Primary and Secondary Tumors of the Parotid Gland: Clinical Features and Prognosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
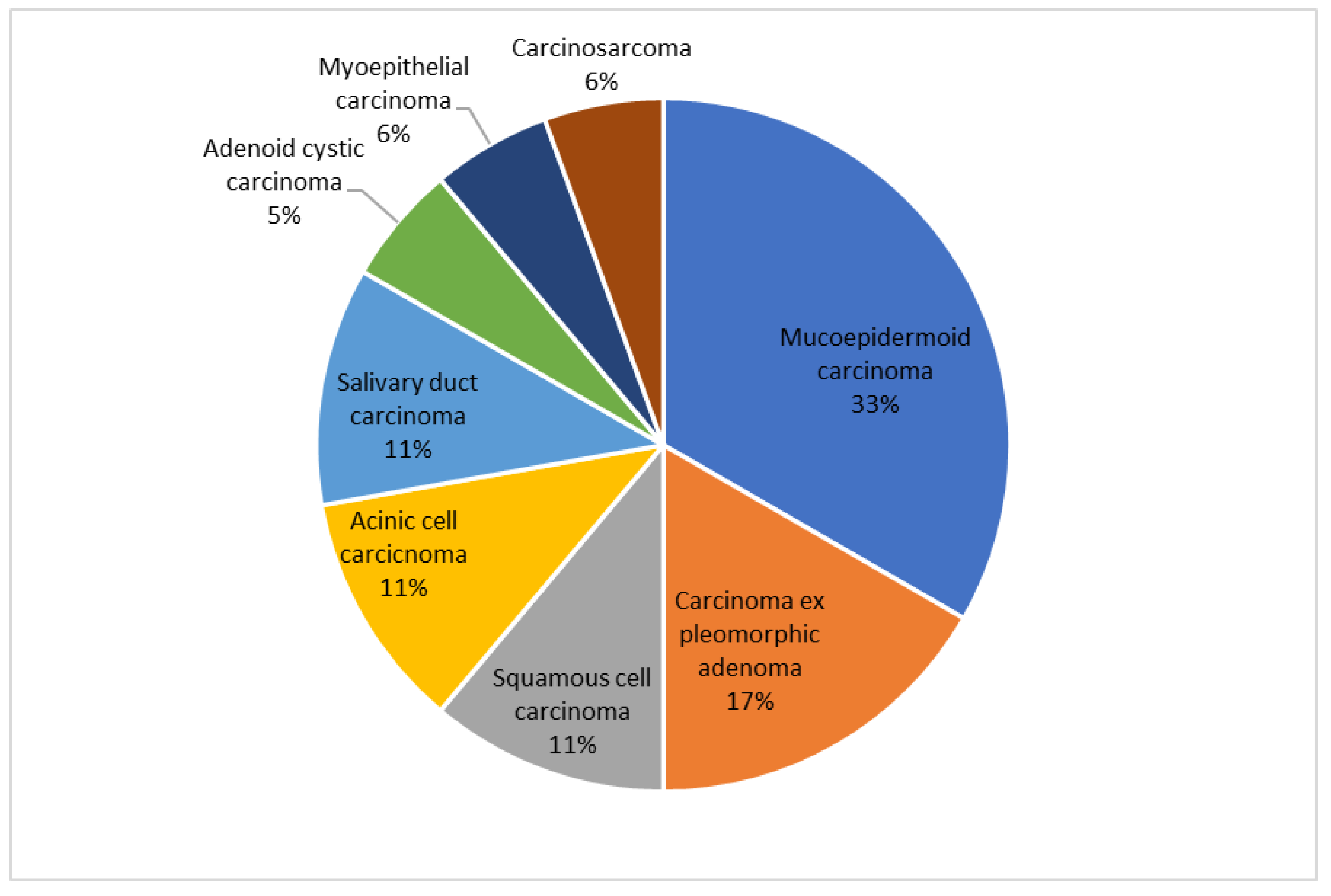
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gatta, G.; Guzzo, M.; Locati, L.D.; McGurk, M.; Prott, F.J. Major and minor salivary gland tumours. Crit. Rev. Oncol. Hematol. 2020, 152, 102959. [Google Scholar] [CrossRef] [PubMed]
- Saravakos, P.; Kourtidis, S.; Hartwein, J.; Preyer, S. Parotid Gland Tumors: A Multicenter Analysis of 1020 Cases. Increasing Incidence of Warthin’s Tumor. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Thoelken, R.; Jering, M.; Märkl, B.; Zenk, J. Metastases of Cutaneous Squamous Cell Carcinoma Seem to be the Most Frequent Malignancies in the Parotid Gland: A Hospital-Based Study From a Salivary Gland Center. Head Neck Pathol. 2021, 15, 843–851. [Google Scholar] [CrossRef]
- Nuyens, M.; Schüpbach, J.; Stauffer, E.; Zbären, P. Metastatic disease to the parotid gland. Otolaryngol. Head. Neck Surg. 2006, 135, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Jering, M.; Mayer, M.; Thölken, R.; Schiele, S.; Müller, G.; Zenk, J. Cancer-specific and overall survival of patients with primary and metastatic malignancies of the parotid gland—A retrospective study. J. Craniomaxillofac. Surg. 2022, 50, 456–461. [Google Scholar] [CrossRef]
- Meyer, M.F.; Wolber, P.; Arolt, C.; Wessel, M.; Quaas, A.; Lang, S.; Klussmann, J.P.; Semrau, R.; Beutner, D. Survival after parotid gland metastases of cutaneous squamous cell carcinoma of the head and neck. Oral Maxillofac. Surg. 2021, 25, 383–388. [Google Scholar] [CrossRef]
- Bobin, C.; Ingrand, P.; Dréno, B.; Rio, E.; Malard, O.; Espitalier, F. Prognostic factors for parotid metastasis of cutaneous squamous cell carcinoma of the head and neck. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 99–103. [Google Scholar] [CrossRef]
- Mooney, C.P.; Clark, J.R.; Shannon, K.; Palme, C.E.; Ebrahimi, A.; Gao, K.; Ch’ng, S.; Elliott, M.; Gupta, R.; Low, T.H. The significance of regional metastasis location in head and neck cutaneous squamous cell carcinoma. Head Neck 2021, 43, 2705–2711. [Google Scholar] [CrossRef]
- O’Brien, C.J.; McNeil, E.B.; McMahon, J.D.; Pathak, I.; Lauer, C.S.; Jackson, M.A. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck 2002, 24, 417–422. [Google Scholar] [CrossRef]
- Badlani, J.; Gupta, R.; Smith, J.; Ashford, B.; Ch’ng, S.; Veness, M.; Clark, J. Metastases to the parotid gland—A review of the clinicopathological evolution, molecular mechanisms and management. Surg. Oncol. 2018, 27, 44–53. [Google Scholar] [CrossRef]
- Ch’ng, S.; Maitra, A.; Lea, R.; Brasch, H.; Tan, S.T. Parotid metastasis—An independent prognostic factor for head and neck cutaneous squamous cell carcinoma. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 1288–1293. [Google Scholar] [CrossRef]
- O’Hara, J.; Ferlito, A.; Takes, R.P.; Rinaldo, A.; Strojan, P.; Shaha, A.R.; Rodrigo, J.P.; Paleri, V. Cutaneous squamous cell carcinoma of the head and neck metastasizing to the parotid gland—A review of current recommendations. Head Neck 2011, 33, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Shigeishi, H.; Ohta, K.; Okui, G.; Seino, S.; Hashikata, M.; Yamamoto, K.; Ishida, Y.; Sasaki, K.; Naruse, T.; Rahman, M.Z.; et al. Clinicopathological analysis of salivary gland carcinomas and literature review. Mol. Clin. Oncol. 2015, 3, 202–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, F.A.; Duarte, E.C.B.; Taveira, C.T.; Máximo, A.A.; de Aquino, É.C.; de Cássia Alencar, R.; Vencio, E.F. Salivary gland tumor: A review of 599 cases in a Brazilian population. Head Neck Pathol. 2009, 3, 271–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzilai, G.; Greenberg, E.; Cohen-Kerem, R.; Doweck, I. Pattern of regional metastases from cutaneous squamous cell carcinoma of the head and neck. Otolaryngol.-Head Neck Surg. 2005, 132, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Czerwonka, L.; De Santis, R.J.; Horowitz, G.; Hong, M.; Orsini, M.; Enepekides, D.; Goldstein, D.P.; Dort, J.; Higgins, K. Staging cutaneous squamous cell carcinoma metastases to the parotid gland. Laryngoscope 2017, 127, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Franzen, A.; Buchali, A.; Lieder, A. The rising incidence of parotid metastases: Our experience from four decades of parotid gland surgery. Acta Otorhinolaryngol. Ital. 2017, 37, 264–269. [Google Scholar] [CrossRef]
- Lombardi, D.; McGurk, M.; Vander Poorten, V.; Guzzo, M.; Accorona, R.; Rampinelli, V.; Nicolai, P. Surgical treatment of salivary malignant tumors. Oral Oncol. 2017, 65, 102–113. [Google Scholar] [CrossRef]
- Thielker, J.; Grosheva, M.; Ihrler, S.; Wittig, A.; Guntinas-Lichius, O. Contemporary Management of Benign and Malignant Parotid Tumors. Front. Surg. 2018, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Kejner, A.E.; Harris, B.N.; Patel, R.; McMullen, C.; Weir, J.; Dahshan, B.A.; Carroll, W.R.; Gillespie, M.B. Management of the parotid for high-risk cutaneous squamous cell carcinoma: A review from the salivary section of the American Head and Neck Society. Am. J. Otolaryngol. 2022, 43, 103374. [Google Scholar] [CrossRef]
- Baker, N.J.; Webb, A.A.C.; Macpherson, D. Surgical management of cutaneous squamous cell carcinoma of the head and neck. Br. J. Oral Maxillofac. Surg. 2001, 39, 87–90. [Google Scholar] [CrossRef]
- Dona, E.; Veness, M.J.; Cakir, B.; Morgan, G.J. Metastatic cutaneous squamous cell carcinoma to the parotid: The role of surgery and adjuvant radiotherapy to achieve best outcome. ANZ J. Surg. 2003, 73, 692–696. [Google Scholar] [CrossRef]
- Hong, T.S.; Kriesel, K.J.; Hartig, G.K.; Harari, P.M. Parotid area lymph node metastases from cutaneous squamous cell carcinoma: Implications for diagnosis, treatment, and prognosis. Head Neck 2005, 27, 851–856. [Google Scholar] [CrossRef]
- Audet, N.; Palme, C.E.; Gullane, P.J.; Gilbert, R.W.; Brown, D.H.; Irish, J.; Neligan, P. Cutaneous metastatic squamous cell carcinoma to the parotid gland: Analysis and outcome. Head Neck 2004, 26, 727–732. [Google Scholar] [CrossRef]
- Givi, B.; Andersen, P.E.; Diggs, B.S.; Wax, M.K.; Gross, N.D. Outcome of patients treated surgically for lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Head Neck 2011, 33, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Coombs, A.C.; Butler, A.; Allison, R. Metastatic cutaneous squamous cell carcinoma of the parotid gland: Prognostic factors. J. Laryngol. Otol. 2018, 132, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Girardi, F.M.; Wagner, V.P.; Martins, M.D.; Abentroth, A.L.; Hauth, L.A. Better outcome for parotid versus neck metastasis of head and neck cutaneous squamous cell carcinoma: A new report on reemerging data. Braz. J. Otorhinolaryngol. 2021, 87, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ch’ng, S.; Maitra, A.; Allison, R.S.; Chaplin, J.M.; Gregor, R.T.; Lea, R.; Tan, S.T. Parotid and cervical nodal status predict prognosis for patients with head and neck metastatic cutaneous squamous cell carcinoma. J. Surg. Oncol. 2008, 98, 101–105. [Google Scholar] [CrossRef]


| Clinical Characteristics | Primary Cancer | Secondary Cancer | p Values |
|---|---|---|---|
| Sex | 0.138 | ||
| Male | 10 (55.6) | 12 (80.0) | |
| Female | 8 (44.4) | 3 (20.0) | |
| Smoking | 0.082 | ||
| Never | 10 (55.6) | 4 (26.7) | |
| Former | 5 (27.8) | 10 (66.7) | |
| Active | 3 (16.7) | 1 (6.7) | |
| Alcoholconsumption * | 3 (16.7) | 3 (20.0) | 0.805 |
| T | - | ||
| 0 | 0 (0.0) | 12 (80.0) | |
| 1 | 8 (44.4) | 0 (0.0) | |
| 2 | 5 (27.8) | 1 (6.7) | |
| 3 | 1 (5.6) | 2 (13.3) | |
| 4 | 4 (22.2) | 0 (0.0) | |
| P | - | ||
| 1 | - | 2 (13.3) | |
| 2 | - | 8 (53.3) | |
| 3 | - | 5 (33.3) | |
| N ** | - | ||
| 0 | 15 (83.3) | 10 (66.7) | |
| 1 | 0 (0.0) | 0 (0.0) | |
| 2 | 2 (11.1) | 5 (33.3) | |
| 3 | 1 (5.6) | - | |
| M | - | ||
| 0 | 18 (100.0) | 15 (100.0) | |
| 1 | 0 (0.0) | 0 (0.0) | |
| Pre-operative facial palsy | 5 (27.8) | 5 (33.3) | 0.730 |
| Surgery | Primary Cancer | Secondary Cancer |
|---|---|---|
| Superficial parotidectomy | 3 (16.7) | 3 (20.0) |
| Total parotidectomy | 14 (77.8) | 7 (46.7) |
| Total parotidectomy with skin removal | 1 (5.6) | 5 (33.3) |
| Neck dissection | 10 (55.6) | 12 (80.0) |
| Reconstruction with deltopectoral flap | 1 (5.6) | 5 (33.3) |
| Pathological Characteristics | Primary Cancer | Secondary Cancer | p Values |
|---|---|---|---|
| Grade | 0.072 | ||
| 1 | 7 (38.9) | 1 (6.7) | |
| 2 | 3 (16.7) | 6 (40.0) | |
| 3 | 8 (44.4) | 8 (53.3) | |
| Margins | 0.887 | ||
| Negative | 7 (38.9) | 7 (46.7) | |
| Close | 6 (33.3) | 4 (26.7) | |
| Positive | 5 (27.8) | 4 (26.7) | |
| Lymphovascular invasion | 4 (22.2) | 5 (33.3) | 0.475 |
| Perineural invasion | 8 (44.4) | 5 (33.3) | 0.515 |
| Nodal extracapsular spread | 3 (16.7) | 2 (13.3) | 0.790 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecorari, G.; Pizzo, C.; Briguglio, M.; Cravero, E.; Riva, G. Primary and Secondary Tumors of the Parotid Gland: Clinical Features and Prognosis. Cancers 2023, 15, 1293. https://doi.org/10.3390/cancers15041293
Pecorari G, Pizzo C, Briguglio M, Cravero E, Riva G. Primary and Secondary Tumors of the Parotid Gland: Clinical Features and Prognosis. Cancers. 2023; 15(4):1293. https://doi.org/10.3390/cancers15041293
Chicago/Turabian StylePecorari, Giancarlo, Claudia Pizzo, Marco Briguglio, Ester Cravero, and Giuseppe Riva. 2023. "Primary and Secondary Tumors of the Parotid Gland: Clinical Features and Prognosis" Cancers 15, no. 4: 1293. https://doi.org/10.3390/cancers15041293
APA StylePecorari, G., Pizzo, C., Briguglio, M., Cravero, E., & Riva, G. (2023). Primary and Secondary Tumors of the Parotid Gland: Clinical Features and Prognosis. Cancers, 15(4), 1293. https://doi.org/10.3390/cancers15041293







