Radiomic Features Associated with Lymphoma Development in the Parotid Glands of Patients with Primary Sjögren’s Syndrome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Standard Reference
2.2. Image Acquisition
2.3. Texture Analysis Protocol and Statistical Analysis
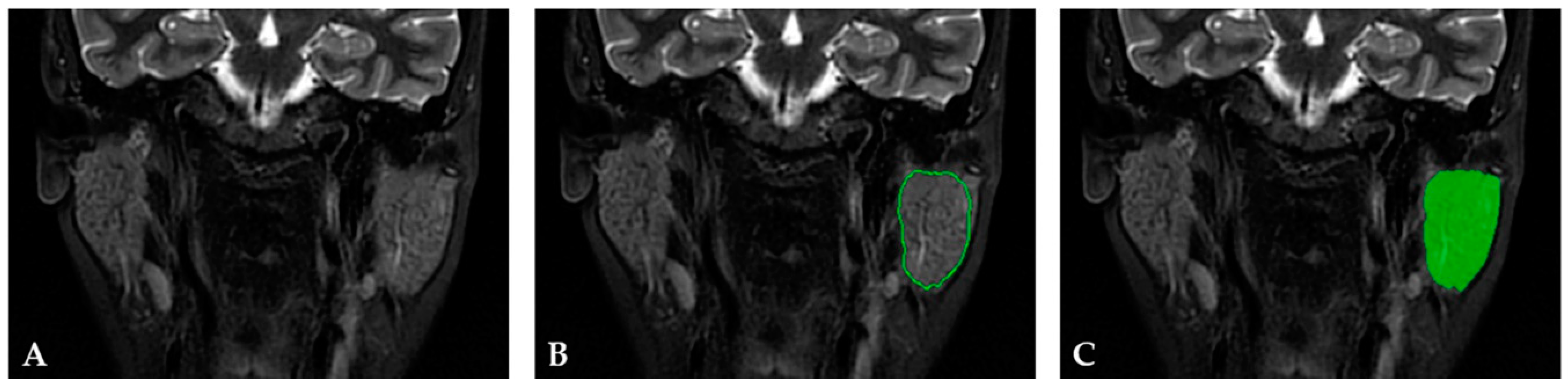
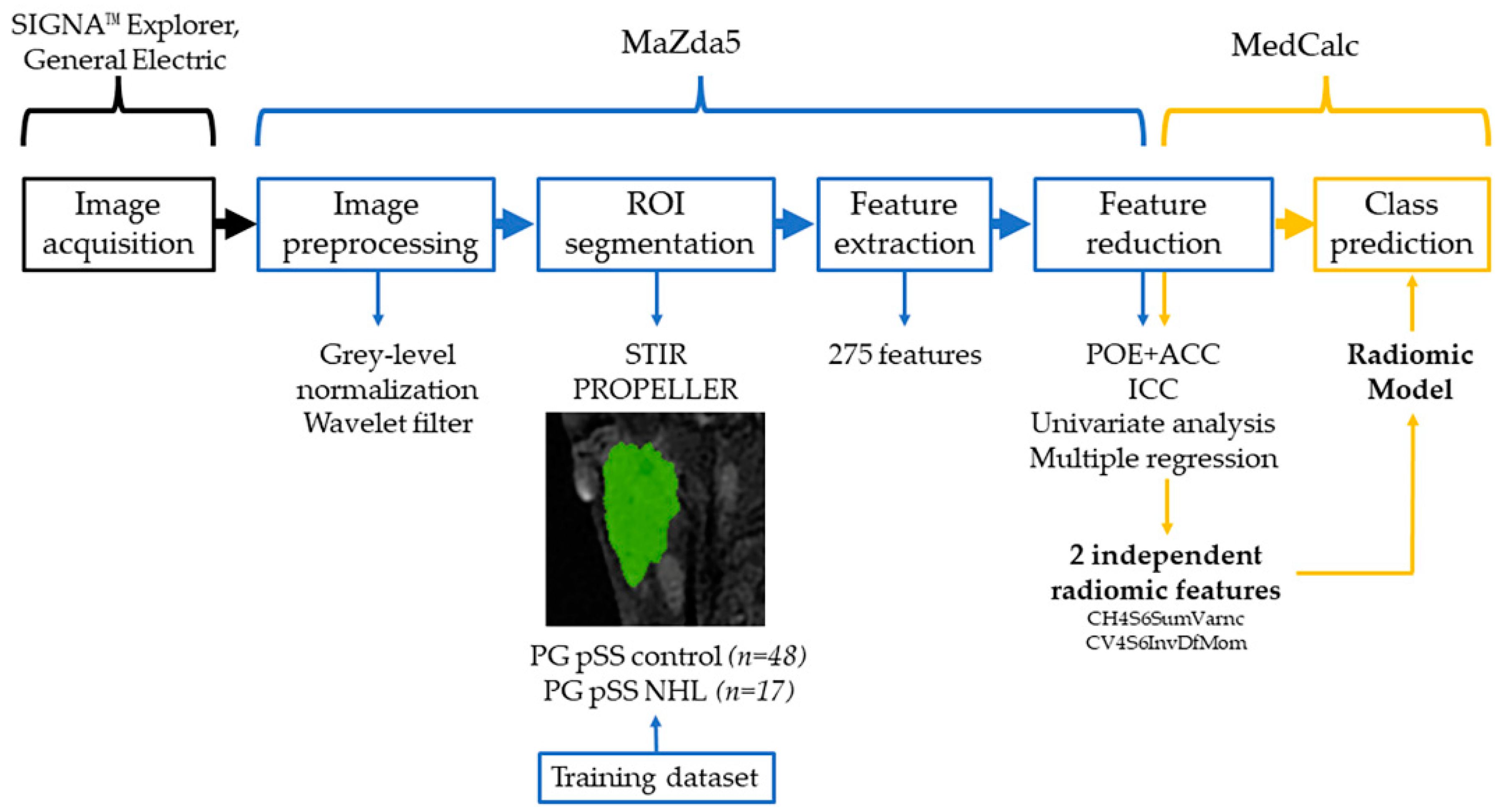
2.3.1. Image Preprocessing and Segmentation
2.3.2. Feature Extraction
2.3.3. Feature Selection and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tzioufas, A.G.; Youinou, P.; Moutsopoulos, H.M. Sjögren’s syndrome. In Oxford Textbook of Rheumatology, 3rd ed.; Isenberg, D.A., Maddison, P., Woo, P., Glass, D., Breedveld, F., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 921–933. [Google Scholar]
- Baldini, C.; Zabotti, A.; Filipovic, N.; Vukicevic, A.; Luciano, N.; Ferro, F.; Lorenzon, M.; De Vita, S. Imaging in primary Sjögren’s syndrome: The ‘obsolete and the new’. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 215–221. [Google Scholar]
- De Vita, S.; Gandolfo, S. Predicting lymphoma development in patients with Sjögren’s syndrome. Expert Rev. Clin. Immunol. 2019, 15, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Skarlis, C.; Raftopoulou, S.; Mavragani, C.P. Sjogren’s Syndrome: Recent Updates. J. Clin. Med. 2022, 11, 399. [Google Scholar] [CrossRef]
- Quartuccio, L.; Isola, M.; Baldini, C.; Priori, R.; Bartoloni Bocci, E.; Carubbi, F.; Maset, M.; Gregoraci, G.; Della Mea, V.; Salvin, S.; et al. Biomarkers of lymphoma in Sjögren’s syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: Results of a multicenter study. J. Autoimmun. 2014, 51, 75–80. [Google Scholar] [CrossRef]
- Fragkioudaki, S.; Mavragani, C.P.; Moutsopoulos, H.M. Predicting the risk for lymphoma development in Sjogren syndrome: An easy tool for clinical use. Medicine 2016, 95, e3766. [Google Scholar] [CrossRef]
- Milic, V.; Colic, J.; Cirkovic, A.; Stanojlovic, S.; Damjanov, N. Disease activity and damage in patients with primary Sjogren’s syndrome: Prognostic value of salivary gland ultrasonography. PLoS ONE 2019, 14, e0226498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coiffier, G.; Martel, A.; Albert, J.D.; Lescoat, A.; Bleuzen, A.; Perdriger, A.; De Bandt, M.; Maillot, F. Ultrasonographic damages of major salivary glands are associated with cryoglobulinemic vasculitis and lymphoma in primary Sjogren’s syndrome: Are the ultrasonographic features of the salivary glands new prognostic markers in Sjogren’s syndrome? Ann. Rheum. Dis. 2021, 80, e111. [Google Scholar] [CrossRef] [Green Version]
- Bădărînză, M.; Serban, O.; Maghear, L.; Bocsa, C.; Micu, M.; Damian, L.; Felea, I.; Fodor, D. Shear wave elastography as a new method to identify parotid lymphoma in primary Sjögren Syndrome patients: An observational study. Rheumatol. Int. 2020, 40, 1275–1281. [Google Scholar] [CrossRef]
- Kato, H.; Kanematsu, M.; Goto, H.; Mizuta, K.; Aoki, M.; Kuze, B.; Hirose, Y. Mucosa-associated lymphoid tissue lymphoma of the salivary glands: MR imaging findings including diffusion-weighted imaging. Eur. J. Radiol. 2012, 81, e612–e617. [Google Scholar] [CrossRef]
- Jousse-Joulin, S.; Coiffier, G. Current status of imaging of Sjogren’s syndrome. Best practice & research. Clin. Rheumatol. 2020, 34, 101592. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.R.; Lee, H.Y.; Jeong, J.Y.; Choi, Y.L.; Kim, J.; Bae, J.; Lee, K.S.; Shim, Y.M. Quantitative image variables reflect the intratumoral pathologic heterogeneity of lung adenocarcinoma. Oncotarget 2016, 7, 67302–67313. [Google Scholar] [CrossRef] [Green Version]
- Caruso, D.; Polici, M.; Zerunian, M.; Pucciarelli, F.; Guido, G.; Polidori, T.; Landolfi, F.; Nicolai, M.; Lucertini, E.; Tarallo, M.; et al. Radiomics in Oncology, Part 1: Technical Principles and Gastrointestinal Application in CT and MRI. Cancers 2021, 13, 2522. [Google Scholar] [CrossRef]
- Caruso, D.; Polici, M.; Zerunian, M.; Pucciarelli, F.; Guido, G.; Polidori, T.; Landolfi, F.; Nicolai, M.; Lucertini, E.; Tarallo, M.; et al. Radiomics in Oncology, Part 2: Thoracic, Genito-Urinary, Breast, Neurological, Hematologic and Musculoskeletal Applications. Cancers 2021, 13, 2681. [Google Scholar] [CrossRef]
- Aringhieri, G.; Fanni, S.C.; Febi, M.; Colligiani, L.; Cioni, D.; Neri, E. The Role of Radiomics in Salivary Gland Imaging: A Systematic Review and Radiomics Quality Assessment. Diagnostics 2022, 12, 3002. [Google Scholar] [CrossRef]
- Vukicevic, A.M.; Milic, V.; Zabotti, A.; Hocevar, A.; De Lucia, O.; Filippou, G.; Frangi, A.F.; Tzioufas, A.; De Vita, S.; Filipovic, N. Radiomics-Based Assessment of Primary Sjögren’s Syndrome from Salivary Gland Ultrasonography Images. IEEE J. Biomed. Health Inform. 2020, 24, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Vukicevic, A.M.; Radovic, M.; Zabotti, A.; Milic, V.; Hocevar, A.; Callegher, S.Z.; De Lucia, O.; De Vita, S.; Filipovic, N. Deep learning segmentation of Primary Sjögren’s syndrome affected salivary glands from ultrasonography images. Comput. Biol. Med. 2021, 129, 104154. [Google Scholar] [CrossRef]
- Muntean, D.D.; Bădărînză, M.; Ștefan, P.A.; Lenghel, M.L.; Rusu, G.M.; Csutak, C.; Coroian, P.A.; Lupean, R.A.; Fodor, D. The Diagnostic Value of MRI-Based Radiomic Analysis of Lacrimal Glands in Patients with Sjögren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 10051. [Google Scholar] [CrossRef]
- Chu, C.; Wang, F.; Zhang, H.; Zhu, Y.; Wang, C.; Chen, W.; He, J.; Sun, L.; Zhou, Z. Whole-volume ADC Histogram and Texture Analyses of Parotid Glands as an Image Biomarker in Evaluating Disease Activity of Primary Sjögren’s Syndrome. Sci. Rep. 2018, 8, 15387. [Google Scholar] [CrossRef] [Green Version]
- van Ginkel, M.S.; Glaudemans, A.W.J.M.; van der Vegt, B.; Mossel, E.; Kroese, F.G.M.; Bootsma, H.; Vissink, A. Imaging in Primary Sjögren’s Syndrome. J. Clin. Med. 2020, 9, 2492. [Google Scholar] [CrossRef]
- Vivino, F.B. Sjogren’s syndrome: Clinical aspects. Clin. Immunol. 2017, 182, 48–54. [Google Scholar] [CrossRef]
- Tonami, H.; Matoba, M.; Kuginuki, Y.; Yokota, H.; Higashi, K.; Yamamoto, I.; Sugai, S. Clinical and imaging findings of lymphoma in patients with Sjögren syndrome. J. Comput. Assist. Tomogr. 2003, 27, 517–524. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Seror, R.; Bowman, S.J.; Brito-Zeron, P.; Theander, E.; Bootsma, H.; Tzioufas, A.; Gottenberg, J.E.; Ramos-Casals, M.; Dörner, T.; Ravaud, P.; et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): A user guide. RMD Open 2015, 1, e000022. [Google Scholar] [CrossRef] [Green Version]
- Michal Strzelecki, M.; Szczypinski, P.; Materka, A.; Klepaczko, A. A software tool for automatic classification and segmentation of 2D/3D medical images. In Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment; Elsevier: Amsterdam, The Netherlands, 2013; Volume 702, pp. 137–140. [Google Scholar] [CrossRef]
- Mărginean, L.; Ștefan, P.A.; Lebovici, A.; Opincariu, I.; Csutak, C.; Lupean, R.A.; Coroian, P.A.; Suciu, B.A. CT in the Differentiation of Gliomas from Brain Metastases: The Radiomics Analysis of the Peritumoral Zone. Brain Sci. 2022, 12, 109. [Google Scholar] [CrossRef]
- Ardakani, A.A.; Rasekhi, A.; Mohammadi, A.; Motevalian, E.; Najafabad, B.K. Differentiation between metastatic and tumour-free cervical lymph nodes in patients with papillary thyroid carcinoma by grey-scale sonographic texture analysis. Pol. J. Radiol. 2018, 83, e37–e46. [Google Scholar] [CrossRef]
- Csutak, C.; Ștefan, P.-A.; Lenghel, L.M.; Moroșanu, C.O.; Lupean, R.-A.; Șimonca, L.; Mihu, C.M.; Lebovici, A. Differentiating High-Grade Gliomas from Brain Metastases at Magnetic Resonance: The Role of Texture Analysis of the Peritumoral Zone. Brain Sci. 2020, 10, 638. [Google Scholar] [CrossRef]
- Lupean, R.A.; Ștefan, P.A.; Feier, D.S.; Csutak, C.; Ganeshan, B.; Lebovici, A.; Petresc, B.; Mihu, C.M. Radiomic Analysis of MRI Images is Instrumental to the Stratification of Ovarian Cysts. J. Pers. Med. 2020, 10, 127. [Google Scholar] [CrossRef]
- De Vita, S.; Gandolfo, S.; Zandonella Callegher, S.; Zabotti, A.; Quartuccio, L. The evaluation of disease activity in Sjögren’s syndrome based on the degree of MALT involvement: Glandular swelling and cryoglobulinaemia compared to ESSDAI in a cohort study. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 112), 150–156. [Google Scholar]
- Takagi, Y.; Sumi, M.; Sumi, T.; Ichikawa, Y.; Nakamura, T. MR microscopy of the parotid glands in patients with Sjogren’s syndrome: Quantitative MR diagnostic criteria. AJNR Am. J. Neuroradiol. 2005, 26, 1207–1214. [Google Scholar]
- Kojima, I.; Sakamoto, M.; Iikubo, M.; Kumamoto, H.; Muroi, A.; Sugawara, Y.; Satoh-Kuriwada, S.; Sasano, T. Diagnostic performance of MR imaging of three major salivary glands for Sjögren’s syndrome. Oral Dis. 2017, 23, 84–90. [Google Scholar] [CrossRef]
- Niemelä, R.K.; Pääkkö, E.; Suramo, I.; Takalo, R.; Hakala, M. Magnetic resonance imaging and magnetic resonance sialography of parotid glands in primary Sjogren’s syndrome. Arthritis Rheum. 2001, 45, 512–518. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, C.; Hua, Y.; Yang, J.; Yu, Q.; Tao, X.; Zheng, J. Dynamic contrast-enhanced MR in the diagnosis of lympho-associated benign and malignant lesions in the parotid gland. Dento Maxillo Facial Radiol. 2016, 45, 20150343. [Google Scholar] [CrossRef]
- Stoia, S.; Băciuț, G.; Lenghel, M.; Badea, R.; Csutak, C.; Rusu, G.M.; Băciuț, M.; Tamaș, T.; Boțan, E.; Armencea, G.; et al. Cross-sectional imaging and cytologic investigations in the preoperative diagnosis of parotid gland tumors–An updated literature review. Bosn. J. Basic Med. Sci. 2021, 21, 19–32. [Google Scholar] [CrossRef]
- Izumi, M.; Eguchi, K.; Nakamura, H.; Nagataki, S.; Nakamura, T. Premature fat deposition in the salivary glands associated with Sjögren syndrome: MR and CT evidence. Am. J. Neuroradiol. 1997, 18, 951–958. [Google Scholar]
- Gadodia, A.; Bhalla, A.S.; Sharma, R.; Thakar, A.; Parshad, R. Bilateral parotid swelling: A radiological review. Dentomaxillofacial Radiol. 2011, 40, 403–414. [Google Scholar] [CrossRef]
- Nakatsu, M.; Hatabu, H.; Itoh, H.; Morikawa, K.; Miki, Y.; Kasagi, K.; Shimono, T.; Shoji, K.; Shimada, Y.; Imamura, M.; et al. Comparison of short inversion time inversion recovery (STIR) and fat-saturated (chemsat) techniques for background fat intensity suppression in cervical and thoracic MR imaging. J. Magn. Reson. Imaging 2000, 11, 56–60. [Google Scholar] [CrossRef]
- Shimamoto, H.; Tsujimoto, T.; Kakimoto, N.; Majima, M.; Iwamoto, Y.; Senda, Y.; Murakami, S. Effectiveness of the periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) technique for reducing motion artifacts caused by mandibular movements on fat-suppressed T2-weighted magnetic resonance (MR) images. Magn. Reson. Imaging 2018, 54, 1–7. [Google Scholar] [CrossRef]
- Dennie, C.; Thornhill, R.; Sethi-Virmani, V.; Souza, C.A.; Bayanati, H.; Gupta, A.; Maziak, D. Role of quantitative computed tomography texture analysis in the differentiation of primary lung cancer and granulomatous nodules. Quant. Imaging Med. Surg. 2016, 6, 6–15. [Google Scholar] [CrossRef]
- van Griethuysen, J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [Green Version]
- Clausi, D.A. An analysis of co-occurrence texture statistics as a function of grey level quantization. Can. J. Remote Sens. 2002, 28, 45–62. [Google Scholar] [CrossRef]
- Unser, M. Sum and difference histograms for texture classification. IEEE Trans. Pattern Anal. Mach. Intell. 1986, 8, 118–125. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. Ann. Intern. Med. 2015, 162, 55–63. [Google Scholar] [CrossRef] [Green Version]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—”How-to” guide and critical reflection. Insights Into Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Shur, J.D.; Doran, S.J.; Kumar, S.; Ap Dafydd, D.; Downey, K.; O’Connor, J.P.B.; Papanikolaou, N.; Messiou, C.; Koh, D.M.; Orton, M.R. Radiomics in Oncology: A Practical Guide. Radiographics 2021, 41, 1717–1732. [Google Scholar] [CrossRef]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef]
- Buch, K.; Kuno, H.; Qureshi, M.M.; Li, B.; Sakai, O. Quantitative variations in texture analysis features dependent on MRI scanning parameters: A phantom model. J. Appl. Clin. Med. Phys. 2018, 19, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Baeßler, B.; Weiss, K.; Pinto Dos Santos, D. Robustness and Reproducibility of Radiomics in Magnetic Resonance Imaging: A Phantom Study. Investig. Radiol. 2019, 54, 221–228. [Google Scholar] [CrossRef]
- Bologna, M.; Corino, V.D.A.; Montin, E.; Messina, A.; Calareso, G.; Greco, F.G.; Sdao, S.; Mainardi, L.T. Assessment of Stability and Discrimination Capacity of Radiomic Features on Apparent Diffusion Coefficient Images. J. Digit. Imaging 2018, 31, 879–894. [Google Scholar] [CrossRef] [Green Version]
- Cattell, R.; Chen, S.; Huang, C. Robustness of radiomic features in magnetic resonance imaging: Review and a phantom study. Vis. Comput. Ind. Biomed. Art 2019, 2, 19. [Google Scholar] [CrossRef]
- Peerlings, J.; Woodruff, H.C.; Winfield, J.M.; Ibrahim, A.; Van Beers, B.E.; Heerschap, A.; Jackson, A.; Wildberger, J.E.; Mottaghy, F.M.; DeSouza, N.M.; et al. Stability of radiomics features in apparent diffusion coefficient maps from a multi-centre test-retest trial. Sci. Rep. 2019, 9, 4800. [Google Scholar] [CrossRef] [Green Version]
- Um, H.; Tixier, F.; Bermudez, D.; Deasy, J.O.; Young, R.J.; Veeraraghavan, H. Impact of image preprocessing on the scanner dependence of multi-parametric MRI radiomic features and covariate shift in multi-institutional glioblastoma datasets. Phys. Med. Biol. 2019, 64, 165011. [Google Scholar] [CrossRef]
- Jethanandani, A.; Lin, T.A.; Volpe, S.; Elhalawani, H.; Mohamed, A.S.R.; Yang, P.; Fuller, C.D. Exploring Applications of Radiomics in Magnetic Resonance Imaging of Head and Neck Cancer: A Systematic Review. Front. Oncol. 2018, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.; Yuan, J.; Zhou, Y.; Wong, O.L.; Cheung, K.Y.; Yu, S.K. Acquisition repeatability of MRI radiomics features in the head and neck: A dual-3D-sequence multi-scan study. Vis. Comput. Ind. Biomed. Art 2022, 5, 10. [Google Scholar] [CrossRef]
- Bianchini, L.; Botta, F.; Origgi, D.; Rizzo, S.; Mariani, M.; Summers, P.; García-Polo, P.; Cremonesi, M.; Lascialfari, A. PETER PHAN: An MRI phantom for the optimisation of radiomic studies of the female pelvis. Phys. Med. 2020, 71, 71–81. [Google Scholar] [CrossRef]



| Category | Feature | Computation | Variation | Number of Features |
|---|---|---|---|---|
| Histogram | Mean, Kurtosis, Percentile 01/10/50/90/99% Skewness, Variance | - | - | 9 |
| Co-occurrence matrix | Angular second moment, Contrast, Correlation, Difference entropy, Difference Variance, Entropy, Inverse difference moment, Sum average, Sum entropy, Sum of squares, Sum variance | 6 bits/pixel | Computed 20 times for distance values from 1 to 5 | 220 |
| Run length matrix | Fraction of image in runs, Grey level nonuniformity, Long run emphasis, Run length nonuniformity, Short run emphasis | 6 bits/pixel | Computed four times for horizontal, vertical, 45°, and 135° directions | 20 |
| Gradient | Kurtosis, Mean, Percentage of pixels with nonzero gradient, Skewness, Variance | 4 bits/pixel | - | 5 |
| Autoregressive model | Sigma, Teta 1–4 | - | - | 5 |
| Wavelet | Wavelet energy with high- and low-pass filters | 8 bits/pixel | 4 scales | 16 |
| Feature | All Patients (n = 36) | pSS Control Group (n = 24) | pSS NHL Group (n = 12) | p |
|---|---|---|---|---|
| Age (years) | 54.93 ± 13.34 | 58.79 ± 12.44 | 46.92 ± 12.50 | 0.013 |
| Gender (female) | 33 (91.6) | 23 (95.8) | 10 (83.3) | 0.207 |
| BMI (kg/m2) | 26.11 ± 4.39 | 25.23 ± 3.98 | 27.39 ± 5.01 | 0.130 |
| Disease duration (months) | 34 [17, 50] | 29 [11, 46] | 37 [20, 69] | 0.416 |
| Disease duration | 0.349 | |||
| <5 years | 24 (66.7) | 21 (87.5) | 3 (25) | |
| ≥5 years | 12 (33.3) | 3 (12.5) | 9 (75) | |
| ESSDAI score | 2 [0, 9] | 0 [0, 2] | 13 [9, 15] | <0.001 |
| Disease activity | <0.001 | |||
| Low (ESSDAI < 5) | 22 (61.1) | 20 (83.3) | 2 (16.6) | |
| Moderate-high (ESSDAI ≥ 5) | 14 (38.9) | 4 (16.7) | 10 (83.4) | |
| Positive Schirmer’s test | 33 (91.6) | 21 (87.5) | 12 (100) | 0.522 |
| UWSF (mL) | 1.24 ± 0.34 | 1.23 ± 0.34 | 1.25 ± 0.28 | 0.861 |
| Anti-Ro/La autoantibodies | 32 (88.9) | 20 (83.3) | 12 (100) | 0.139 |
| Rheumatoid factor | 27 (75) | 15 (62.5) | 12 (100) | 0.016 |
| Texture Parameters | PG pSS Control Group (n = 48) | PG pSS NHL Group (n = 17) | p | ICC | ||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | |||
| CH4S6SumVarnc | 199.24 | 175.39–221.77 | 234.60 | 214.35–257.19 | 0.0004 | 0.956 |
| WavEnHL_s-4 | 270.38 | 186.15–370.19 | 497.10 | 283.74–727.07 | 0.0094 | 0.910 |
| Perc90 | 33,280.50 | 33,186.00–33,354.50 | 33,512.00 | 33,359.75–33,563.50 | 0.0001 | 0.922 |
| Mean | 33,147.03 | 33,101.45–33,229.68 | 33,377.18 | 33,230.06–33,389.89 | 0.0001 | 0.933 |
| CV4S6InvDfMom | 0.18 | 0.16–0.22 | 0.10 | 0.10–0.13 | <0.0001 | 0.924 |
| CH3S6Correlat | 0.14 | 0.04–0.25 | 0.405 | 0.17–0.49 | 0.0027 | 0.901 |
| CN1S6SumVarnc | 349.22 | 327.72–369.30 | 360.04 | 320.52–388.26 | 0.3547 | 0.897 |
| RNS6RLNonUni | 1371.17 | 1104.56–1720.21 | 1603.50 | 1272.27–2083.16 | 0.2383 | 0.905 |
| Perc1 | 32,990.50 | 32,942.00–33,050.00 | 33,109.00 | 33,028.25–33,157.25 | 0.0005 | 0.911 |
| CV5S6SumAverg | 65.00 | 64.50–65.29 | 62.86 | 59.58 to 65.09 | 0.4737 | 0.934 |
| Parameter | Cutoff | AUC | Se (%) | Sp (%) | +LR | −LR | Youden Index | p |
|---|---|---|---|---|---|---|---|---|
| CH4S6SumVarnc | >207.62 | 0.800 | 88.24 (63.6–98.5) | 64.58 (49.5–77.8) | 2.49 (1.64–3.79) | 0.18 (0.04–0.68) | 0.528 | <0.0001 |
| WavEnHL_s-4 | >388.84 | 0.713 | 58.82 (32.9–81.6) | 81.25 (67.4–91.1) | 3.14 (1.54–6.39) | 0.51 (0.28–0.91) | 0.400 | 0.0021 |
| Perc90 | >33,363 | 0.816 | 76.47 (50.1–93.2) | 79.17 (65.0–89.5) | 3.67 (1.99–6.76) | 0.30 (0.12–0.71) | 0.554 | <0.0001 |
| Mean | >33,233.87 | 0.821 | 76.47 (50.1–93.2) | 81.25 (67.4–91.1) | 4.08 (2.14–7.78) | 0.29 (0.12–0.69) | 0.577 | <0.0001 |
| CV4S6InvDfMom | <0.145 | 0.875 | 88.24 (63.6–98.5) | 77.08 (62.7–88.0) | 3.85 (2.23–6.65) | 0.15 (0.04–0.57) | 0.653 | <0.0001 |
| CH3S6Correlat | >0.321 | 0.746 | 52.94 (27.8–77.0) | 89.58 (77.3–96.5) | 5.08 (1.98–13.05) | 0.53 (0.31–0.88) | 0.425 | 0.0008 |
| Perc1 | >33,006 | 0.787 | 88.24 (63.6–98.5) | 62.50 (47.4–76.0) | 2.35 (1.57–3.53) | 0.19 (0.05–0.70) | 0.507 | <0.0001 |
| Independent Variables | Coefficient | Std. Error | p | VIF |
|---|---|---|---|---|
| (Constant) | −27.7065 | |||
| CH4S6SumVarnc | 0.00417 | 0.001495 | 0.0072 | 3.284 |
| WavEnHL_s-4 | 0.00009 | 0.0001514 | 0.5478 | 1.303 |
| Perc90 | −0.00242 | 0.0006944 | 0.001 | 15.771 |
| Mean | 0.00423 | 0.001474 | 0.0058 | 27.424 |
| CV4S6InvDfMom | 3.3534 | 0.8530 | 0.0002 | 1.529 |
| CH3S6Correlat | 0.0356 | 0.3662 | 0.9229 | 3.776 |
| Perc1 | −0.001 | 0.001147 | 0.3841 | 7.217 |
| R2 | 0.5524 | |||
| R2-adjusted | 0.4975 | |||
| MCC | 0.7433 | |||
| RSD | 0.3140 |
| Parameter | Cutoff | AUC | Se (%) | Sp (%) | Youden Index | p |
|---|---|---|---|---|---|---|
| Radiomic Model | ≥1.556 | 0.931 | 94.12 (71.3–99.9) | 85.42 (72.2–93.9) | 0.795 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntean, D.D.; Lenghel, L.M.; Ștefan, P.A.; Fodor, D.; Bădărînză, M.; Csutak, C.; Dudea, S.M.; Rusu, G.M. Radiomic Features Associated with Lymphoma Development in the Parotid Glands of Patients with Primary Sjögren’s Syndrome. Cancers 2023, 15, 1380. https://doi.org/10.3390/cancers15051380
Muntean DD, Lenghel LM, Ștefan PA, Fodor D, Bădărînză M, Csutak C, Dudea SM, Rusu GM. Radiomic Features Associated with Lymphoma Development in the Parotid Glands of Patients with Primary Sjögren’s Syndrome. Cancers. 2023; 15(5):1380. https://doi.org/10.3390/cancers15051380
Chicago/Turabian StyleMuntean, Delia Doris, Lavinia Manuela Lenghel, Paul Andrei Ștefan, Daniela Fodor, Maria Bădărînză, Csaba Csutak, Sorin Marian Dudea, and Georgeta Mihaela Rusu. 2023. "Radiomic Features Associated with Lymphoma Development in the Parotid Glands of Patients with Primary Sjögren’s Syndrome" Cancers 15, no. 5: 1380. https://doi.org/10.3390/cancers15051380
APA StyleMuntean, D. D., Lenghel, L. M., Ștefan, P. A., Fodor, D., Bădărînză, M., Csutak, C., Dudea, S. M., & Rusu, G. M. (2023). Radiomic Features Associated with Lymphoma Development in the Parotid Glands of Patients with Primary Sjögren’s Syndrome. Cancers, 15(5), 1380. https://doi.org/10.3390/cancers15051380









