Prognostic Factors in Pseudomyxoma Peritonei with Emphasis on the Predictive Role of Peritoneal Cancer Index and Tumor Markers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Population
2.3. Statistical Analysis
3. Results
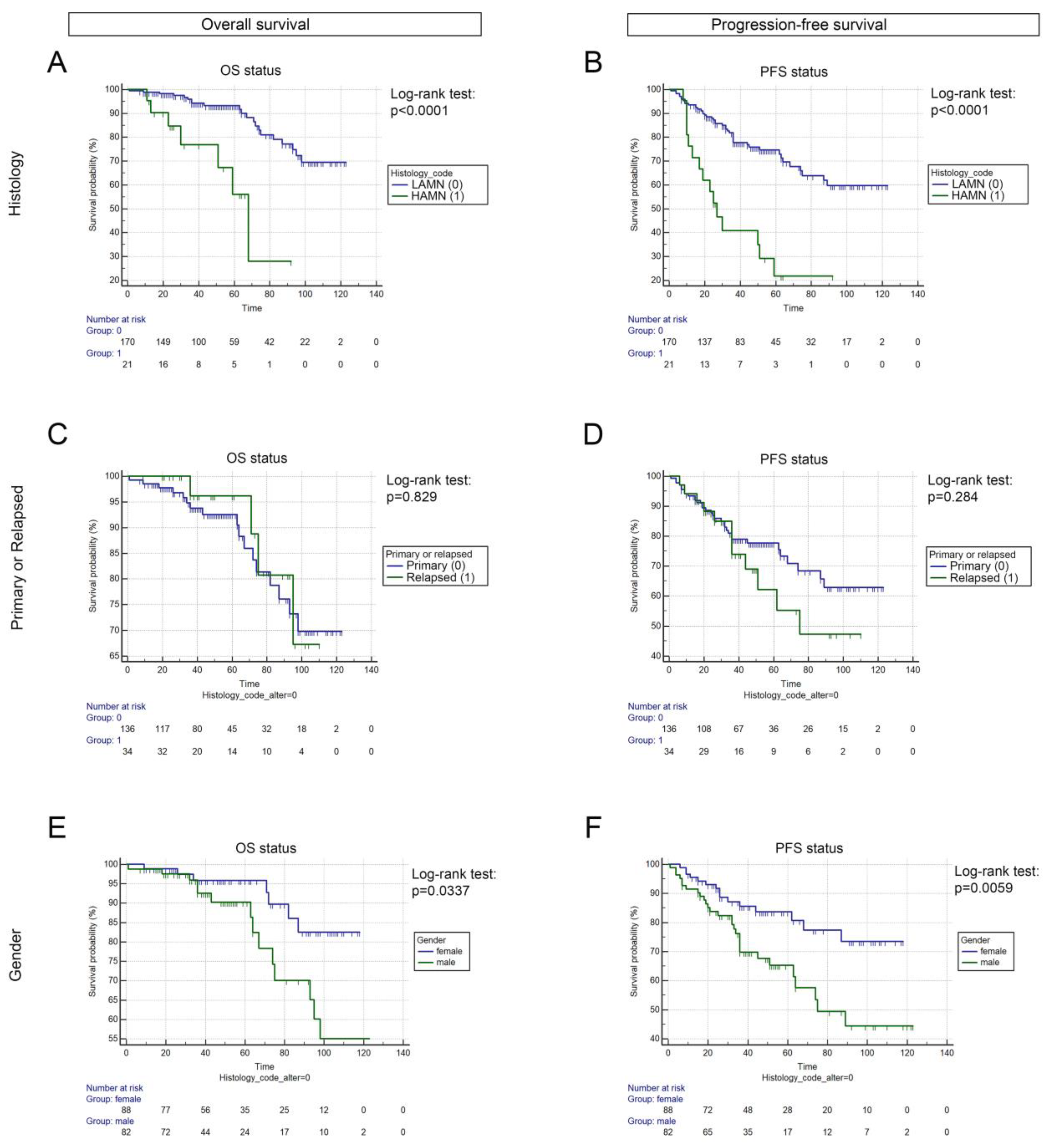
3.1. Cohort Characteristics, Baseline Parameters, and Survival
3.2. Plasma Concentration of Tumor Markers CA19-9 and CEA Correlates with Histology and PCI Ccore
3.3. CC Score Is Prognostic in LAMN Patients
3.4. Prognostic Role of PCI Score Status
3.5. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smeenk, R.M.; van Velthuysen, M.L.; Verwaal, V.J.; Zoetmulder, F.A. Appendiceal neoplasms and pseudomyxoma peritonei: A population based study. Eur. J. Surg. Oncol. 2008, 34, 196–201. [Google Scholar] [CrossRef]
- Kusamura, S.; Barretta, F.; Yonemura, Y.; Sugarbaker, P.H.; Moran, B.J.; Levine, E.A.; Goere, D.; Baratti, D.; Nizri, E.; Morris, D.L.; et al. The role of hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei after cytoreductive surgery. JAMA Surg. 2021, 156, e206363. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Ledakis, P.; El Halabi, H.; Gushchin, V.; Sardi, A. Neoplasms of the appendix: Current treatment guidelines. Hematol. Oncol. Clin. N. Am. 2012, 26, 1261–1290. [Google Scholar] [CrossRef]
- Munoz-Zuluaga, C.A.; King, M.C.; Diaz-Sarmiento, V.S.; Studeman, K.; Sittig, M.; MacDonald, R.; Nieroda, C.; Zambrano-Vera, K.; Gushchin, V.; Sardi, A. Defining "complete cytoreduction" after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (crs/hipec) for the histopathologic spectrum of appendiceal carcinomatosis. Ann. Surg. Oncol. 2020, 27, 5026–5036. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Cytoreductive surgery and perioperative intraperitoneal chemotherapy: A new standard of care for appendiceal mucinous tumors with peritoneal dissemination. Clin. Colon Rectal Surg. 2005, 18, 204–214. [Google Scholar] [CrossRef]
- Carr, N.J.; Cecil, T.D.; Mohamed, F.; Sobin, L.H.; Sugarbaker, P.H.; Gonzalez-Moreno, S.; Taflampas, P.; Chapman, S.; Moran, B.J.; Peritoneal Surface Oncology Group, I. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: The results of the peritoneal surface oncology group international (psogi) modified delphi process. Am. J. Surg. Pathol. 2016, 40, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Carr, N.J.; Bibeau, F.; Bradley, R.F.; Dartigues, P.; Feakins, R.M.; Geisinger, K.R.; Gui, X.; Isaac, S.; Milione, M.; Misdraji, J.; et al. The histopathological classification, diagnosis and differential diagnosis of mucinous appendiceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei. Histopathology 2017, 71, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Honore, C.; Caruso, F.; Dartigues, P.; Benhaim, L.; Chirica, M.; Goere, D.; Elias, D. Strategies for preventing pseudomyxoma peritonei after resection of a mucinous neoplasm of the appendix. Anticancer Res. 2015, 35, 4943–4947. [Google Scholar] [PubMed]
- Tiselius, C.; Kindler, C.; Shetye, J.; Letocha, H.; Smedh, K. Computed tomography follow-up assessment of patients with low-grade appendiceal mucinous neoplasms: Evaluation of risk for pseudomyxoma peritonei. Ann. Surg. Oncol. 2017, 24, 1778–1782. [Google Scholar] [CrossRef] [PubMed]
- Screaton, R.A.; Penn, L.Z.; Stanners, C.P. Carcinoembryonic antigen, a human tumor marker, cooperates with myc and bcl-2 in cellular transformation. J. Cell Biol. 1997, 137, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Shi, S.; Liang, C.; Liang, D.; Xu, W.; Ji, S.; Zhang, B.; Ni, Q.; Xu, J.; Yu, X. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: A systematic review and meta-analysis. Onco. Targets Ther. 2017, 10, 4591–4598. [Google Scholar] [CrossRef]
- Anoop, T.M.; Joseph, P.R.; Soman, S.; Chacko, S.; Mathew, M. Significance of serum carcinoembryonic antigen in metastatic breast cancer patients: A prospective study. World J. Clin. Oncol. 2022, 13, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Xu, W.; Zhang, G.; Liu, J.; Zheng, Z.; Chen, Y.; Niu, P.; Xu, X. The role of tissue and serum carcinoembryonic antigen in stages i to iii of colorectal cancer-a retrospective cohort study. Cancer Med. 2018, 7, 5327–5338. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Han, S.S.; Park, S.J.; Lee, W.J.; Woo, S.M.; Yoo, T.; Moon, S.H.; Kim, S.H.; Hong, E.K.; Kim, D.Y.; et al. Ca 19-9 level as indicator of early distant metastasis and therapeutic selection in resected pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, e743–e748. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.X.; Liu, L.R.; Yang, X.Y.; Liu, F.; Zhang, Z.G. Serum ca19-9 as a marker of circulating tumor cells in first reflux blood of colorectal cancer patients. Oncotarget 2017, 8, 67918–67932. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.L.; Wang, Z.R.; Shi, J.S.; Lu, M.; Wang, L.; He, Q.R. Utility of serum ca19-9 in diagnosis of cholangiocarcinoma: In comparison with cea. World J. Gastroenterol. 2004, 10, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Sachan, A.; Saluja, S.S.; Nekarakanti, P.K.; Nimisha; Mahajan, B.; Nag, H.H.; Mishra, P.K. Raised ca19-9 and cea have prognostic relevance in gallbladder carcinoma. BMC Cancer 2020, 20, 826. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Teng, T.Z.J.; Shelat, V.G. Carbohydrate antigen 19-9-tumor marker: Past, present, and future. World J. Gastrointest Surg. 2020, 12, 468–490. [Google Scholar] [CrossRef] [PubMed]
- Kaji, M.; Ishikura, H.; Kishimoto, T.; Omi, M.; Ishizu, A.; Kimura, C.; Takahashi, T.; Kato, H.; Yoshiki, T. E-selectin expression induced by pancreas-carcinoma-derived interleukin-1 alpha results in enhanced adhesion of pancreas-carcinoma cells to endothelial cells. Int. J. Cancer 1995, 60, 712–717. [Google Scholar] [CrossRef]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (ca 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [PubMed]
- Mittal, R.; Chandramohan, A.; Moran, B. Pseudomyxoma peritonei: Natural history and treatment. Int. J. Hyperth. 2017, 33, 511–519. [Google Scholar] [CrossRef]
- Taflampas, P.; Dayal, S.; Chandrakumaran, K.; Mohamed, F.; Cecil, T.D.; Moran, B.J. Pre-operative tumour marker status predicts recurrence and survival after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for appendiceal pseudomyxoma peritonei: Analysis of 519 patients. Eur. J. Surg. Oncol. 2014, 40, 515–520. [Google Scholar] [CrossRef] [PubMed]
- van Ruth, S.; Hart, A.A.; Bonfrer, J.M.; Verwaal, V.J.; Zoetmulder, F.A. Prognostic value of baseline and serial carcinoembryonic antigen and carbohydrate antigen 19.9 measurements in patients with pseudomyxoma peritonei treated with cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2002, 9, 961–967. [Google Scholar] [CrossRef]
- Wagner, P.L.; Austin, F.; Sathaiah, M.; Magge, D.; Maduekwe, U.; Ramalingam, L.; Jones, H.L.; Holtzman, M.P.; Ahrendt, S.A.; Zureikat, A.H.; et al. Significance of serum tumor marker levels in peritoneal carcinomatosis of appendiceal origin. Ann. Surg. Oncol. 2013, 20, 506–514. [Google Scholar] [CrossRef]
- van Eden, W.J.; Kok, N.F.M.; Snaebjornsson, P.; Jozwiak, K.; Woensdregt, K.; Bottenberg, P.D.; Boot, H.; Aalbers, A.G.J. Factors influencing long-term survival after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei originating from appendiceal neoplasms. BJS Open. 2019, 3, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Weiss, S.; von Winterfeld, M.; Krannich, A.; Feist, M.; Pratschke, J.; Raue, W.; Rau, B. Predictive value of peritoneal cancer index for survival in patients with mucinous peritoneal malignancies treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A single centre experience. Int. J. Hyperth. 2018, 34, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Quenet, F.; Elias, D.; Roca, L.; Goere, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (prodige 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Bhatt, A.; Mehta, S.; Kammar, P.; Saklani, A. Limitations oft he PRODIGE 7 trial. Lancet Oncol. 2021, 22, e175. [Google Scholar] [CrossRef]
- Herold, Z.; Acs, M.; Szasz, A.M.; Olasz, K.; Hussong, J.; Mayr, M.; Dank, M.; Piso, P. Metachronous Peritoneal Metastatic Mucinous Colorectal Adenocarcinoma Benefit More from Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) than Their Synchronous Counterparts. Cancers 2022, 14, 3978. [Google Scholar] [CrossRef]
- Delhorme, J.B.; Sauvinet, G.; Séverac, F.; Diab, S.; Liu, D.; Rohr, S.; Romain, B.; Brigand, C. Peritoneal Metastases of Colorectal Origin Treated with Complete Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy: The Efficiency of Mitomycin C. Ann. Surg. Oncol. 2022, 29, 7568–7576. [Google Scholar] [CrossRef] [PubMed]



| Parameter | N | Percent |
|---|---|---|
| Gender Male Female | 100 93 | 51.9% 48.1% |
| Age <60 ≥60 | 89 104 | 46.2% 53.8% |
| Tumor type at CRS primary recurrence | 168 25 | 86.8% 13.2% |
| Disease recurrence no yes | 150 43 | 77.8% 22.2% |
| Tumor histology LAMN HAMN | 178 15 | 92.2% 7.8% |
| ASA status ASA I ASA II ASA III | 4 33 156 | 2.1% 17.1% 80.8% |
| BMI * <30 kg/m2 ≥30 kg/m2 | 105 22 | 54.4% 11.3% |
| CA19-9 * <40 IU/mL ≥40 IU/ml | 91 32 | 47.1% 16.5% |
| CEA * <3 ng/mL ≥3 ng/ml | 66 58 | 34.1% 30% |
| HIPEC Duration 30 min 60 min 90 min | 124 20 49 | 64.3% 10.4% 25.3% |
| HIPEC drug * Mytomycin C Oxaliplatin | 67 122 | 34.7% 63.3% |
| CC score CC0 CC1 CC2 | 163 22 8 | 84.5% 11.6% 3.9% |
| PCI ≤12 >12 | 91 102 | 47.2% 52.8% |
| Covariate | p-Value | HR | 95% CI of HR |
|---|---|---|---|
| Gender | 0.0875 | 1.9429 | 0.9105–4.1461 |
| Age | 0.0284 | 1.0337 | 1.0037–1.0647 |
| ASA status | 0.9988 | 0.9995 | 0.5360–1.8639 |
| BMI (kg/m2) | 0.9077 | 1.0078 | 0.8845–1.1483 |
| CA19-9 IU/mL | 0.0192 | 1.0006 | 1.0001–1.0011 |
| CEA ng/mL | 0.0003 | 1.0086 | 1.0040–1.0133 |
| Histology (LAMN vs. HAMN) | 0.0002 | 5.6928 | 2.3236–13.947 |
| Primary/Recurrence | 0.8298 | 1.0986 | 0.4680–2.5789 |
| HIPEC Duration | 0.3905 | 0.4012 | 0.0504–3.1916 |
| HIPEC Drug | 0.3529 | 0.4929 | 0.1116–2.1761 |
| CC score | 0.0011 | 2.3344 | 1.4079–3.8708 |
| PCI | 0.0038 | 1.0575 | 1.0183–1.0981 |
| Covariate | p-Value | HR | 95% CI of HR |
|---|---|---|---|
| Gender | 0.0056 | 2.1289 | 1.2506–3.6240 |
| Age | 0.9672 | 1.0004 | 0.9823–1.0188 |
| ASA status | 0.9408 | 1.0170 | 0.6541–1.5811 |
| BMI (kg/m2) | 0.1831 | 0.9448 | 0.8694–1.0267 |
| CA19-9 IU/mL | 0.0001 | 1.0005 | 1.0003–1.0008 |
| CEA ng/mL | <0.0001 | 1.0063 | 1.0038–1.0088 |
| Histology (LAMN vs. HAMN) | <0.0001 | 3.8875 | 2.1563–7.0085 |
| Primary/Recurrence | 0.3154 | 1.3421 | 0.7579–2.3767 |
| HIPEC Duration | 0.0705 | 1.8378 | 0.9535–3.5423 |
| HIPEC Drug | 0.4260 | 1.2857 | 0.6947–2.3794 |
| CC score | 0.0002 | 1.9795 | 1.3922–2.8144 |
| PCI | <0.0001 | 1.0719 | 1.0455–1.0990 |
| Covariate | p-Value | HR | 95% CI of HR |
|---|---|---|---|
| Age | 0.0156 | 1.0519 | 1.0099–1.0958 |
| CC score | 0.0379 | 2.1954 | 1.0490–4.5947 |
| Histology (LAMN vs. HAMN) | 0.0091 | 6.6807 | 1.6139–27.6553 |
| PCI | 0.0037 | 1.1006 | 1.0319–1.1740 |
| Covariate | p-Value | HR | 95% CI of HR |
|---|---|---|---|
| PCI | <0.0001 | 1.1122 | 1.0707–1.1553 |
| Histology (LAMN vs. HAMN) | 0.0112 | 2.6247 | 1.2503–5.5100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaj, S.; Dora, D.; Lohinai, Z.; Herold, Z.; Szasz, A.M.; Herzberg, J.; Kodacsi, R.; Baransi, S.; Schlitt, H.J.; Hornung, M.; et al. Prognostic Factors in Pseudomyxoma Peritonei with Emphasis on the Predictive Role of Peritoneal Cancer Index and Tumor Markers. Cancers 2023, 15, 1326. https://doi.org/10.3390/cancers15041326
Blaj S, Dora D, Lohinai Z, Herold Z, Szasz AM, Herzberg J, Kodacsi R, Baransi S, Schlitt HJ, Hornung M, et al. Prognostic Factors in Pseudomyxoma Peritonei with Emphasis on the Predictive Role of Peritoneal Cancer Index and Tumor Markers. Cancers. 2023; 15(4):1326. https://doi.org/10.3390/cancers15041326
Chicago/Turabian StyleBlaj, Sebastian, David Dora, Zoltan Lohinai, Zoltan Herold, Attila Marcell Szasz, Jonas Herzberg, Roland Kodacsi, Saher Baransi, Hans Jürgen Schlitt, Matthias Hornung, and et al. 2023. "Prognostic Factors in Pseudomyxoma Peritonei with Emphasis on the Predictive Role of Peritoneal Cancer Index and Tumor Markers" Cancers 15, no. 4: 1326. https://doi.org/10.3390/cancers15041326
APA StyleBlaj, S., Dora, D., Lohinai, Z., Herold, Z., Szasz, A. M., Herzberg, J., Kodacsi, R., Baransi, S., Schlitt, H. J., Hornung, M., Werner, J. M., Slowik, P., Acs, M., & Piso, P. (2023). Prognostic Factors in Pseudomyxoma Peritonei with Emphasis on the Predictive Role of Peritoneal Cancer Index and Tumor Markers. Cancers, 15(4), 1326. https://doi.org/10.3390/cancers15041326






