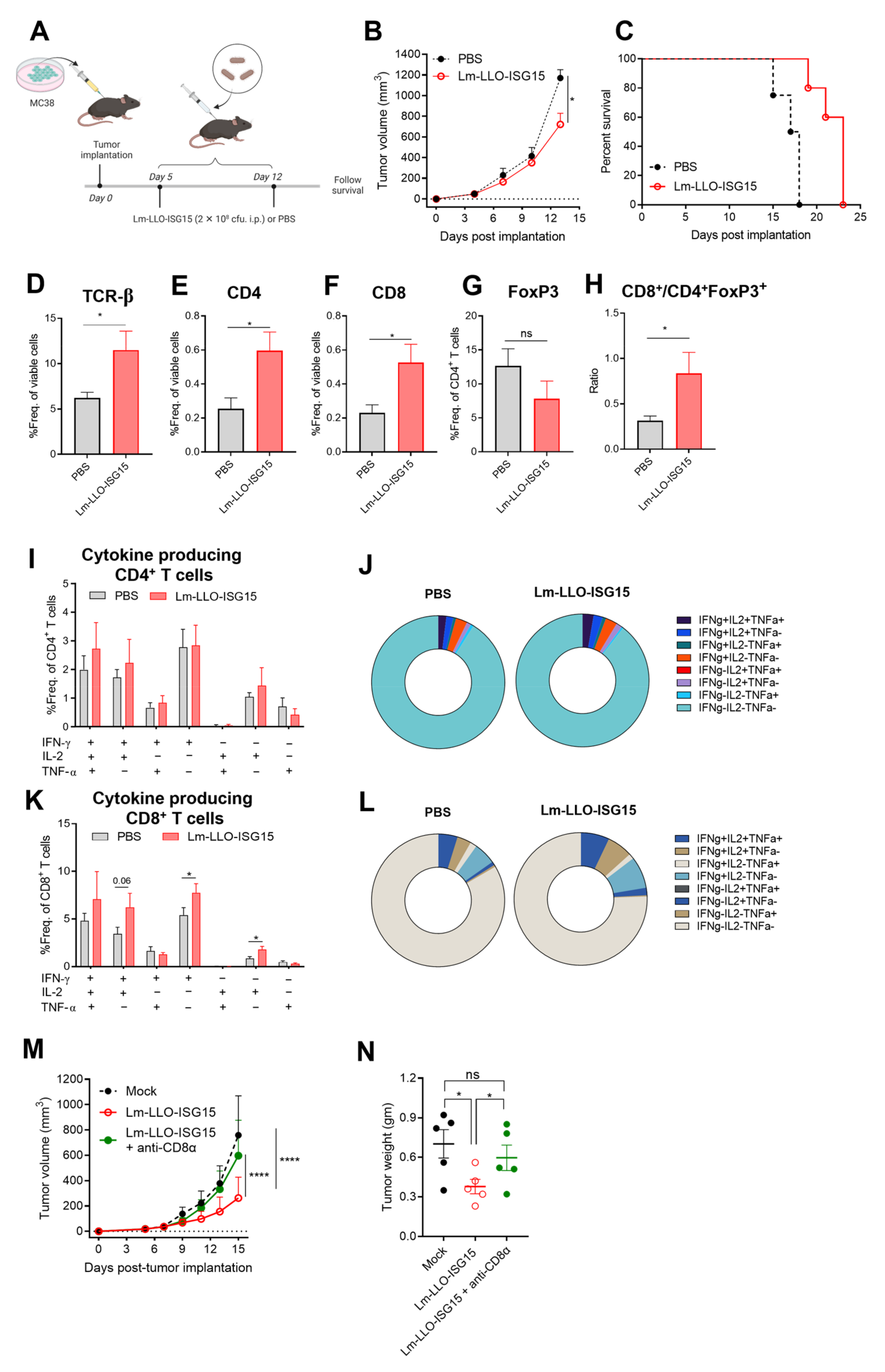
3.3. Lm-LLO-ISG15 Demonstrates Potential Anti-Tumor Effects in Subcutaneous CRC Mouse Model in a CD8+ T Cell-Dependent Manner
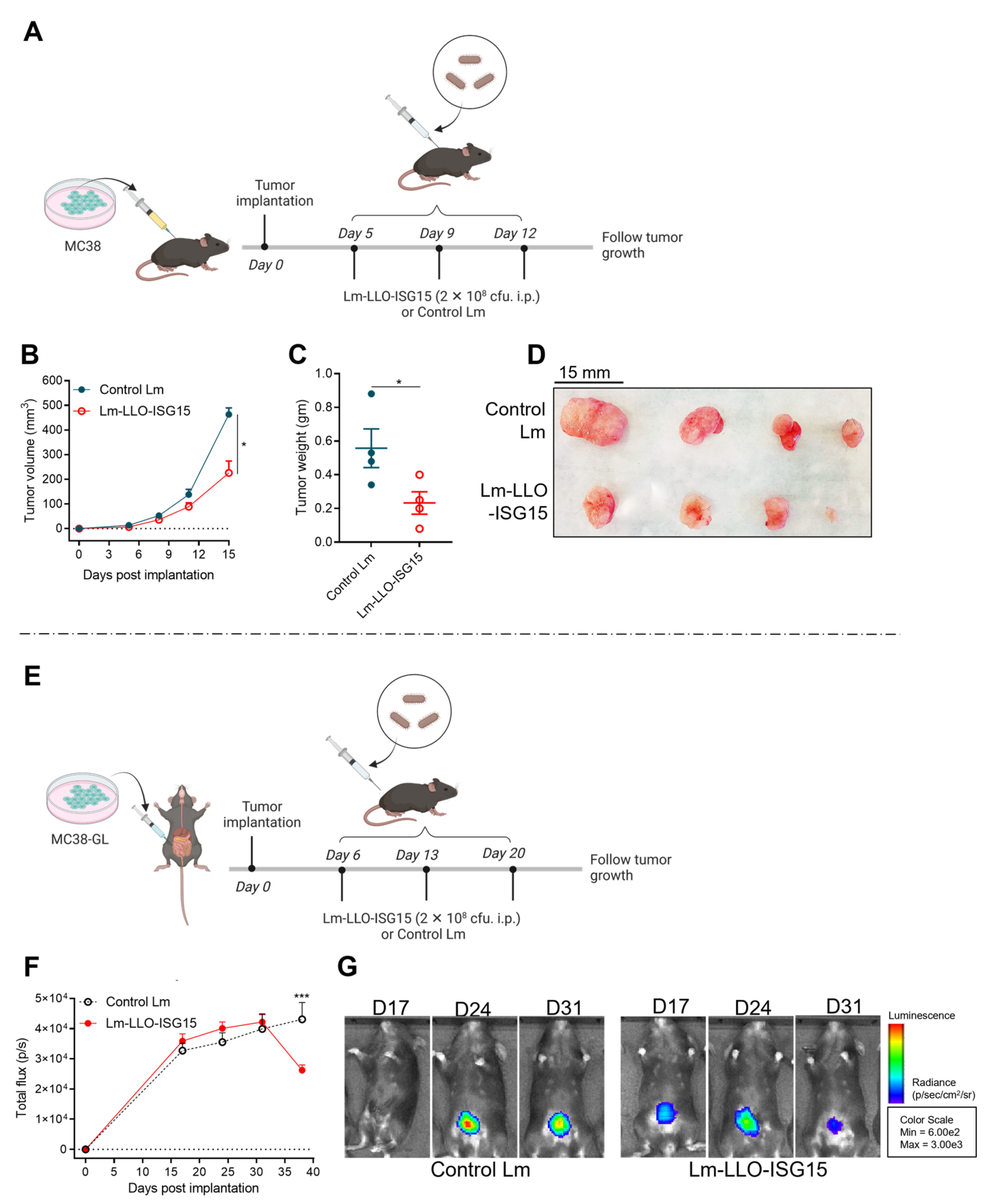
3.4. Induction of Anti-Tumor Immune Response with Lm-LLO-ISG15 by Oral and Intraperitoneal Administration in Orthotopic CRC Tumors
3.5. Comparison of Efficacy of Lm-LLO-ISG15 versus Control Lm in Subcutaneous and Orthotopic CRC Mouse Models
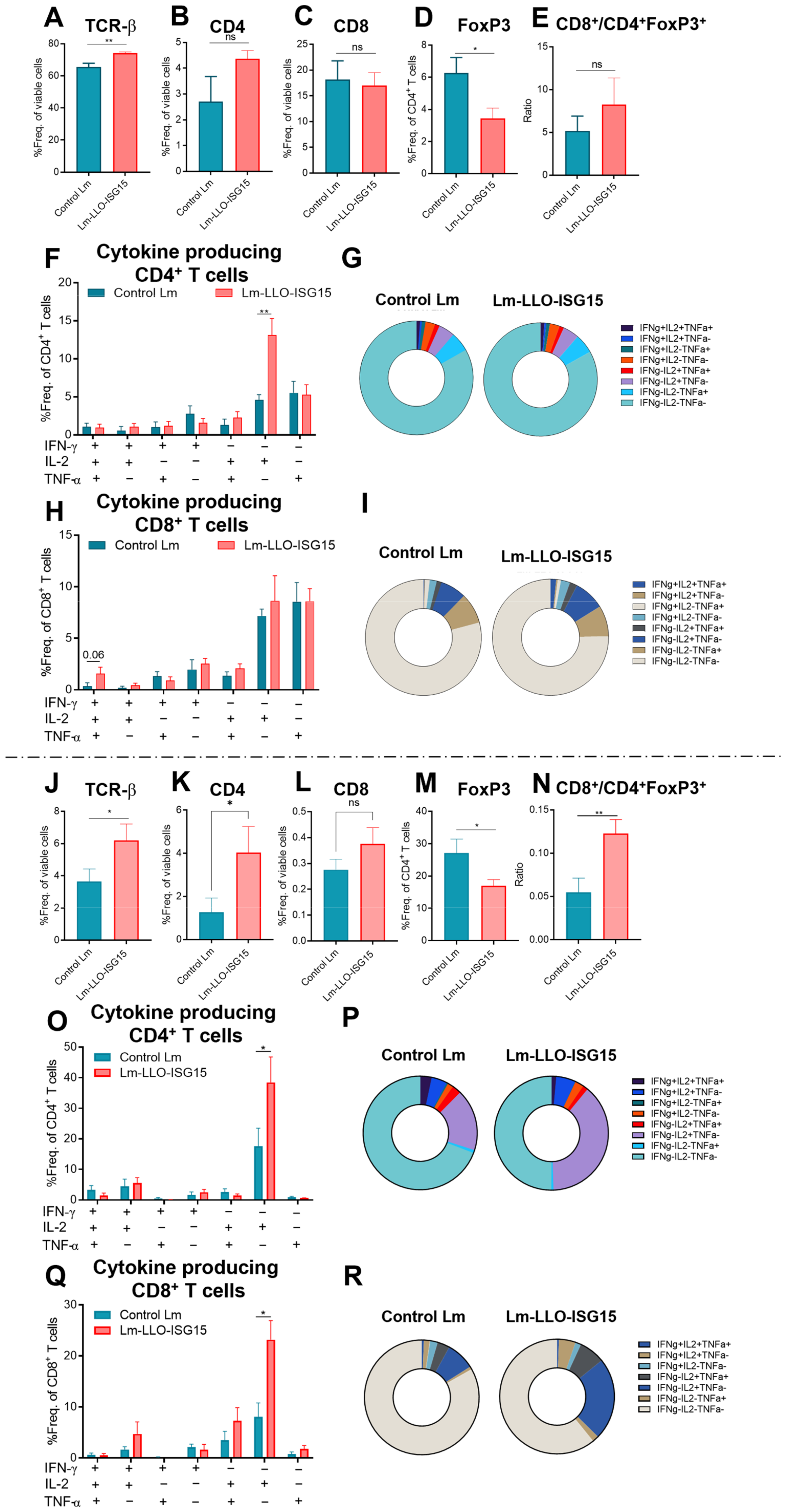
3.6. Induction of Anti-Tumor Immune Response with Lm-LLO-ISG15 in Subcutaneous and Orthotopic CRC Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ciombor, K.; Jones, J.; Strickler, J.; Bekaii-Saab, T.; Wu, C. The Current Molecular Treatment Landscape of Advanced Colorectal Cancer and Need for the COLOMATE Platform. Oncology 2021, 35, 553–559. [Google Scholar] [PubMed]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Chen, L.; Yang, F.; Chen, S.; Tai, J. Mechanisms on chemotherapy resistance of colorectal cancer stem cells and research progress of reverse transformation: A mini-review. Front. Med. 2022, 9, 995882. [Google Scholar] [CrossRef]
- Ruan, W.-C.; Che, Y.-P.; Ding, L.; Li, H.-F. Efficacy and Toxicity of Addition of Bevacizumab to Chemotherapy in Patients with Metastatic Colorectal Cancer. Comb. Chem. High Throughput Screen. 2018, 21, 718–724. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, W.; Yue, L.; Dai, X.; Rong, D.; Wu, F.; Gu, J.; Qian, X. Perspectives on Immunotherapy of Metastatic Colorectal Cancer. Front. Oncol. 2021, 11, 659964. [Google Scholar] [CrossRef]
- Motta, R.; Cabezas-Camarero, S.; Torres-Mattos, C.; Riquelme, A.; Calle, A.; Figueroa, A.; Sotelo, M.J. Immunotherapy in microsatellite instability metastatic colorectal cancer: Current status and future perspectives. J. Clin. Transl. Res. 2021, 7, 511–522. [Google Scholar]
- Overman, M.J.; Ernstoff, M.; Morse, M. Where We Stand with Immunotherapy in Colorectal Cancer: Deficient Mismatch Repair, Proficient Mismatch Repair, and Toxicity Management. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 239–247. [Google Scholar] [CrossRef]
- Kotoula, V.; Fostira, F.; Fountzilas, E. Primary Resistance to Immune Checkpoint Inhibitors in Metastatic Colorectal Cancer—Beyond the Misdiagnosis. JAMA Oncol. 2019, 5, 740–741. [Google Scholar] [CrossRef]
- Cohen, R.; Hain, E.; Buhard, O.; Guilloux, A.; Bardier, A.; Kaci, R.; Bertheau, P.; Renaud, F.; Bibeau, F.; Fléjou, J.-F.; et al. Association of Primary Resistance to Immune Checkpoint Inhibitors in Metastatic Colorectal Cancer with Misdiagnosis of Microsatellite Instability or Mismatch Repair Deficiency Status. JAMA Oncol. 2019, 5, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Sarvizadeh, M.; Ghasemi, F.; Tavakoli, F.; Khatami, S.S.; Razi, E.; Sharifi, H.; Biouki, N.M.; Taghizadeh, M. Vaccines for colorectal cancer: An update. J. Cell. Biochem. 2019, 120, 8815–8828. [Google Scholar] [CrossRef]
- Oladejo; Paterson, Y.; Wood, L. Clinical Experience and Recent Advances in the Development of Listeria-Based Tumor Immunotherapies. Front. Immunol. 2021, 12, 642316. [Google Scholar] [CrossRef]
- Wood, L.M.; Paterson, Y. Attenuated Listeria monocytogenes: A powerful and versatile vector for the future of tumor immunotherapy. Front. Cell. Infect. Microbiol. 2014, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Flickinger, J.C., Jr.; Staudt, R.E.; Singh, J.; Carlson, R.D.; Barton, J.R.; Baybutt, T.R.; Rappaport, J.A.; Zalewski, A.; Pattison, A.; Waldman, S.A.; et al. Chimeric adenoviral (Ad5.F35) and listeria vector prime-boost immunization is safe and effective for cancer immunotherapy. NPJ Vaccines 2022, 7, 61. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, H.; Xiang, T.; Wang, G. Clinical Application of Adaptive Immune Therapy in MSS Colorectal Cancer Patients. Front. Immunol. 2021, 12, 762341. [Google Scholar] [CrossRef]
- Perng, Y.C.; Lenschow, D. ISG15 in antiviral immunity and beyond. Nat. Rev. Microbiol. 2018, 16, 423–439. [Google Scholar] [CrossRef]
- Wood, L.; Seavey, M.; Pan, Z.-K.; Paterson, Y. ISG15 as a Novel Target for Tumor Immunotherapy (95.13). J. Immunol. 2010, 184 (Suppl. 1), 95.13. [Google Scholar] [CrossRef]
- Desai, S.D. ISG15: A double edged sword in cancer. Oncoimmunology 2015, 4, e1052935. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.-M.; Gaikwad, S.; Oladejo, M.; Agrawal, M.Y.; Srivastava, S.K.; Wood, L.M. Interferon stimulated gene 15 (ISG15) in cancer: An update. Cancer Lett. 2023, 556, 216080. [Google Scholar] [CrossRef]
- Oladejo, M.; Nguyen, H.-M.; Silwal, A.; Reese, B.; Paulishak, W.; Markiewski, M.M.; Wood, L.M. Listeria-based immunotherapy directed against CD105 exerts anti-angiogenic and anti-tumor efficacy in renal cell carcinoma. Front. Immunol. 2022, 13, 1038807. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.; Leong, X.; Engleman, E. Orthotopic mouse model of colorectal cancer. J. Vis. Exp. 2007, 10, 484. [Google Scholar]
- Kochall, S.; Thepkaysone, M.L.; García, S.A.; Betzler, A.M.; Weitz, J.; Reissfelder, C.; Schölch, S. Isolation of Circulating Tumor Cells in an Orthotopic Mouse Model of Colorectal Cancer. J. Vis. Exp. 2017, 125, 55357. [Google Scholar]
- Ghouse, S.M.; Nguyen, H.-M.; Bommareddy, P.K.; Guz-Montgomery, K.; Saha, D. Oncolytic Herpes Simplex Virus Encoding IL12 Controls Triple-Negative Breast Cancer Growth and Metastasis. Front. Oncol. 2020, 10, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaud, C.; Westwood, J.A.; John, L.B.; Flynn, J.K.; Paquet-Fifield, S.; Duong, C.P.M.; Yong, C.S.M.; Pegram, H.J.; Stacker, S.A.; Achen, M.G.; et al. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol. Ther. 2014, 22, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Fan, W.; Rachagani, S.; Zhou, Z.; Lele, S.M.; Batra, S.K.; Garrison, J.C. Comparative Study of Subcutaneous and Orthotopic Mouse Models of Prostate Cancer: Vascular Perfusion, Vasculature Density, Hypoxic Burden and BB2r-Targeting Efficacy. Sci. Rep. 2019, 9, 11117. [Google Scholar] [CrossRef] [Green Version]
- Pan, Z.K.; Ikonomidis, G.; Pardoll, D.; Paterson, Y. Regression of established tumors in mice mediated by the oral administration of a recombinant Listeria monocytogenes vaccine. Cancer Res. 1995, 55, 4776–4779. [Google Scholar]
- Pan, Z.K.; Weiskirch, L.; Paterson, Y. Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Res. 1999, 59, 5264–5269. [Google Scholar]
- Lin, C.-W.; Lee, J.-Y.; Tsao, Y.-P.; Shen, C.-P.; Lai, H.-C.; Chen, S.-L. Oral vaccination with recombinant Listeria monocytogenes expressing human papillomavirus type 16 E7 can cause tumor growth in mice to regress. Int. J. Cancer 2002, 102, 629–637. [Google Scholar] [CrossRef]
- Sinha, S.; Kuo, C.-Y.; Ho, J.K.; White, P.J.; Jazayeri, J.A.; Pouton, C.W. A suicidal strain of Listeria monocytogenes is effective as a DNA vaccine delivery system for oral administration. Vaccine 2017, 35, 5115–5122. [Google Scholar] [CrossRef]
- Borelli, B.; Antoniotti, C.; Carullo, M.; Germani, M.M.; Conca, V.; Masi, G. Immune-Checkpoint Inhibitors (ICIs) in Metastatic Colorectal Cancer (mCRC) Patients beyond Microsatellite Instability. Cancers 2022, 14, 4974. [Google Scholar] [CrossRef]
- Bolado-Carrancio, A.; Lee, M.; Ewing, A.; Muir, M.; Macleod, K.G.; Gallagher, W.M.; Nguyen, L.K.; Carragher, N.O.; Semple, C.A.; Brunton, V.G.; et al. ISGylation drives basal breast tumour progression by promoting EGFR recycling and Akt signalling. Oncogene 2021, 40, 6235–6247. [Google Scholar] [CrossRef]
- Alcalá, S.; Sancho, P.; Martinelli, P.; Navarro, D.; Pedrero, C.; Martín-Hijano, L.; Valle, S.; Earl, J.; Rodríguez-Serrano, M.; Ruiz-Cañas, L.; et al. ISG15 and ISGylation is required for pancreatic cancer stem cell mitophagy and metabolic plasticity. Nat. Commun. 2020, 11, 2682. [Google Scholar] [CrossRef]
- Desai, S.D.; Reed, R.E.; Burks, J.; Wood, L.M.; Pullikuth, A.K.; Haas, A.L.; Liu, L.; Breslin, J.W.; Meiners, S.; Sankar, S. ISG15 disrupts cytoskeletal architecture and promotes motility in human breast cancer cells. Exp. Biol. Med. 2012, 237, 38–49. [Google Scholar] [CrossRef]
- Chen, R.-H.; Xiao, Z.-W.; Yan, X.-Q.; Han, P.; Liang, F.-Y.; Wang, J.-Y.; Yu, S.-T.; Zhang, T.-Z.; Chen, S.-Q.; Zhong, Q.; et al. Tumor Cell-Secreted ISG15 Promotes Tumor Cell Migration and Immune Suppression by Inducing the Macrophage M2-Like Phenotype. Front. Immunol. 2020, 11, 594775. [Google Scholar] [CrossRef]
- Nguyen, H.-M.; Oladejo, M.; Paulishak, W.; Wood, L.M. A Listeria-based vaccine targeting ISG15 exerts anti-tumor efficacy in renal cell carcinoma. Cancer Immunol. Immunother. 2022. [Google Scholar] [CrossRef]
- Hochnadel, I.; Hoenicke, L.; Petriv, N.; Neubert, L.; Reinhard, E.; Hirsch, T.; Alfonso, J.C.L.; Suo, H.; Longerich, T.; Geffers, R.; et al. Safety and efficacy of prophylactic and therapeutic vaccine based on live-attenuated Listeria monocytogenes in hepatobiliary cancers. Oncogene 2022, 41, 2039–2053. [Google Scholar] [CrossRef]
- Keenan, B.P.; Saenger, Y.; Kafrouni, M.I.; Leubner, A.; Lauer, P.; Maitra, A.; Rucki, A.A.; Gunderson, A.J.; Coussens, L.M.; Brockstedt, D.G.; et al. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology 2014, 146, 1784–1794. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.-M.; Wood, L. 1370 Distinct anti-tumor response to Listeria-based vaccines between orthotopic and subcutaneous syngeneic mouse models of renal cell carcinoma. J. Immunol. Ther. Cancer 2022, 10 (Suppl. 2), A1420–A1421. [Google Scholar]
- Wood, L.M.; Pan, Z.-K.; Seavey, M.M.; Muthukumaran, G.; Paterson, Y. The ubiquitin-like protein, ISG15, is a novel tumor-associated antigen for cancer immunotherapy. Cancer Immunol. Immunother. 2012, 61, 689–700. [Google Scholar] [CrossRef] [Green Version]
- Lecuit, M.; Dramsi, S.; Gottardi, C.; Fedor-Chaiken, M.; Gumbiner, B.; Cossart, P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999, 18, 3956–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Li, L.; Starr, T.K.; Subramanian, S. Tumor location impacts immune response in mouse models of colon cancer. Oncotarget 2017, 8, 54775–54787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.; Yin, Y.; Duan, F.; Fu, H.; Hu, M.; Gao, Y.; Pan, Z.; Jiao, X. Prophylactic and therapeutic efficacy of an attenuated Listeria monocytogenes-based vaccine delivering HPV16 E7 in a mouse model. Int. J. Mol. Med. 2012, 30, 1335–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.-M.; Gaikwad, S.; Oladejo, M.; Paulishak, W.; Wood, L.M. Targeting Ubiquitin-like Protein, ISG15, as a Novel Tumor Associated Antigen in Colorectal Cancer. Cancers 2023, 15, 1237. https://doi.org/10.3390/cancers15041237
Nguyen H-M, Gaikwad S, Oladejo M, Paulishak W, Wood LM. Targeting Ubiquitin-like Protein, ISG15, as a Novel Tumor Associated Antigen in Colorectal Cancer. Cancers. 2023; 15(4):1237. https://doi.org/10.3390/cancers15041237
Chicago/Turabian StyleNguyen, Hong-My, Shreyas Gaikwad, Mariam Oladejo, Wyatt Paulishak, and Laurence M. Wood. 2023. "Targeting Ubiquitin-like Protein, ISG15, as a Novel Tumor Associated Antigen in Colorectal Cancer" Cancers 15, no. 4: 1237. https://doi.org/10.3390/cancers15041237
APA StyleNguyen, H. -M., Gaikwad, S., Oladejo, M., Paulishak, W., & Wood, L. M. (2023). Targeting Ubiquitin-like Protein, ISG15, as a Novel Tumor Associated Antigen in Colorectal Cancer. Cancers, 15(4), 1237. https://doi.org/10.3390/cancers15041237






