Current Trends in Mucosal Melanomas: An Overview
Abstract
:Simple Summary
Abstract
1. Introduction
2. Mucosal Melanomas
2.1. Epidemiology
2.2. Risk Factors
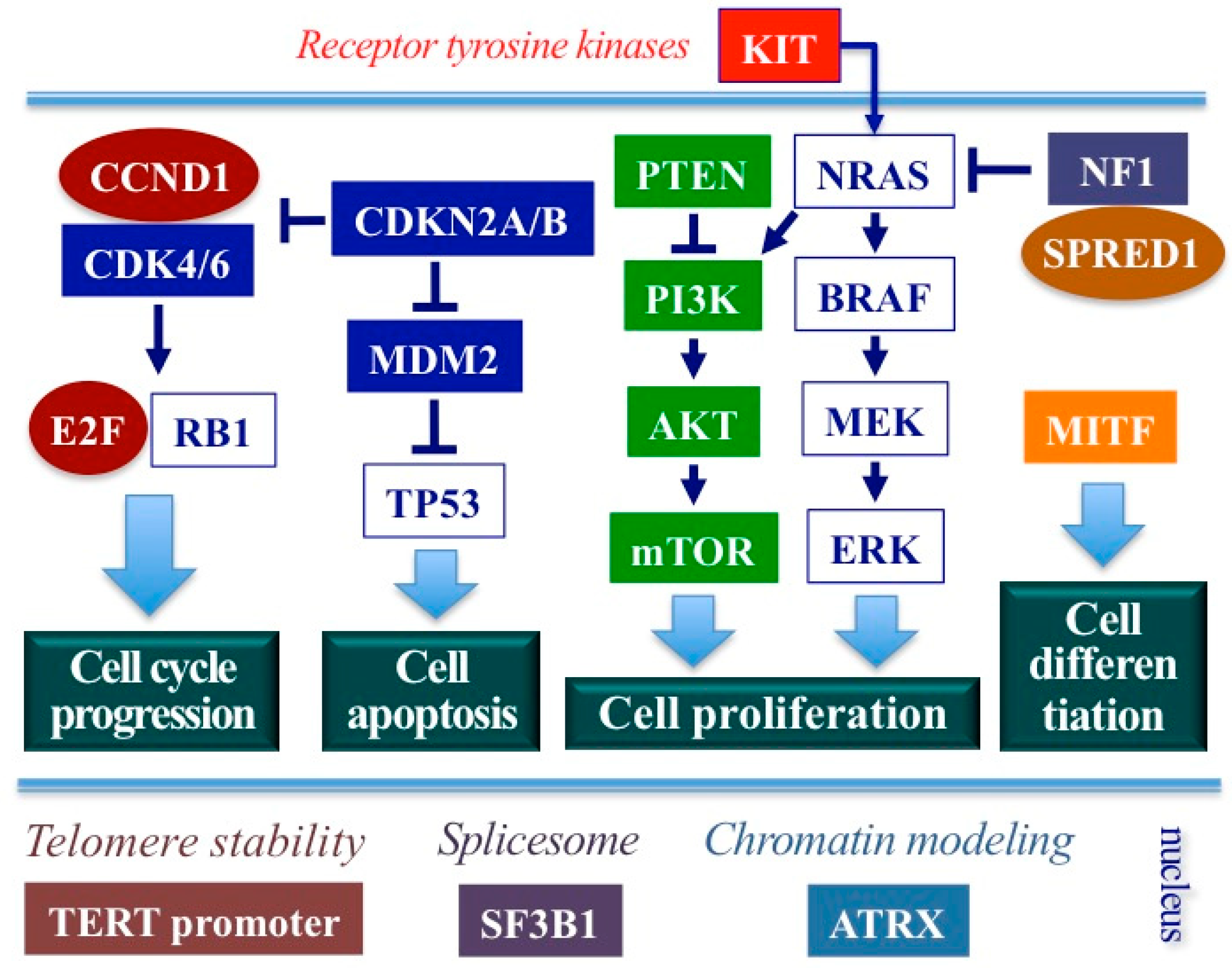
2.3. Molecular Features
2.4. Clinical Behavior
3. Clinical Presentation
3.1. Head and Neck
3.2. Gastrointestinal MMs
3.3. Genitourinary MMs
4. Diagnosis
5. Staging
6. Management
6.1. Surgery
6.2. Radiotherapy
6.3. Systemic Therapy
6.4. Future Therapeutic Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary mucosal melanomas: A comprehensive review. Int. J. Clin. Exp. Pathol. 2012, 5, 739–753. [Google Scholar] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Lerner, B.A.; Stewart, L.A.; Horowitz, D.P.; Carvajal, R.D. Mucosal Melanoma: New Insights and Therapeutic Options for a Unique and Aggressive Disease. Oncology 2017, 31, e23–e32. [Google Scholar] [PubMed]
- Dupin, E.; Le Douarin, N.M. Development of melanocyte precursors from the vertebrate neural crest. Oncogene 2003, 22, 3016–3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postow, M.A.; Hamid, O.; Carvajal, R.D. Mucosal melanoma: Pathogenesis, clinical behavior, and management. Curr. Oncol. Rep. 2012, 14, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Accorona, R.; Botti, G.; Farina, D.; Fossati, P.; Gatta, G.; Gogas, H.; Lombardi, D.; Maroldi, R.; Nicolai, P.; et al. Mucosal melanoma of the head and neck. Crit. Rev. Oncol. Hematol. 2017, 112, 136–152. [Google Scholar] [CrossRef]
- Kuk, D.; Shoushtari, A.N.; Barker, C.A.; Panageas, K.S.; Munhoz, R.R.; Momtaz, P.; Ariyan, C.E.; Brady, M.S.; Coit, D.G.; Bogatch, K.; et al. Prognosis of mucosal, uveal, acral, nonacral cutaneous, and unknown primary melanoma from the time of first metastasis. Oncologist 2016, 21, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Furney, S.J.; Turajlic, S.; Stamp, G.; Nohadani, M.; Carlisle, A.; Thomas, J.M.; Hayes, A.; Strauss, D.; Gore, M.; van den Oord, J.; et al. Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J. Pathol. 2013, 230, 261–269. [Google Scholar] [CrossRef]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- McLaughlin, C.C.; Wu, X.C.; Jemal, A.; Martin, H.J.; Roche, L.M.; Chen, V.W. Incidence of noncutaneous melanomas in the U.S. Cancer 2005, 103, 1000–1007. [Google Scholar] [CrossRef]
- Scott, J.F.; Gerstenblith, M.R. Noncutaneous Melanoma; Codon Publications: Brisbane, Australia, 2018; Chapters 2–5. [Google Scholar]
- Zhou, R.; Shi, C.; Tao, W.; Li, J.; Wu, J.; Han, Y.; Yang, G.; Gu, Z.; Xu, S.; Wang, Y.; et al. Analysis of mucosal melanoma whole-genome landscapes reveals clinically relevant genomic aberrations. Clin. Cancer Res. 2019, 25, 3548–3560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombino, M.; Lissia, A.; Franco, R.; Botti, G.; Ascierto, P.A.; Manca, A.; Sini, M.C.; Pisano, M.; Paliogiannis, P.; Tanda, F.; et al. Unexpected distribution of cKIT and BRAF mutations among southern Italian patients with sinonasal melanoma. Dermatology 2013, 226, 279–284. [Google Scholar] [CrossRef]
- Colombino, M.; Paliogiannis, P.; Cossu, A.; De Re, V.; Miolo, G.; Botti, G.; Scognamiglio, G.; Ascierto, P.A.; Santeufemia, D.A.; Fraggetta, F.; et al. BRAF Mutations and Dysregulation of the MAP Kinase Pathway Associated to Sinonasal Mucosal Melanomas. J. Clin. Med. 2019, 8, 1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tas, F.; Keskin, S.; Karadeniz, A.; Dağoğlu, N.; Sen, F.; Kilic, L.; Yildiz, I. Noncutaneous Melanoma Have Distinct Features from Each Other and Cutaneous Melanoma. Oncology 2011, 81, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Fara, A.M.; Pintus, G.; Abdel-Rahman, W.M.; Colombino, M.; Casula, M.; Palmieri, G.; Cossu, A. Primary Melanoma of the Lung: A Systematic Review. Medicina 2020, 56, 576. [Google Scholar] [CrossRef] [PubMed]
- Hintzsche, J.D.; Gorden, N.T.; Amato, C.M.; Kim, J.; Wuensch, K.E.; Robinson, S.E.; Applegate, A.J.; Couts, K.L.; Medina, T.M.; Wells, K.R.; et al. Whole-exome sequencing identifies recurrent SF3B1 R625 mutation and comutation of NF1 and KIT in mucosal melanoma. Melanoma Res. 2017, 27, 189–199. [Google Scholar] [CrossRef]
- Chacón, M.; Pfluger, Y.; Angel, M.; Waisberg, F.; Enrico, D. Uncommon Subtypes of Malignant Melanomas: A Review Based on Clinical and Molecular Perspectives. Cancers 2020, 12, 2362. [Google Scholar] [CrossRef] [PubMed]
- Axéll, T.; Hedin, C.A. Epidemiologic study of excessive oral melanin pigmentation with special reference to the influence of tobacco habits. Scand. J. Dent. Res. 1982, 90, 434–442. [Google Scholar] [CrossRef]
- Rapini, R.P. Oral melanoma: Diagnosis and treatment. Semin. Cutan. Med. Surg. 1997, 16, 320–322. [Google Scholar] [CrossRef]
- Newell, F.; Kong, Y.; Wilmott, J.S.; Johansson, P.A.; Ferguson, P.M.; Cui, C.; Li, Z.; Kazakoff, S.H.; Burke, H.; Dodds, T.J.; et al. Whole-genome landscape of mucosal melanoma reveals diverse drivers and therapeutic targets. Nat. Commun. 2019, 10, 3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.-M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Guo, J.; Si, L.; Bai, X. Evolving treatment approaches to mucosal melanoma. Curr. Oncol. Rep. 2022, 24, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, X.; Yan, X.; Chi, Z.; Si, L.; Cui, C.; Tang, B.; Li, S.; Mao, L.; Lian, B.; Wang, X.; et al. Axitinib in Combination with Toripalimab, a Humanized Immunoglobulin G4 Monoclonal Antibody against Programmed Cell Death-1, in Patients with Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial. J. Clin. Oncol. 2019, 37, 2987–2999. [Google Scholar] [CrossRef]
- Klemen, N.D.; Wang, M.; Rubinstein, J.C.; Olino, K.; Clune, J.; Ariyan, S.; Cha, C.; Weiss, S.A.; Kluger, H.M.; Sznol, M. Survival after checkpoint inhibitors for metastatic acral, mucosal and uveal melanoma. J. Immunother. Cancer 2020, 8, e000341. [Google Scholar] [CrossRef]
- Palmieri, G.; Colombino, M.; Casula, M.; Manca, A.; Mandalà, M.; Cossu, A.; Italian Melanoma Intergroup. Molecular pathways in melanomagenesis: What we learned from next-generation sequencing approaches. Curr. Oncol. Rep. 2018, 20, 86. [Google Scholar] [CrossRef] [Green Version]
- Nassar, K.W.; Tan, A.C. The mutational landscape of mucosal melanoma. Semin. Cancer Biol. 2020, 61, 139–148. [Google Scholar] [CrossRef]
- Broit, N.; Johansson, P.A.; Rodgers, C.B.; Walpole, S.T.; Newell, F.; Hayward, N.K.; Pritchard, A.L. Meta-Analysis and systematic review of the genomics of mucosal melanoma. Mol. Cancer Res. 2021, 19, 991–1004. [Google Scholar] [CrossRef]
- Beaudoux, O.; Oudart, J.B.; Riffaud, L.; Visseaux, L.; Marchal, A.; Lebre, A.S.; Grange, F. Mutational characteristics of primary mucosal melanoma: A systematic review. Mol. Diagn. Ther. 2022, 26, 189–202. [Google Scholar] [CrossRef]
- Koeller, D.R.; Schwartz, A.; DeSimone, M.S.; Rana, H.Q.; Rojas-Rudilla, V.; Russell-Goldman, E.; Laga, A.C.; Lindeman, N.I.; Garber, J.E.; Ghazani, A.A. Vulvar Melanoma in association with germline MITF p.E318K variant. Cancer Genet. 2022, 262–263, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Dumaz, N.; Jouenne, F.; Delyon, J.; Mourah, S.; Bensussan, A.; Lebbé, C. Atypical BRAF and NRAS mutations in mucosal melanoma. Cancers 2019, 11, 1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Banik, I.; Shain, A.H.; Yeh, I.; Bastian, B.C. Integrated genomic analyses of acral and mucosal melanomas nominate novel driver genes. Genome Med. 2022, 14, 65. [Google Scholar] [CrossRef]
- Maldonado, J.L.; Fridlyand, J.; Patel, H.; Jain, A.N.; Busam, K.; Kageshita, T.; Ono, T.; Albertson, D.G.; Pinkel, D.; Bastian, B.C. Determinants of BRAF mutations in primary melanomas. J. Natl. Cancer Inst. 2003, 95, 1878–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chłopek, M.; Lasota, J.; Thompson, L.D.R.; Szczepaniak, M.; Kuźniacka, A.; Hińcza, K.; Kubicka, K.; Kaczorowski, M.; Newford, M.; Liu, Y.; et al. Alterations in key signaling pathways in sinonasal tract melanoma. A molecular genetics and immunohistochemical study of 90 cases and comprehensive review of the literature. Mod. Pathol. 2022, 35, 1609–1617. [Google Scholar] [CrossRef]
- Schoenewolf, N.L.; Bull, C.; Belloni, B.; Holzmann, D.; Tonolla, S.; Lang, R.; Mihic-Probst, D.; Andres, C.; Dummer, R. Sinonasal, genital and acrolentiginous melanomas show distinct characteristics of KIT expression and mutations. Eur. J. Cancer 2012, 48, 1842–1852. [Google Scholar] [CrossRef]
- Wróblewska, J.P.; Dias-Santagata, D.; Ustaszewski, A.; Wu, C.L.; Fujimoto, M.; Selim, M.A.; Biernat, W.; Ryś, J.; Marszalek, A.; Hoang, M.P. Prognostic Roles of BRAF, KIT, NRAS, IGF2R and SF3B1 Mutations in Mucosal Melanomas. Cells 2021, 10, 2216. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, Z.; Cui, C.; Wu, X.; Yu, H.; Guo, J.; Kong, Y. Frequent genetic aberrations in the cell cycle related genes in mucosal melanoma indicate the potential for targeted therapy. J. Transl. Med. 2019, 17, 245. [Google Scholar] [CrossRef] [Green Version]
- Lian, B.; Cui, C.L.; Zhou, L.; Song, X.; Zhang, X.S.; Wu, D.; Si, L.; Chi, Z.H.; Sheng, X.N.; Mao, L.L.; et al. The natural history and patterns of metastases from mucosal melanoma: An analysis of 706 prospectively-followed patients. Ann. Oncol. 2017, 28, 868–873. [Google Scholar] [CrossRef]
- Tyrrell, H.; Payne, M. Combatting mucosal melanoma: Recent advances and future perspectives. Melanoma Manag. 2018, 5, MMT11. [Google Scholar] [CrossRef] [Green Version]
- Malapelle, U.; Rossi, G.; Pisapia, P.; Barberis, M.; Buttitta, F.; Castiglione, F.; Cecere, F.L.; Grimaldi, A.M.; Iaccarino, A.; Marchetti, A.; et al. BRAF as a positive predictive biomarker: Focus on lung cancer and melanoma patients. Crit. Rev. Oncol. Hematol. 2020, 156, 103–118. [Google Scholar] [CrossRef]
- Tayshetye, P.; Miller, K.; Monga, D.; Brem, C.; Silverman, J.F.; and Finley, G.G. Molecular Profiling of Advanced Malignancies: A Community Oncology Network Experience and Review of Literature. Front. Med. 2020, 7, 314. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.A.; Fridlyand, J.; Kageshita, T.; Patel, H.N.; Busam, K.J.; Kutzner, H.; Cho, K.H.; Aiba, S.; Bröcker, E.B.; Leboit, P.E.; et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 2005, 353, 2135–2147. [Google Scholar] [CrossRef]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Harlow, A.; Cruz, F.; Azar, S.; et al. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indini, A.; Roila, F.; Grossi, F.; Massi, D.; Mandalà, M. Molecular profiling and novel therapeutic strategies for mucosal melanoma: A comprehensive review. Int. J. Mol. Sci. 2021, 23, 147. [Google Scholar] [CrossRef]
- Palmieri, G.; Colombino, M.; Casula, M.; Sini, M.C.; Manca, A.; Pisano, M.; Paliogiannis, P.; Cossu, A. Molecular landscape profile of melanoma. In New Therapies in Advanced Cutaneous Malignancies; Rutkowski, P., Mandalà, M., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Nissan, M.H.; Pratilas, C.A.; Jones, A.M.; Ramirez, R.; Won, H.; Liu, C.; Tiwari, S.; Kong, L.; Hanrahan, A.J.; Yao, Z.; et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res. 2014, 74, 2340–2350. [Google Scholar] [CrossRef] [Green Version]
- Halaban, R.; Krauthammer, M. RASopathy gene mutations in melanoma. J. Investig. Dermatol. 2016, 136, 1755–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Kan, H.; Zhao, L.; Sun, Z.; Bai, C. Immune checkpoint inhibitors in advanced or metastatic mucosal melanoma: A systematic review. Ther. Adv. Med. Oncol. 2020, 12. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Spencer, S.A.; Lydiatt, W. Mucosal melanoma: A clinically and biologically unique disease entity. J. Natl. Compr. Cancer Netw. 2012, 3, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Lian, B.; Zhou, L.; Song, X.; Zhang, X.; Wu, D.; Chi, Z.; Si, L.; Sheng, X.; Kong, Y.; et al. Multifactorial Analysis of Prognostic Factors and Survival Rates Among 706 Mucosal Melanoma Patients. Ann. Surg. Oncol. 2018, 8, 2184–2192. [Google Scholar] [CrossRef]
- López, F.; Rodrigo, J.P.; Cardesa, A.; Triantafyllou, A.; Devaney, K.O.; Mendenhall, W.M.; Haigentz, M., Jr.; Strojan, P.; Pellitteri, P.K.; Bradford, C.R.; et al. Update on primary head and neck mucosal melanoma. Head Neck 2016, 1, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, M.A.; Roberts, D.B.; Kupferman, M.E.; DeMonte, F.; El-Naggar, A.K.; Williams, M.; Rosenthal, D.S.; Hanna, E.Y. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M. D. Anderson Cancer Center. Cancer 2010, 9, 2215–2223. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kaushal, V.; Singla, S.; Sen, R. Primary glottic malignant melanoma of the larynx (PGMML): A very rare entity. BMJ Case. Rep. 2015, 2015, bcr2015211317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oztürk, O.; Ozek, H.; Cansiz, H.; Karakullukçu, B. Primary malignant melanoma of the pharynx. J. Laryngol. Otol. 2001, 11, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Ota, Y.; Yamada, E.; Takahashi, K.; Watanabe, T.; Makuuchi, Y.; Suda, T.; Osaka, Y.; Seshimo, A.; Katsumata, K.; et al. Primary malignant melanoma of the esophagus with multiple lymph node metastases: A case report and literature review. Medicine 2020, 22, e18573. [Google Scholar] [CrossRef]
- Thompson, L.D.; Wieneke, J.A.; Miettinen, M. Sinonasal tract and nasopharyngeal melanomas: A clinicopathologic study of 115 cases with a proposed staging system. Am. J. Surg. Pathol. 2003, 5, 594–611. [Google Scholar] [CrossRef]
- Manolidis, S.; Donald, P.J. Malignant mucosal melanoma of the head and neck: Review of the literature and report of 14 patients. Cancer 1997, 8, 1373–1386. [Google Scholar] [CrossRef]
- Patel, S.G.; Prasad, M.L.; Escrig, M.; Singh, B.; Shaha, A.R.; Kraus, D.H.; Boyle, J.O.; Huvos, A.G.; Busam, K.; Shah, J.P. Primary mucosal malignant melanoma of the head and neck. Head Neck 2002, 3, 247–257. [Google Scholar] [CrossRef]
- Femiano, F.; Lanza, A.; Buonaiuto, C.; Gombos, F.; Di Spirito, F.; Cirillo, N. Oral malignant melanoma: A review of the literature. J. Oral Pathol. Med. 2008, 7, 383–388. [Google Scholar] [CrossRef]
- Penel, N.; Mallet, Y.; Mirabel, X.; Van, J.T.; Lefebvre, J.L. Primary mucosal melanoma of head and neck: Prognostic value of clear margins. Laryngoscope 2006, 6, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Didona, D.; Macrì, G.; Calvieri, S.; Mercuri, S.R. Anorectal Melanoma. In Noncutaneous Melanoma; Scott, J.F., Gerstenblith, M.R., Eds.; Codon Publications: Brisbane, Australia, 2018; Chapter 6. [Google Scholar] [CrossRef] [Green Version]
- Roumen, R.M. Anorectal melanoma in The Netherlands: A report of 63 patients. Eur. J. Surg. Oncol. 1996, 6, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Roesch, A.; Weide, B.; Gutzmer, R.; Meier, F.; Loquai, C.; Kähler, K.C.; Gesierich, A.; Meissner, M.; von Bubnoff, D.; et al. Prognostic factors and treatment outcomes in 444 patients with mucosal melanoma. Eur. J. Cancer 2017, 81, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Vyas, R.; Thompson, C.L.; Zargar, H.; Selph, J.; Gerstenblith, M.R. Epidemiology of genitourinary melanoma in the United States: 1992 through 2012. J. Am. Acad. Dermatol. 2016, 75, 144–150. [Google Scholar] [CrossRef]
- Disease, A.N.; Cooper, S.M.; Wojnarowska, F. Anogenital (non-venereal) disease. In Dermatology: 2-Volume Set, 4th ed.; Bolognia, J.L., Schaffer, J.V., Cerroni, L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2017; pp. 1243–1258.e1. [Google Scholar] [CrossRef]
- Schaefer, T.; Satzger, I.; Gutzmer, R. Clinics, prognosis and new therapeutic options in patients with mucosal melanoma: A retrospective analysis of 75 patients. Medicine 2017, 1, e5753. [Google Scholar] [CrossRef]
- Amin, M.; Edge, S.; Greene, F. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Ballantyne, A.J. Malignant melanoma of the skin of the head and neck. An analysis of 405 cases. Am. J. Surg. 1970, 4, 425–431. [Google Scholar] [CrossRef]
- Nilsson, P.J.; Ragnarsson-Olding, B.K. Importance of clear resection margins in anorectal malignant melanoma. Br. J. Surg. 2010, 1, 98–103. [Google Scholar] [CrossRef]
- Seifried, S.; Haydu, L.E.; Quinn, M.J.; Scolyer, R.A.; Stretch, J.R.; Thompson, J.F. Melanoma of the vulva and vagina: Principles of staging and their relevance to management based on a clinicopathologic analysis of 85 cases. Ann. Surg. Oncol. 2015, 22, 1959–1966. [Google Scholar] [CrossRef]
- Hanna, E.; DeMonte, F.; Ibrahim, S.; Roberts, D.; Levine, N.; Kupferman, M. Endoscopic resection of sinonasal cancers with and without craniotomy: Oncologic results. Arch. Otolaryngol. Head Neck Surg. 2009, 12, 1219–1224. [Google Scholar] [CrossRef] [Green Version]
- Krengli, M.; Jereczek-Fossa, B.A.; Kaanders, J.H.; Masini, L.; Beldì, D.; Orecchia, R. What is the role of radiotherapy in the treatment of mucosal melanoma of the head and neck? Crit. Rev. Oncol. Hematol. 2008, 2, 121–128. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.-J.; et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Munhoz, R.R.; Kuk, D.; Ott, P.A.; Johnson, D.B.; Tsai, K.K.; Rapisuwon, S.; Eroglu, Z.; Sullivan, R.J.; Luke, J.J.; et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer 2016, 21, 3354–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. RELATIVITY-047 Investigators. Relatlimab and Nivolumab versus Nivolumab in untreated advanced melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.-J.; Gangadhar, T.C.; et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; Larkin, J.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 11, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Moya-Plana, A.; Herrera Gómez, R.G.; Rossoni, C.; Dercle, L.; Ammari, S.; Girault, I.; Roy, S.; Scoazec, J.Y.; Vagner, S.; Janot, F.; et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019, 68, 1171–1178. [Google Scholar] [CrossRef]
- Del Vecchio, M.; Di Guardo, L.; Ascierto, P.A.; Grimaldi, A.M.; Sileni, V.C.; Pigozzo, J.; Ferraresi, V.; Nuzzo, C.; Rinaldi, G.; Testori, A.; et al. Efficacy and safety of ipilimumab 3 mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur. J. Cancer 2014, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbé, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: A pooled analysis. J. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Yan, X.; Chi, Z.; Si, L.; Cui, C.; Tang, B.; Li, S.; Mao, L.; Lian, B.; Wang, X.; et al. Overall survival and biomarker analysis of a phase Ib combination study of toripalimab, a humanized IgG4 mAb against programmed death-1 (PD-1) with axitinib in patients with metastatic mucosal melanoma. J. Clin. Oncol. 2020, 38, 10007. [Google Scholar] [CrossRef]
- Postow, M.A.; Luke, J.J.; Bluth, M.J.; Ramaiya, N.; Panageas, K.S.; Lawrence, D.P.; Ibrahim, N.; Flaherty, K.T.; Sullivan, R.J.; Ott, P.A.; et al. Ipilimumab for patients with advanced mucosal melanoma. Oncologist 2013, 6, 726–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, M.; Mellor, J.D.; McArthur, G.; Kee, D. Ipilimumab in pretreated patients with unresectable or metastatic cutaneous, uveal and mucosal melanoma. Med. J. Aust. 2014, 201, 49–53. [Google Scholar] [CrossRef]
- Mignard, C.; Deschamps Huvier, A.; Gillibert, A.; Duval Modeste, A.B.; Dutriaux, C.; Khammari, A.; Avril, M.-F.; Kramkimel, N.; Mortier, L.; Marcant, P.; et al. Efficacy of immunotherapy in patients with metastatic mucosal or uveal melanoma. J. Oncol. 2018, 2018, 1908065. [Google Scholar] [CrossRef] [Green Version]
- Hamid, O.; Robert, C.; Ribas, A.; Hodi, F.S.; Walpole, E.; Daud, A.; Arance, A.S.; Brown, E.; Hoeller, C.; Mortier, L.; et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: A post-hoc analysis of KEYNOTE-001, 002, 006. Br. J. Cancer 2018, 119, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Shoushtari, A.N.; Wagstaff, J.; Ascierto, P.A.; Butler, M.O.; Lao, C.D.; Marquez-Rodas, I.; Chiarion-Sileni, V.; Dummer, R.; Ferrucci, P.F.; Lorigan, P.; et al. CheckMate 067: Long-term outcomes in patients with mucosal melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Dimitriou, F.; Namikawa, K.; Reijers, I.L.M.; Buchbinder, E.I.; Soon, J.A.; Zaremba, A.; Teterycz, P.; Mooradian, M.J.; Armstrong, E.; Nakamura, Y.; et al. Single-agent anti-PD-1 or combined with ipilimumab in patients with mucosal melanoma: An international, retrospective, cohort study. Ann. Oncol. 2022, 33, 968–980. [Google Scholar] [CrossRef]
- Lian, B.; Si, L.; Chi, Z.H.; Sheng, X.N.; Kong, Y.; Wang, X.; Tian, H.; Li, K.; Mao, L.L.; Bai, X.; et al. Toripalimab (anti-PD-1) versus high-dose interferon-α2b as adjuvant therapy in resected mucosal melanoma: A phase II randomized trial. Ann. Oncol. 2022, 33, 1061–1070. [Google Scholar] [CrossRef]
- Ho, J.; Mattei, J.; Tetzlaff, M.; Williams, M.D.; Davies, M.A.; Diab, A.; Oliva, I.C.G.; McQuade, J.; Patel, S.P.; Tawbi, H.; et al. Neoadjuvant checkpoint inhibitor immunotherapy for resectable mucosal melanoma. Front. Oncol. 2022, 12, 1001150. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Mao, L.L.; Chi, Z.H.; Sheng, X.N.; Cui, C.L.; Kong, Y.; Dai, J.; Wang, X.; Li, S.M.; Tang, B.X.; et al. BRAF inhibitors: Efficacious and tolerable in BRAF-mutant acral and mucosal melanoma. Neoplasma 2017, 64, 626–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodi, F.S.; Corless, C.L.; Giobbie-Hurder, A.; Fletcher, J.A.; Zhu, M.; Marino-Enriquez, A.; Friedlander, P.; Gonzalez, R.; Weber, J.S.; Gajewski, T.F.; et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J. Clin. Oncol. 2013, 31, 3182–3190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.H.; Kim, K.M.; Kwon, M.; Kim, J.H.; Lee, J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Investig. New Drugs 2012, 30, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Lawrence, D.P.; Weber, J.S.; Gajewski, T.F.; Gonzalez, R.; Lutzky, J.; O’Day, S.J.; Hamid, O.; Wolchok, J.D.; Chapman, P.B.; et al. Phase II Study of Nilotinib in Melanoma Harboring KIT Alterations Following Progression to Prior KIT Inhibition. Clin. Cancer Res. 2015, 21, 2289–2296. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Carvajal, R.D.; Dummer, R.; Hauschild, A.; Daud, A.; Bastian, B.C.; Markovic, S.N.; Queirolo, P.; Arance, A.; Berking, C.; et al. Efficacy and safety of nilotinib in patients with KIT-mutated metastatic or inoperable melanoma: Final results from the global, single-arm, phase II TEAM trial. Ann. Oncol. 2017, 28, 1380–1387. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Yan, X.; Zhou, L.; Chi, Z.; Si, L.; Cui, C.; Tang, B.; Mao, L.; Lian, B.; et al. Toripalimab plus axitinib in patients with metastatic mucosal melanoma: 3-year survival update and biomarker analysis. J. Immunother. Cancer 2022, 10, e004036. [Google Scholar] [CrossRef]
- Yan, X.; Sheng, X.; Chi, Z.; Cui, C.; Kong, Y.; Tang, B.; Mao, L.; Wang, X.; Lian, B.; Li, S.; et al. Randomized phase II study of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced mucosal melanoma. J. Clin. Oncol. 2021, 39, 881–889. [Google Scholar] [CrossRef]
- Graells, J.; Vinyals, A.; Figueras, A.; Llorens, A.; Moreno, A.; Marcoval, J.; Gonzalez, F.J.; Fabra, A. Overproduction of VEGF concomitantly expressed with its receptors promotes growth and survival of melanoma cells through MAPK and PI3K signaling. J. Investig. Dermatol. 2004, 123, 1151–1161. [Google Scholar] [CrossRef] [Green Version]
- Simonetti, O.; Lucarini, G.; Rubini, C.; Lazzarini, R.; Di Primio, R.; Offidani, A. Clinical and prognostic significance of survivin, AKT and VEGF in primary mucosal oral melanoma. Anticancer Res. 2015, 35, 2113–2120. [Google Scholar] [PubMed]
- Johnson, B.F.; Clay, T.M.; Hobeika, A.C.; Lyerly, H.K.; Morse, M.A. Vascular endothelial growth factor and immunosuppression in cancer: Current knowledge and potential for new therapy. Expert Opin. Biol. Ther. 2007, 7, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.-L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N. Incorporating VEGF Blockade Into a Shifting Treatment Paradigm for Mucosal Melanoma. J. Clin. Oncol. 2021, 8, 867–869. [Google Scholar] [CrossRef]
- Lin, W.; Lu, X.; Yang, H.; Huang, L.; Huang, W.; Tang, Y.; Liu, S.; Wang, H.; Zhang, Y. Metabolic heterogeneity protects metastatic mucosal melanomas cells from ferroptosis. Int. J. Mol. Med. 2022, 50, 124. [Google Scholar] [CrossRef]
- Iga, N.; Otsuka, A.; Hirata, M.; Kataoka, T.R.; Irie, H.; Nakashima, C.; Matsushita, S.; Uchi, H.; Yamamoto, Y.; Funakoshi, T.; et al. Variable indoleamine 2,3-dioxygenase expression in acral/mucosal melanoma and its possible link to immunotherapy. Cancer Sci. 2019, 110, 3434–3441. [Google Scholar] [CrossRef]
- Zakharia, Y.; McWilliams, R.R.; Rixe, O.; Drabick, J.; Shaheen, M.F.; Grossmann, K.F.; Kolhe, R.; Pacholczyk, R.; Sadek, R.; Tennant, L.L.; et al. Phase II trial of the ID pathway inhibitor indoximod plus pembrolizumab for the treatment of patients with advanced melanoma. J. Immunother. Cancer 2021, 9, e002057. [Google Scholar] [CrossRef]
- Shikuma, K.; Omasa, M.; Yutaka, Y.; Okuda, M.; Taki, T. Treatment of primary melanoma of the lung monitored by 5-S-cysteinyldopa levels. Ann. Thorac. Surg. 2009, 87, 1264–1266. [Google Scholar] [CrossRef]
- Soma, T.; Nakamura, Y.; Fukui, N.; Sakai, Y.; Kageyama, Y. Malignant melanoma of the male urethra with increased 5-S-cysteinyldopa: A case report. IJU Case Rep. 2019, 2, 215–217. [Google Scholar] [CrossRef] [Green Version]
- Wakamatsu, K.; Fukushima, S.; Minagawa, A.; Omodaka, T.; Hida, T.; Hatta, N.; Takata, M.; Uhara, H.; Okuyama, R.; Ihn, H. Significance of 5-S-Cysteinyldopa as a marker for melanoma. Int. J. Mol. Sci. 2020, 21, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Ma, M.; Dai, J.; Cui, C.; Si, L.; Sheng, X.; Chi, Z.; Xu, L.; Yu, S.; Xu, T.; et al. miR-let-7b and miR-let-7c suppress tumourigenesis of human mucosal melanoma and enhance the sensitivity to chemotherapy. J. Exp. Clin. Cancer Res. 2019, 38, 212. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Dai, J.; Tang, H.; Xu, T.; Yu, S.; Si, L.; Cui, C.; Sheng, X.; Chi, Z.; Mao, L.; et al. MicroRNA-23a-3p inhibits mucosal melanoma growth and progression through targeting adenylate cyclase 1 and attenuating cAMP and MAPK pathways. Theranostics 2019, 9, 945–960. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Hu, D.; Li, Y.; Yang, L. Development of therapeutic vaccines for the treatment of diseases. Mol. Biomed. 2022, 3, 40. [Google Scholar] [CrossRef]
- Sittplangkoon, C.; Alameh, M.-G.; Weissman, D.; Lin, P.J.C.; Tam, Y.K.; Prompetchara, E.; Palaga, T. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front. Immunol. 2022, 13, 983000. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef]
- The National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/show/NCT03897881 (accessed on 18 December 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santeufemia, D.A.; Palmieri, G.; Miolo, G.; Colombino, M.; Doro, M.G.; Frogheri, L.; Paliogiannis, P.; Capobianco, G.; Madonia, M.; Cossu, A.; et al. Current Trends in Mucosal Melanomas: An Overview. Cancers 2023, 15, 1356. https://doi.org/10.3390/cancers15051356
Santeufemia DA, Palmieri G, Miolo G, Colombino M, Doro MG, Frogheri L, Paliogiannis P, Capobianco G, Madonia M, Cossu A, et al. Current Trends in Mucosal Melanomas: An Overview. Cancers. 2023; 15(5):1356. https://doi.org/10.3390/cancers15051356
Chicago/Turabian StyleSanteufemia, Davide Adriano, Giuseppe Palmieri, Gianmaria Miolo, Maria Colombino, Maria Grazia Doro, Laura Frogheri, Panagiotis Paliogiannis, Giampiero Capobianco, Massimo Madonia, Antonio Cossu, and et al. 2023. "Current Trends in Mucosal Melanomas: An Overview" Cancers 15, no. 5: 1356. https://doi.org/10.3390/cancers15051356
APA StyleSanteufemia, D. A., Palmieri, G., Miolo, G., Colombino, M., Doro, M. G., Frogheri, L., Paliogiannis, P., Capobianco, G., Madonia, M., Cossu, A., Lo Re, G., & Corona, G. (2023). Current Trends in Mucosal Melanomas: An Overview. Cancers, 15(5), 1356. https://doi.org/10.3390/cancers15051356








