Mechanistic Insights on Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Hyperthermia in Cancer Therapy—The Clinical Picture
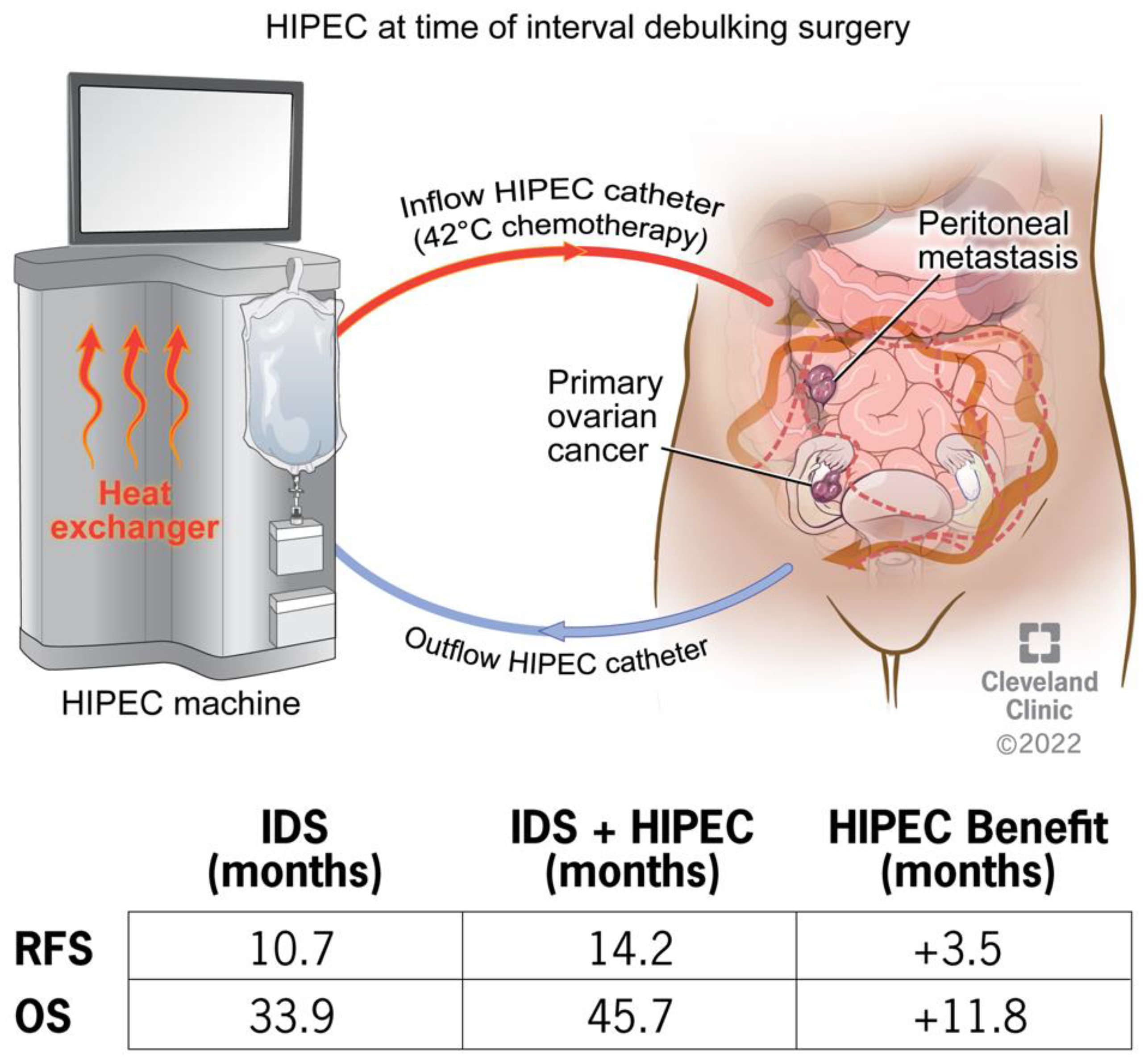
2.1. Ovarian Cancer
| Author | Year | Study Type | N | Study Details | OS Benefit | PFS Benefit | RFS Benefit |
|---|---|---|---|---|---|---|---|
| Lim et al. [12] | 2022 | Single-Blind Randomized | 184 | HIPEC + interval CRS after NACT in ovarian cancer | 13.6 months | 2 months | N/A |
| Ghirardi et al. [23] | 2022 | Retrospective | 70 | HIPEC + BRCA mutational status in EOC | No difference between BRCA status | No difference between BRCA status | N/A |
| Costales et al. [18] | 2021 | Retrospective | 48 | PS vs. PR EOC patients given HIPEC after CRS | median 26.9 months in PR patients | N/A | 11.2 months in PS patients |
| Van Driel et al. [3] | 2018 | Open-Label Randomized | 245 | Interval CRS ± HIPEC for EOC | 11.8 months | N/A | 3.5 months |
| Spiliotis et al. [16] | 2015 | Open-Label Randomized | 120 | CRS ± HIPEC for recurrent EOC | 13.3 months | N/A | N/A |
| Safra et al. [25] | 2014 | Case-Control Study | 27 | CRS ± HIPEC ± BRCA mutation in EOC | Not reached at time of analysis (70% patients alive) | 9 months, no difference in BRCA status | N/A |
2.2. Additional Applications/Future Directions of HIPEC Therapy
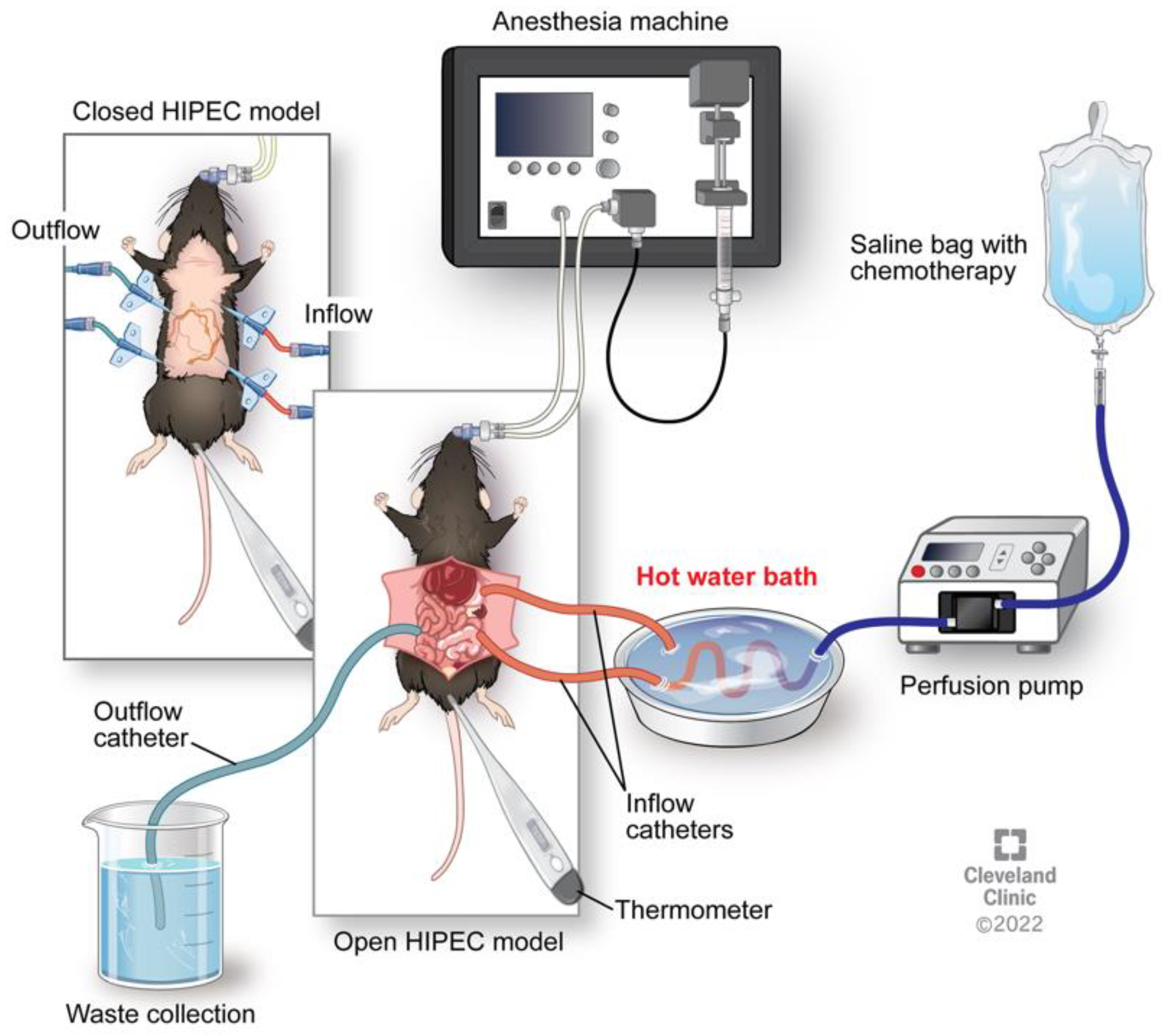
3. Animal Models of HIPEC
Summary of Pre-Clinical Findings and Challenges
4. Mechanisms of Hyperthermia with or without Chemotherapy
4.1. Hyperthermic Impact on Chemotherapeutics
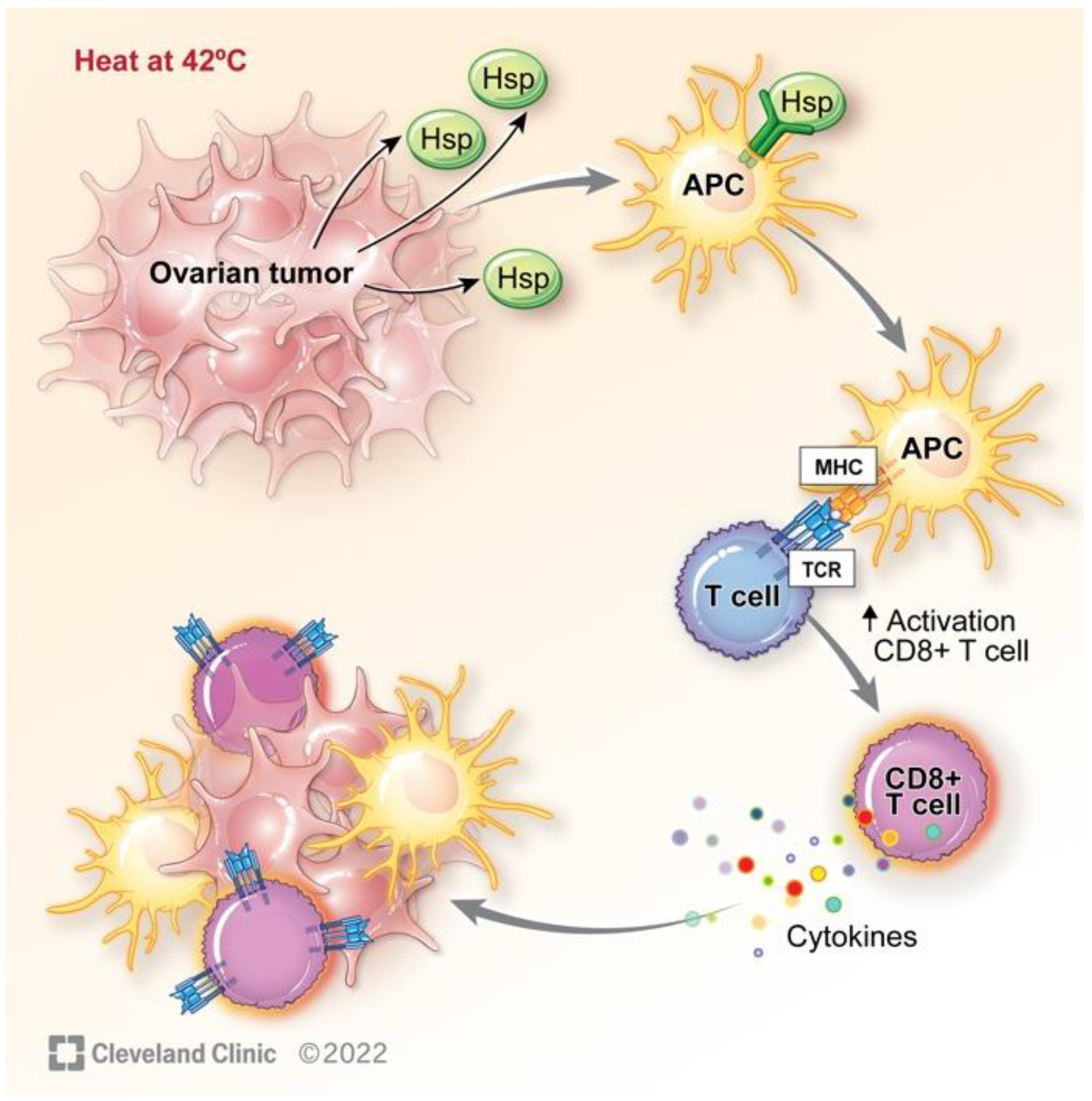
4.2. Heat Shock Response
5. Hyperthermia Impact on the Immune System
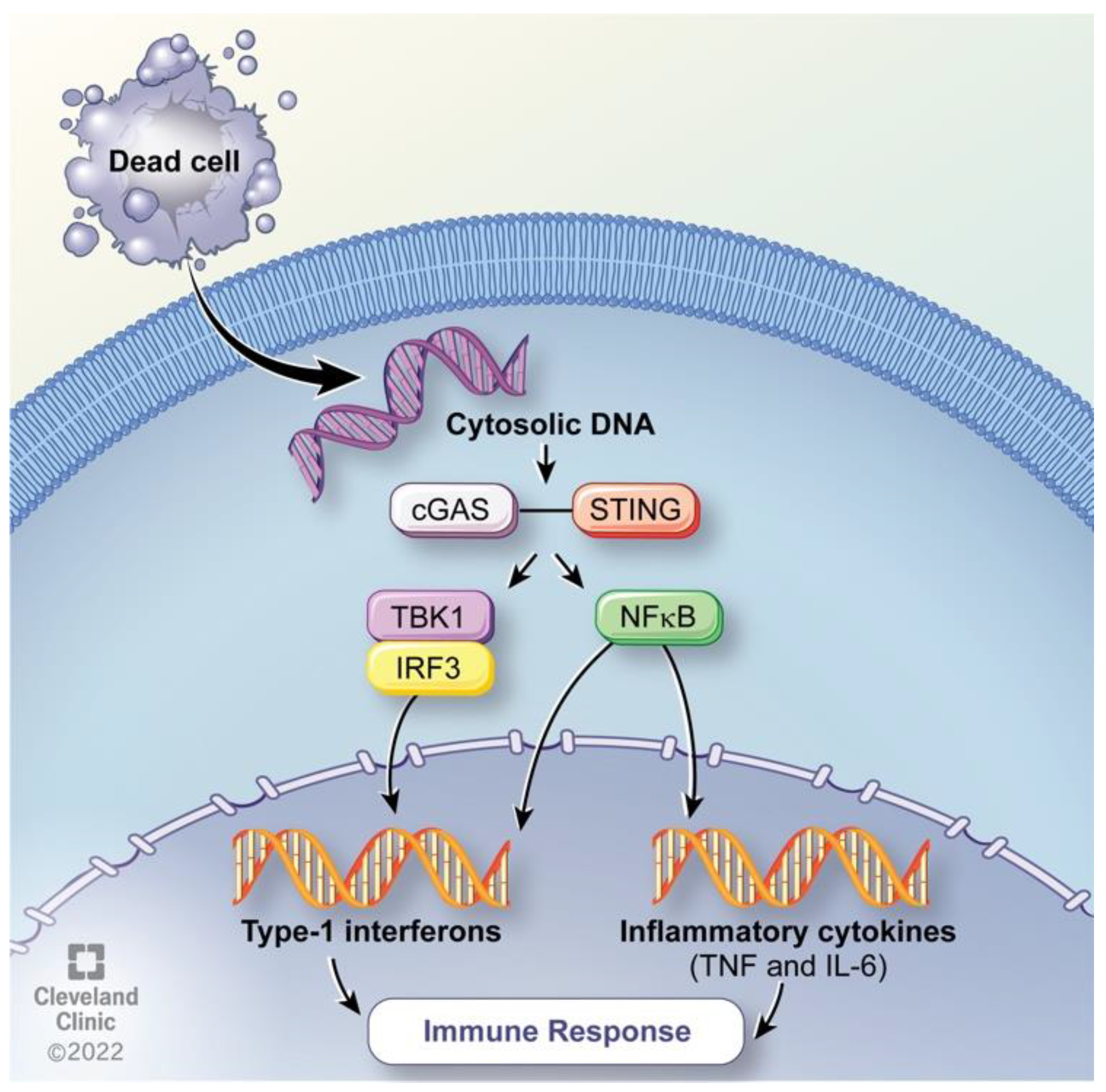
6. Hyperthermia Impact on Genome Instability
7. Clinical Correlates on Immune and DNA Repair Activity in HIPEC
8. Conclusions and Prospects of Future Therapeutic Strategy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Singh, N.; Ghatage, P. Past, Present, and Future of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Ovarian Cancer. Cureus 2021, 13, e15563. [Google Scholar] [CrossRef] [PubMed]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.; van der Velden, J.; Arts, H.J.; Massuger, L.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Clinic, C. Epithelial Ovarian Cancer. 2022. Available online: https://my.clevelandclinic.org/health/diseases/22250-epithelial-ovarian-cancer (accessed on 26 October 2022).
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [Green Version]
- Society, A.C. Cancer Statistics Center. Available online: http://cancerstatisticscenter.cancer.org (accessed on 26 October 2022).
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet. Gynecol. 2021, 137, 108–121. [Google Scholar] [CrossRef]
- Bristow, R.E.; Tomacruz, R.S.; Armstrong, D.K.; Trimble, E.L.; Montz, F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J. Clin. Oncol. 2002, 20, 1248–1259. [Google Scholar] [CrossRef]
- Chi, D.S.; Eisenhauer, E.L.; Lang, J.; Huh, J.; Haddad, L.; Abu-Rustum, N.R.; Sonoda, Y.; Levine, D.A.; Hensley, M.; Barakat, R.R. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol. Oncol. 2006, 103, 559–564. [Google Scholar] [CrossRef]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, 2011, CD007565. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.M.; Cristea, M.; DeRosa, M.; et al. NCCN Guidelines Insights: Ovarian Cancer, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.C.; Chang, S.J.; Park, B.; Yoo, H.J.; Yoo, C.W.; Nam, B.H.; Park, S.Y. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022, 157, 374–383. [Google Scholar] [CrossRef]
- Chambers, L.; Horowitz, M.; Costales, A.; Yao, M.; Chichura, A.; Morton, M.; Gruner, M.; Rose, P.; Michener, C.; Debernardo, R. Cisplatin and paclitaxel are associated with improved progression-free survival compared to cisplatin alone during interval debulking surgery with hyperthermic intraperitoneal chemotherapy in women with advanced epithelial ovarian cancer. Gynecol. Oncol. 2021, 162, S58–S59. [Google Scholar] [CrossRef]
- Zivanovic, O.; Chi, D.S.; Zhou, Q.; Iasonos, A.; Konner, J.A.; Makker, V.; Grisham, R.N.; Brown, A.K.; Nerenstone, S.; Diaz, J.P.; et al. Secondary Cytoreduction and Carboplatin Hyperthermic Intraperitoneal Chemotherapy for Platinum-Sensitive Recurrent Ovarian Cancer: An MSK Team Ovary Phase II Study. J. Clin. Oncol. 2021, 39, 2594–2604. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Tinker, A.V.; Friedlander, M. “Platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Spiliotis, J.; Halkia, E.; Lianos, E.; Kalantzi, N.; Grivas, A.; Efstathiou, E.; Giassas, S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: A prospective randomized phase III study. Ann. Surg. Oncol. 2015, 22, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Bakrin, N.; Bereder, J.M.; Decullier, E.; Classe, J.M.; Msika, S.; Lorimier, G.; Abboud, K.; Meeus, P.; Ferron, G.; Quenet, F.; et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: A French multicentre retrospective cohort study of 566 patients. Eur. J. Surg. Oncol. 2013, 39, 1435–1443. [Google Scholar] [CrossRef]
- Costales, A.B.; Chambers, L.; Chichura, A.; Rose, P.G.; Mahdi, H.; Michener, C.M.; Yao, M.; Debernardo, R. Effect of platinum sensitivity on the efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) in recurrent epithelial ovarian cancer. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101844. [Google Scholar] [CrossRef]
- Norquist, B.M.; Brady, M.F.; Harrell, M.I.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Burger, R.A.; Tewari, K.S.; et al. Mutations in Homologous Recombination Genes and Outcomes in Ovarian Carcinoma Patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin. Cancer Res. 2018, 24, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Zhang, Y.; Shi, Y.; Yao, S.; Dai, M.; Cai, H. Cytoreductive Surgery (CRS) Combined with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Platinum-Sensitive Recurrence Epithelial Ovarian Cancer with HRR Mutation: A Phase III Randomized Clinical Trial. Technol. Cancer Res. Treat. 2022, 21, 15330338221104565. [Google Scholar] [CrossRef]
- Toh, M.; Ngeow, J. Homologous Recombination Deficiency: Cancer Predispositions and Treatment Implications. Oncologist 2021, 26, e1526–e1537. [Google Scholar] [CrossRef]
- Foulkes, W.D. BRCA1 and BRCA2: Chemosensitivity, treatment outcomes and prognosis. Fam. Cancer 2006, 5, 135–142. [Google Scholar] [CrossRef]
- Ghirardi, V.; De Felice, F.; D’Indinosante, M.; Bernardini, F.; Giudice, M.T.; Fagotti, A.; Scambia, G. Hyperthermic intraperitoneal chemotherapy (HIPEC) after primary debulking surgery in advanced epithelial ovarian cancer: Is BRCA mutational status making the difference? Cancer Treat. Res. Commun. 2022, 31, 100518. [Google Scholar] [CrossRef]
- Koole, S.N.; Schouten, P.C.; Hauke, J.; Kluin, R.J.C.; Nederlof, P.; Richters, L.K.; Krebsbach, G.; Sikorska, K.; Alkemade, M.; Opdam, M.; et al. Effect of HIPEC according to HRD/BRCAwt genomic profile in stage III ovarian cancer: Results from the phase III OVHIPEC trial. Int. J. Cancer 2022, 151, 1394–1404. [Google Scholar] [CrossRef]
- Safra, T.; Grisaru, D.; Inbar, M.; Abu-Abeid, S.; Dayan, D.; Matceyevsky, D.; Weizman, A.; Klausner, J.M. Cytoreduction surgery with hyperthermic intraperitoneal chemotherapy in recurrent ovarian cancer improves progression-free survival, especially in BRCA-positive patients- a case-control study. J. Surg. Oncol. 2014, 110, 661–665. [Google Scholar] [CrossRef]
- Colombo, N.; Lorusso, D.; Scollo, P. Impact of Recurrence of Ovarian Cancer on Quality of Life and Outlook for the Future. Int. J. Gynecol. Cancer 2017, 27, 1134–1140. [Google Scholar] [CrossRef] [Green Version]
- Chambers, L.M.; Yao, M.; Morton, M.; Gruner, M.; Chichura, A.B.; Horowitz, M.; Costales, A.; Rose, P.G.; Michener, C.M.; Debernardo, R. Patterns of recurrence in women with advanced and recurrent epithelial ovarian cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Gynecol. Oncol. 2021, 161, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.S.; Gelli, M.; Agarwal, D.; Goéré, D. Complications of Cytoreductive Surgery and HIPEC in the Treatment of Peritoneal Metastases. Indian J. Surg. Oncol. 2016, 7, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, D.E.; Lee, Y.; Ha, H.I.; Chang, Y.J.; Chang, S.J.; Park, S.Y.; Lim, M.C. Quality of life outcomes from the randomized trial of hyperthermic intraperitoneal chemotherapy following cytoreductive surgery for primary ovarian cancer (KOV-HIPEC-01). J. Gynecol. Oncol. 2022, 33, e54. [Google Scholar] [CrossRef]
- Graversen, M.; Detlefsen, S.; Ainsworth, A.P.; Fristrup, C.W.; Knudsen, A.O.; Pfeiffer, P.; Tarpgaard, L.S.; Mortensen, M.B. Treatment of Peritoneal Metastasis with Pressurized Intraperitoneal Aerosol Chemotherapy: Results from the Prospective PIPAC-OPC2 Study. Ann. Surg. Oncol. 2023. [Google Scholar] [CrossRef]
- Graversen, M.; Lundell, L.; Fristrup, C.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) as an outpatient procedure. Pleura Peritoneum 2018, 3, 20180128. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, M.; Cui, S.; Ba, M.; Chen, Z.; Ruan, Q. Efficacy and safety of ultrasound-guided continuous hyperthermic intraperitoneal perfusion chemotherapy for the treatment of malignant ascites: A midterm study of 36 patients. Onco Targets Ther. 2016, 9, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Helderman, R.; Loke, D.R.; Tanis, P.J.; Tuynman, J.B.; Ceelen, W.; de Hingh, I.H.; van der Speeten, K.; Franken, N.A.P.; Oei, A.L.; Kok, H.P.; et al. Preclinical In Vivo-Models to Investigate HIPEC; Current Methodologies and Challenges. Cancers 2021, 13, 3430. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.; McCarthy, E.M.-L.B.; Levi-Polyachenko, B. Survival Mouse Model of Intraperitoneal Perfusion Mimicking Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Acad. Surg. Congr. 2018. Available online: https://www.asc-abstracts.org/abs2018/80-07-survival-mouse-model-of-inraperitoneal-perfusion-mimicking-hyperthermic-intraperitoneal-chemotherapy-hipec/ (accessed on 1 November 2022).
- McCabe-Lankford, E.; Peterson, M.; McCarthy, B.; Brown, A.J.; Terry, B.; Galarza-Paez, L.; Levi-Polyachenko, N. Murine Models of Intraperitoneal Perfusion for Disseminated Colorectal Cancer. J. Surg. Res. 2019, 233, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Liesenfeld, L.F.; Wagner, B.; Hillebrecht, H.C.; Brune, M.; Eckert, C.; Klose, J.; Schmidt, T.; Büchler, M.W.; Schneider, M. HIPEC-Induced Acute Kidney Injury: A Retrospective Clinical Study and Preclinical Model. Ann. Surg. Oncol. 2022, 29, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Miailhe, G.; Arfi, A.; Mirshahi, M.; Eveno, C.; Pocard, M.; Touboul, C. A new animal model for hyperthermic intraperitoneal chemotherapy (HIPEC) in tumor-bearing mice in the treatment of peritoneal carcinomatosis of ovarian origin. J. Visc. Surg. 2018, 155, 183–189. [Google Scholar] [CrossRef]
- Pelz, J.O.; Doerfer, J.; Hohenberger, W.; Meyer, T. A new survival model for hyperthermic intraperitoneal chemotherapy (HIPEC) in tumor-bearing rats in the treatment of peritoneal carcinomatosis. BMC Cancer 2005, 5, 56. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.C.; Hsu, Y.T.; Chang, C.L. Hyperthermic intraperitoneal chemotherapy enhances antitumor effects on ovarian cancer through immune-mediated cancer stem cell targeting. Int. J. Hyperth. 2021, 38, 1013–1022. [Google Scholar] [CrossRef]
- Liu, H.; Lv, L.; Yang, K. Chemotherapy targeting cancer stem cells. Am. J. Cancer Res. 2015, 5, 880–893. [Google Scholar]
- Wagner, B.R.; Adamus, A.L.; Sönnecken, D.; Vahdad, R.; Jank, P.; Denkert, C.; Mahnken, A.H.; Seitz, G. Establishment of a new valid animal model for the evaluation of hyperthermic intraperitoneal chemotherapy (HIPEC) in pediatric rhabdomyosarcoma. Pediatr. Blood Cancer 2021, 68, e29202. [Google Scholar] [CrossRef]
- Graziosi, L.; Mencarelli, A.; Renga, B.; Santorelli, C.; Cantarella, F.; Bugiantella, W.; Cavazzoni, E.; Donini, A.; Fiorucci, S. Gene expression changes induced by HIPEC in a murine model of gastric cancer. In Vivo 2012, 26, 39–45. [Google Scholar]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef]
- Dunne, M.; Regenold, M.; Allen, C. Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv. Drug Deliv. Rev. 2020, 163–164, 98–124. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wessalowski, R.; Reichardt, P.; Wust, P.; Ghadjar, P.; Hohenberger, P.; Angele, M.; Salat, C.; et al. Effect of Neoadjuvant Chemotherapy Plus Regional Hyperthermia on Long-term Outcomes Among Patients with Localized High-Risk Soft Tissue Sarcoma: The EORTC 62961-ESHO 95 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 483–492. [Google Scholar] [CrossRef]
- Hyperthermic Cencer Institute. Hyperthermia and Chemotherapy. 2023. Available online: https://hcioncology.com/hyperthermia-and-chemotherapy/ (accessed on 4 December 2022).
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia Treatment as a Promising Anti-Cancer Strategy: Therapeutic Targets, Perspective Mechanisms and Synergistic Combinations in Experimental Approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Wallin, R.P.; Lundqvist, A.; Moré, S.H.; von Bonin, A.; Kiessling, R.; Ljunggren, H.G. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002, 23, 130–135. [Google Scholar] [CrossRef]
- Dubey, A.; Prajapati, K.S.; Swamy, M.; Pachauri, V. Heat shock proteins: A therapeutic target worth to consider. Vet. World 2015, 8, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Dubrez, L.; Causse, S.; Borges Bonan, N.; Dumétier, B.; Garrido, C. Heat-shock proteins: Chaperoning DNA repair. Oncogene 2020, 39, 516–529. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Gong, J.; Murshid, A. Extracellular HSPs: The Complicated Roles of Extracellular HSPs in Immunity. Front. Immunol. 2016, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Brenu, E.W.; Staines, D.R.; Tajouri, L.; Huth, T.; Ashton, K.J.; Marshall-Gradisnik, S.M. Heat shock proteins and regulatory T cells. Autoimmune Dis. 2013, 2013, 813256. [Google Scholar] [CrossRef] [Green Version]
- Tsan, M.F.; Gao, B. Heat shock protein and innate immunity. Cell Mol. Immunol. 2004, 1, 274–279. [Google Scholar]
- Park, H.K.; Yoon, N.G.; Lee, J.E.; Hu, S.; Yoon, S.; Kim, S.Y.; Hong, J.H.; Nam, D.; Chae, Y.C.; Park, J.B.; et al. Unleashing the full potential of Hsp90 inhibitors as cancer therapeutics through simultaneous inactivation of Hsp90, Grp94, and TRAP1. Exp. Mol. Med. 2020, 52, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Bae, S.C. Histone deacetylase inhibitors: Molecular mechanisms of action and clinical trials as anti-cancer drugs. Am. J. Transl. Res. 2011, 3, 166–179. [Google Scholar]
- Yano, M.; Yasuda, M.; Sakaki, M.; Nagata, K.; Fujino, T.; Arai, E.; Hasebe, T.; Miyazawa, M.; Miyazawa, M.; Ogane, N.; et al. Association of histone deacetylase expression with histology and prognosis of ovarian cancer. Oncol. Lett. 2018, 15, 3524–3531. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Skitzki, J.J.; Repasky, E.A.; Evans, S.S. Hyperthermia as an immunotherapy strategy for cancer. Curr. Opin. Investig. Drugs 2009, 10, 550–558. [Google Scholar]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Khan, I.U.; Brooks, G.; Guo, N.N.; Chen, J.; Guo, F. Fever-range hyperthermia promotes cGAS-STING pathway and synergizes DMXAA-induced antiviral immunity. Int. J. Hyperth. 2021, 38, 30–37. [Google Scholar] [CrossRef]
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef]
- Miller, K.N.; Victorelli, S.G.; Salmonowicz, H.; Dasgupta, N.; Liu, T.; Passos, J.F.; Adams, P.D. Cytoplasmic DNA: Sources, sensing, and role in aging and disease. Cell 2021, 184, 5506–5526. [Google Scholar] [CrossRef]
- Bai, H.; Lester, G.M.S.; Petishnok, L.C.; Dean, D.A. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci. Rep. 2017, 37, BSR20160616. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef] [Green Version]
- Motwani, M.; Pesiridis, S.; Fitzgerald, K.A. DNA sensing by the cGAS-STING pathway in health and disease. Nat. Rev. Genet. 2019, 20, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020, 10, 26–39. [Google Scholar] [CrossRef]
- Oei, A.L.; Vriend, L.E.; Crezee, J.; Franken, N.A.; Krawczyk, P.M. Effects of hyperthermia on DNA repair pathways: One treatment to inhibit them all. Radiat. Oncol. 2015, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.; Hou, Z.; Wang, M.; Li, C.; Lin, J. Recent Advances in Hyperthermia Therapy-Based Synergistic Immunotherapy. Adv. Mater. 2021, 33, 2004788. [Google Scholar] [CrossRef]
- Krawczyk, P.M.; Eppink, B.; Essers, J.; Stap, J.; Rodermond, H.; Odijk, H.; Zelensky, A.; van Bree, C.; Stalpers, L.J.; Buist, M.R.; et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc. Natl. Acad. Sci. USA 2011, 108, 9851–9856. [Google Scholar] [CrossRef] [Green Version]
- Chen, A. PARP inhibitors: Its role in treatment of cancer. Chin. J. Cancer 2011, 30, 463–471. [Google Scholar] [CrossRef]
- Schaaf, L.; Schwab, M.; Ulmer, C.; Heine, S.; Mürdter, T.E.; Schmid, J.O.; Sauer, G.; Aulitzky, W.E.; van der Kuip, H. Hyperthermia Synergizes with Chemotherapy by Inhibiting PARP1-Dependent DNA Replication Arrest. Cancer Res. 2016, 76, 2868–2875. [Google Scholar] [CrossRef] [Green Version]
- Moukarzel, L.A.; Ferrando, L.; Dopeso, H.; Stylianou, A.; Basili, T.; Pareja, F.; Da Cruz Paula, A.; Zoppoli, G.; Abu-Rustum, N.R.; Reis-Filho, J.S.; et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) with carboplatin induces distinct transcriptomic changes in ovarian tumor and normal tissues. Gynecol. Oncol. 2022, 165, 239–247. [Google Scholar] [CrossRef]
- Dellinger, T.H.; Han, E.S.; Raoof, M.; Lee, B.; Wu, X.; Cho, H.; He, T.F.; Lee, P.; Razavi, M.; Liang, W.S.; et al. Hyperthermic Intraperitoneal Chemotherapy-Induced Molecular Changes in Humans Validate Preclinical Data in Ovarian Cancer. JCO Precis. Oncol. 2022, 6, e2100239. [Google Scholar] [CrossRef]
- Fiorentini, G.; Sarti, D.; Patriti, A.; Eugeni, E.; Guerra, F.; Masedu, F.; Mackay, A.R.; Guadagni, S. Immune response activation following hyperthermic intraperitoneal chemotherapy for peritoneal metastases: A pilot study. World J. Clin. Oncol. 2020, 11, 397–404. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huffman, O.G.; Chau, D.B.; Dinicu, A.I.; DeBernardo, R.; Reizes, O. Mechanistic Insights on Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. Cancers 2023, 15, 1402. https://doi.org/10.3390/cancers15051402
Huffman OG, Chau DB, Dinicu AI, DeBernardo R, Reizes O. Mechanistic Insights on Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. Cancers. 2023; 15(5):1402. https://doi.org/10.3390/cancers15051402
Chicago/Turabian StyleHuffman, Olivia G., Danielle B. Chau, Andreea I. Dinicu, Robert DeBernardo, and Ofer Reizes. 2023. "Mechanistic Insights on Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer" Cancers 15, no. 5: 1402. https://doi.org/10.3390/cancers15051402
APA StyleHuffman, O. G., Chau, D. B., Dinicu, A. I., DeBernardo, R., & Reizes, O. (2023). Mechanistic Insights on Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. Cancers, 15(5), 1402. https://doi.org/10.3390/cancers15051402






