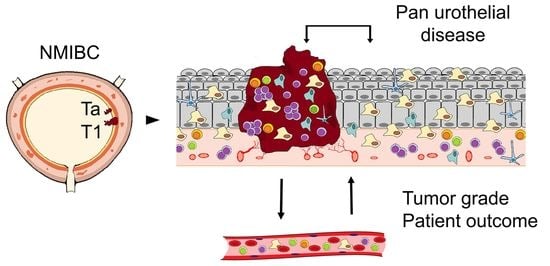
A Comprehensive Analysis of Immune Response in Patients with Non-Muscle-Invasive Bladder Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Processing
2.3. Flow Cytometry Analysis
- Panel 1 (T lymphocytes 1): CD49b, CD127, CD3, LAG3, CD4, CD25 and CD8;
- Panel 2 (T lymphocytes 2): TIM3, PD1, CD3, ICOS, CD27, CD4 and CD8;
- Panel 3 (myeloid cells): MHCII, CD 127a/b (SIRP), IDO1, CD14, CD11c and CD123;
- Panel 4 (NK cells): KIR, NKG2a, NKG2d and CD56.
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Differential Immune Profile in Samples from Patients with Urothelial Bladder Cancer
3.2. Immune Markers in Tumor Progression and Patient Outcome
3.3. CD4+ CD27+ Cells and IDO+ Monocytes Are Independent Markers for Tumor Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faguet, G.B. A brief history of cancer: Age-old milestones underlying our current knowledge database. Int. J. Cancer. 2015, 136, 2022–2036. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; López, M.; Lillo, C.; Vargas, M.J. The Edwin Smith papyrus in the history of medicine. Rev. Méd. Chile 2012, 140, 1357–1362. [Google Scholar] [CrossRef] [Green Version]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Prev. Biomark. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allard, P.; Bernard, P.; Fradet, Y.; Têtu, B. The early clinical course of primary Ta and T1 bladder cancer: A proposed prognostic index. Br. J. Urol. 1998, 81, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Chamie, K.; Litwin, M.S.; Bassett, J.C.; Daskivich, T.J.; Lai, J.; Hanley, J.M.; Konety, B.R.; Saigal, C.S.; the Urologic Diseases in America Project. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer 2013, 119, 3219–3227. [Google Scholar] [CrossRef] [Green Version]
- Svatek, R.S.; Hollenbeck, B.K.; Holmäng, S.; Lee, R.; Kim, S.P.; Stenzl, A.; Lotan, Y. The Economics of Bladder Cancer: Costs and Considerations of Caring for This Disease. Eur. Urol. 2014, 66, 253–262. [Google Scholar] [CrossRef]
- Malats, N.; Real, F.X. Epidemiology of bladder cancer. Hematol. Oncol. Clin. N. Am. 2015, 29, 177–189. [Google Scholar] [CrossRef]
- Burger, M.; Catto, J.W.F.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and Risk Factors of Urothelial Bladder Cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef]
- Cumberbatch, M.G.K.; Jubber, I.; Black, P.C.; Esperto, F.; Figueroa, J.D.; Kamat, A.M.; Kiemeney, L.; Lotan, Y.; Pang, K.; Silverman, D.T.; et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur. Urol. 2018, 74, 784–795. [Google Scholar] [CrossRef] [Green Version]
- Palucka, A.K.; Coussens, L.M. The Basis of Oncoimmunology. Cell 2016, 164, 1233–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietzak, E.J.; Mucksavage, P.; Guzzo, T.J.; Malkowicz, S.B. Heavy Cigarette Smoking and Aggressive Bladder Cancer at Initial Presentation. Urology 2015, 86, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, L.M.; Svatek, R.S.; Shariat, S.F.; Messing, E.; Lotan, Y. Bladder cancer risk: Use of the PLCO and NLST to identify a suitable screening cohort. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 65.e19–65.e25. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zeng, D.; Ou, Q.; Liu, S.; Li, A.; Chen, Y.; Lin, D.; Gao, Q.; Zhou, H.; Liao, W.; et al. Association of Survival and Immune-Related Biomarkers with Immunotherapy in Patients with Non–Small Cell Lung Cancer: A Meta-analysis and Individual Patient–Level Analysis. JAMA Netw. Open 2019, 2, e196879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.S.; Kang, M.; Baek, J.H.; Lee, J.I.; Ha, S.Y. Clinical impact of tumor-infiltrating lymphocytes for survival in curatively resected stage IV colon cancer with isolated liver or lung metastasis. Ann. Surg. Oncol. 2013, 20, 697–702. [Google Scholar] [CrossRef]
- Gielen, P.R.; Schulte, B.M.; Kers-Rebel, E.D.; Verrijp, K.; Bossman, S.A.J.F.H.; Ter Laan, M.; Wesseling, P.; Adema, G.J. Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. Neuro-Oncol. 2016, 18, 1253–1264. [Google Scholar] [CrossRef] [Green Version]
- Zalfa, C.; Paust, S. Natural Killer Cell Interactions with Myeloid Derived Suppressor Cells in the Tumor Microenvironment and Implications for Cancer Immunotherapy. Front. Immunol. 2021, 12, 633205. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccin. Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Ruffo, E.; Wu, R.C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Lymphocyte-activation gene 3 (LAG3): The next immune checkpoint receptor. Semin. Immunol. 2019, 42, 101305. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Tang, X.; Kim, H.J.; Jalali, S.; Pritchett, J.C.; Villasboas, J.C.; Novak, A.J.; Yang, Z.; Ansell, S.M. Expression of KLRG1 and CD127 defines distinct CD8 + subsets that differentially impact patient outcome in follicular lymphoma. J. Immunother. Cancer 2021, 9, e002662. [Google Scholar] [CrossRef] [PubMed]
- Ayari, C.; LaRue, H.; Hovington, H.; Decobert, M.; Harel, F.; Bergeron, A.; Têtu, B.; Lacombe, L.; Fradet, Y. Bladder tumor infiltrating mature dendritic cells and macrophages as predictors of response to bacillus Calmette-Guérin immunotherapy. Eur. Urol. 2009, 55, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Xiao, J.F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Strandgaard, T.; Lindskrog, S.V.; Nordentoft, I.; Christensen, E.; Birkenkamp-Demtröder, K.; Andreasen, T.G.; Lamy, P.; Kjær, A.; Ranti, D.; Wang, Y.A.; et al. Elevated T-cell Exhaustion and Urinary Tumor DNA Levels Are Associated with Bacillus Calmette-Guérin Failure in Patients with Non-muscle-invasive Bladder Cancer. Eur. Urol. 2022, 82, 646–656. [Google Scholar] [CrossRef]
- Castellano, E.; Samba, C.; Esteso, G.; Simpson, L.; Vendrame, E.; García-Cuesta, E.M.; López-Cobo, S.; Álvarez-Maestro, M.; Linares, A.; Leibar, A.; et al. CyTOF analysis identifies unusual immune cells in urine of BCG-treated bladder cancer patients. Front. Immunol. 2022, 13, 970931. [Google Scholar] [CrossRef] [PubMed]
- Dixon, K.O.; Tabaka, M.; Schramm, M.A.; Xiao, S.; Tang, R.; Dionne, D.; Anderson, A.C.; Rozenblatt-Rosen, O.; Regev, A.; Kuchroo, V.K. TIM-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature 2021, 595, 101–106. [Google Scholar] [CrossRef]
- Hastings, W.D.; Anderson, D.E.; Kassam, N.; Koguchi, K.; Greenfield, E.A.; Kent, S.C.; Zheng, X.X.; Strom, T.B.; Hafler, D.A.; Kuchroo, V.K. TIM-3 is Expressed on Activated Human CD4+ T Cells and Regulates Th1 and Th17 Cytokines. Eur. J. Immunol. 2009, 39, 2492–2501. [Google Scholar] [CrossRef] [Green Version]




| Patients | |
|---|---|
| n | 41 |
| Sex | Female 11 (25.9%) Male 39 (73.1%) |
| Age | Mean ± SD: 74.97 ± 38.89 (39–94) |
| WHO/ISUP TNM 8th edition | pTa 27 (65.8%) pT1 14 (34.2%) High grade 25 (60.9%) Low grade 16 (39.1%) |
| Recurrences at 4 years | Yes 5 (12.2%) (22, 6, 11, 5 and 4 months after diagnosis) No 36 (87.8%) |
| Tumor Size | ≥3 cm: 13 (31.7%) <3 cm: 28 (68.3%) |
| Risk factor: tobacco | Smokers: 7 (17%) Nonsmokers: 12 (29.4%) Ex-smokers: 22 (53.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celada Luis, G.; Albers Acosta, E.; de la Fuente, H.; Velasco Balanza, C.; Arroyo Correas, M.; Romero-Laorden, N.; Alfranca, A.; Olivier Gómez, C. A Comprehensive Analysis of Immune Response in Patients with Non-Muscle-Invasive Bladder Cancer. Cancers 2023, 15, 1364. https://doi.org/10.3390/cancers15051364
Celada Luis G, Albers Acosta E, de la Fuente H, Velasco Balanza C, Arroyo Correas M, Romero-Laorden N, Alfranca A, Olivier Gómez C. A Comprehensive Analysis of Immune Response in Patients with Non-Muscle-Invasive Bladder Cancer. Cancers. 2023; 15(5):1364. https://doi.org/10.3390/cancers15051364
Chicago/Turabian StyleCelada Luis, Guillermo, Eduardo Albers Acosta, Hortensia de la Fuente, Clara Velasco Balanza, Montserrat Arroyo Correas, Nuria Romero-Laorden, Arantzazu Alfranca, and Carlos Olivier Gómez. 2023. "A Comprehensive Analysis of Immune Response in Patients with Non-Muscle-Invasive Bladder Cancer" Cancers 15, no. 5: 1364. https://doi.org/10.3390/cancers15051364