CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression
Abstract
:Simple Summary
Abstract
1. Introduction
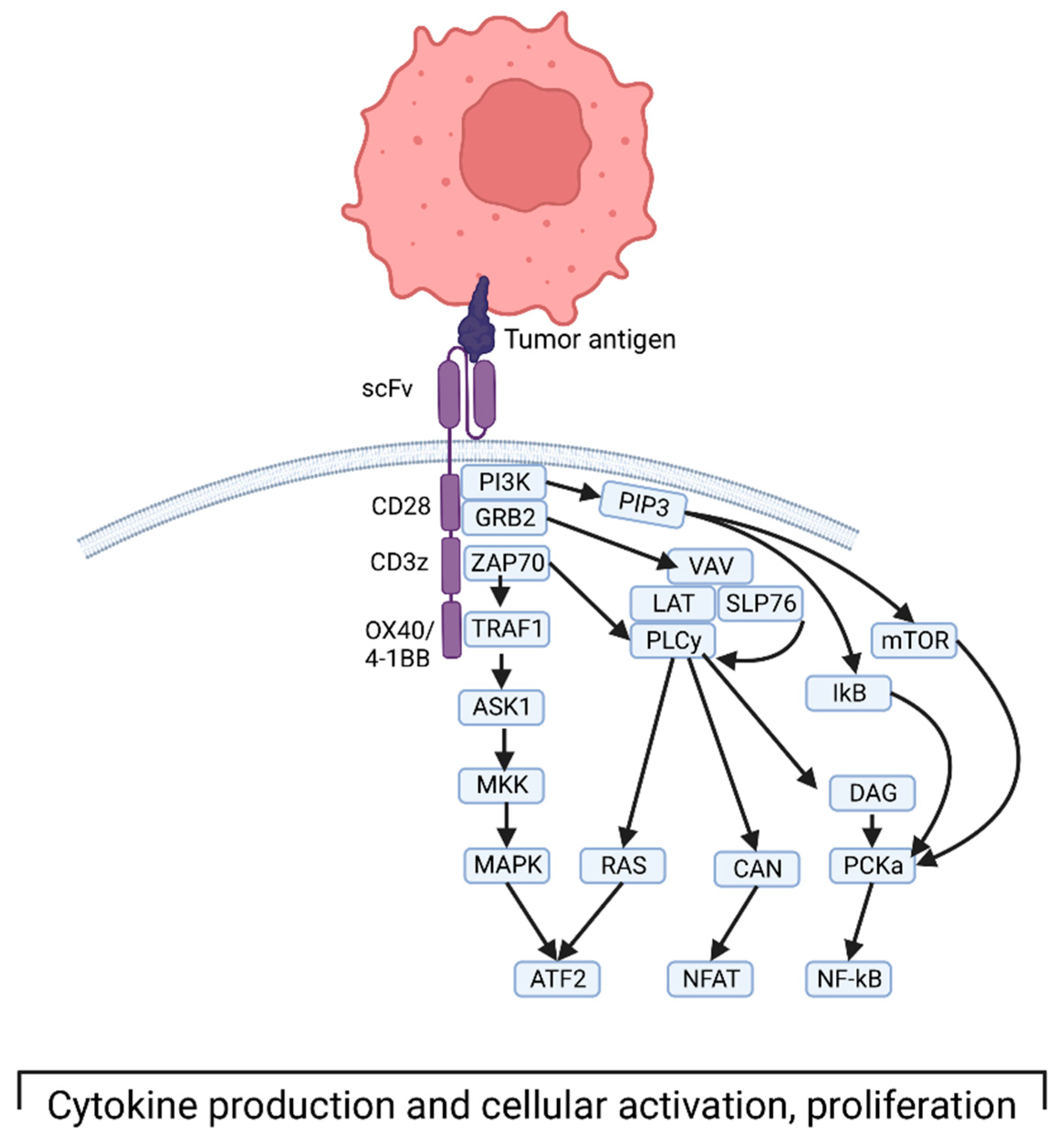
2. CAR T Therapy Background
3. CAR T Therapy in GBM
4. CAR T Therapy Evasion in GBM
5. Improving CAR T Therapy in GBM
5.1. Targeting Alternative Antigens
5.2. Targeting Multiple Antigens
5.3. Targeting Antigens of Cancer Stem Cells
5.4. Adjuvant Therapies to Increase Antigen Availability and CAR T Efficacy
5.5. CAR T Cells and Immune Exhaustion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Grossman, S.A.; Ye, X.; Piantadosi, S.; Desideri, S.; Nabors, L.B.; Rosenfeld, M.; Fisher, J.; NABTT CNS Consortium. Survival of Patients with Newly Diagnosed Glioblastoma Treated with Radiation and Temozolomide in Research Studies in the United States. Clin. Cancer Res. 2010, 16, 2443–2449. [Google Scholar] [CrossRef] [Green Version]
- Holland, E.C. Glioblastoma Multiforme: The Terminator. Proc. Natl. Acad. Sci. USA 2000, 97, 6242–6244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to Improve Delivery of Anticancer Drugs across the Blood-Brain Barrier to Treat Glioblastoma. Neuro-Oncology 2016, 18, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of Immunotherapy Resistance: Lessons from Glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef]
- Bagley, S.J.; Kothari, S.; Rahman, R.; Lee, E.Q.; Dunn, G.P.; Galanis, E.; Chang, S.M.; Nabors, L.B.; Ahluwalia, M.S.; Stupp, R.; et al. Glioblastoma Clinical Trials: Current Landscape and Opportunities for Improvement. Clin. Cancer Res. 2021, 28, 594–602. [Google Scholar] [CrossRef]
- Bausart, M.; Préat, V.; Malfanti, A. Immunotherapy for Glioblastoma: The Promise of Combination Strategies. J. Exp. Clin. Cancer Res. 2022, 41, 35. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Hwang, W.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric Antigen Receptor T Cells Persist and Induce Sustained Remissions in Relapsed Refractory Chronic Lymphocytic Leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef] [Green Version]
- Boyiadzis, M.M.; Dhodapkar, M.V.; Brentjens, R.J.; Kochenderfer, J.N.; Neelapu, S.S.; Maus, M.V.; Porter, D.L.; Maloney, D.G.; Grupp, S.A.; Mackall, C.L.; et al. Chimeric Antigen Receptor (CAR) T Therapies for the Treatment of Hematologic Malignancies: Clinical Perspective and Significance. J. ImmunoTher. Cancer. 2018, 6, 137. [Google Scholar] [CrossRef] [Green Version]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Beyar-Katz, O.; Gill, S. Advances in Chimeric Antigen Receptor T Cells. Curr. Opin. Hematol. 2020, 27, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Maus, M.V.; June, C.H.; Sampson, J.H. Immunotherapy for Glioblastoma: Adoptive T-Cell Strategies. Clin. Cancer Res. 2019, 25, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8(+) T Cells are Abundant and Phenotypically Distinct in Human Tumour Infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- Quattrocchi, K.B.; Miller, C.H.; Cush, S.; Bernard, S.A.; Dull, S.T.; Smith, M.; Gudeman, S.; Varia, M.A. Pilot Study of Local Autologous Tumor Infiltrating Lymphocytes for the Treatment of Recurrent Malignant Gliomas. J. Neurooncol. 1999, 45, 141–157. [Google Scholar] [CrossRef]
- Feins, S.; Kong, W.; Williams, E.F.; Milone, M.C.; Fraietta, J.A. An Introduction to Chimeric Antigen Receptor (CAR) T-Cell Immunotherapy for Human Cancer. Am. J. Hematol. 2019, 94, S3–S9. [Google Scholar] [CrossRef] [Green Version]
- Amini, L.; Silbert, S.K.; Maude, S.L.; Nastoupil, L.J.; Ramos, C.A.; Brentjens, R.J.; Sauter, C.S.; Shah, N.N.; Abou-El-Enein, M. Preparing for CAR T Cell Therapy: Patient Selection, Bridging Therapies and Lymphodepletion. Nat. Rev. Clin. Oncol. 2022, 19, 342–355. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T Cell Therapy for H3K27M-Mutated Diffuse Midline Gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Xin Yu, J.; Hubbard-Lucey, V.M.; Tang, J. The Global Pipeline of Cell Therapies for Cancer. Nat. Rev. Drug Discov. 2019, 18, 821–822. [Google Scholar] [CrossRef]
- Chmielewski, M.; Hombach, A.A.; Abken, H. Antigen-Specific T-Cell Activation Independently of the MHC: Chimeric Antigen Receptor-Redirected T Cells. Front. Immunol. 2013, 4, 371. [Google Scholar] [CrossRef] [Green Version]
- Guedan, S.; Calderon, H.; Posey, A.D.J.; Maus, M.V. Engineering and Design of Chimeric Antigen Receptors. Mol. Ther. Methods Clin. Dev. 2018, 12, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kershaw, M.H.; Westwood, J.A.; Darcy, P.K. Gene-Engineered T Cells for Cancer Therapy. Nat. Rev. Cancer 2013, 13, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Emtage, P.C.R.; Lo, A.S.Y.; Gomes, E.M.; Liu, D.L.; Gonzalo-Daganzo, R.M.; Junghans, R.P. Second-Generation Anti-Carcinoembryonic Antigen Designer T Cells Resist Activation-Induced Cell Death, Proliferate on Tumor Contact, Secrete Cytokines, and Exhibit Superior Antitumor Activity In Vivo: A Preclinical Evaluation. Clin. Cancer Res. 2008, 14, 8112–8122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheykhhasan, M.; Manoochehri, H.; Dama, P. Use of CAR T-Cell for Acute Lymphoblastic Leukemia (ALL) Treatment: A Review Study. Cancer Gene Ther. 2022, 29, 1080–1096. [Google Scholar] [CrossRef]
- Wang, K.; Wei, G.; Liu, D. CD19: A Biomarker for B Cell Development, Lymphoma Diagnosis and Therapy. Exp. Hematol. Oncol. 2012, 1, 36. [Google Scholar] [CrossRef] [Green Version]
- Westin, J.; Sehn, L.H. CAR T Cells as a Second-Line Therapy for Large B-Cell Lymphoma: A Paradigm Shift? Blood 2022, 139, 2737–2746. [Google Scholar] [CrossRef]
- Shargian, L.; Raanani, P.; Yeshurun, M.; Gafter-Gvili, A.; Gurion, R. Chimeric Antigen Receptor T-Cell Therapy is Superior to Standard of Care as Second-Line Therapy for Large B-Cell Lymphoma: A Systematic Review and Meta-Analysis. Br. J. Haematol. 2022, 198, 838–846. [Google Scholar] [CrossRef]
- Shah, N.N.; Johnson, B.D.; Schneider, D.; Zhu, F.; Szabo, A.; Keever-Taylor, C.A.; Krueger, W.; Worden, A.A.; Kadan, M.J.; Yim, S.; et al. Bispecific Anti-CD20, Anti-CD19 CAR T Cells for Relapsed B Cell Malignancies: A Phase 1 Dose Escalation and Expansion Trial. Nat. Med. 2020, 26, 1569–1575. [Google Scholar] [CrossRef]
- Frey, N.; Porter, D. Cytokine Release Syndrome with Chimeric Antigen Receptor T Cell Therapy. Biol. Blood Marrow Transplant. 2019, 25, e123–e127. [Google Scholar] [CrossRef] [Green Version]
- Roschewski, M.; Longo, D.L.; Wilson, W.H. CAR T-Cell Therapy for Large B-Cell Lymphoma—Who, when, and how? N. Engl. J. Med. 2022, 386, 692–696. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Ge, H.; Lin, Y.; Kang, D. Glioblastoma Vaccine Tumor Therapy Research Progress. Chin. Neurosurg. J. 2022, 8, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Bagley, S.J.; Desai, A.S.; Linette, G.P.; June, C.H.; O’Rourke, D.M. CAR T-Cell Therapy for Glioblastoma: Recent Clinical Advances and Future Challenges. Neuro-Oncology 2018, 20, 1429–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, S.A.; Ye, X.; Lesser, G.; Sloan, A.; Carraway, H.; Desideri, S.; Piantadosi, S.; NABTT CNS Consortium. Immunosuppression in Patients with High-Grade Gliomas Treated with Radiation and Temozolomide. Clin. Cancer Res. 2011, 17, 5473–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woroniecka, K.I.; Rhodin, K.E.; Chongsathidkiet, P.; Keith, K.A.; Fecci, P.E. T-Cell Dysfunction in Glioblastoma: Applying a New Framework. Clin. Cancer Res. 2018, 24, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Chongsathidkiet, P.; Jackson, C.; Koyama, S.; Loebel, F.; Cui, X.; Farber, S.H.; Woroniecka, K.; Elsamadicy, A.A.; Dechant, C.A.; Kemeny, H.R.; et al. Sequestration of T Cells in Bone Marrow in the Setting of Glioblastoma and Other Intracranial Tumors. Nat. Med. 2018, 24, 1459–1468. [Google Scholar] [CrossRef]
- Nejo, T.; Yamamichi, A.; Almeida, N.D.; Goretsky, Y.E.; Okada, H. Tumor Antigens in Glioma. Semin. Immunol. 2020, 47, 101385. [Google Scholar] [CrossRef]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T Cell Therapy for Solid Tumors. Annu. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef]
- Douglass, J.; Hsiue, E.H.; Mog, B.J.; Hwang, M.S.; DiNapoli, S.R.; Pearlman, A.H.; Miller, M.S.; Wright, K.M.; Azurmendi, P.A.; Wang, Q.; et al. Bispecific Antibodies Targeting Mutant RAS Neoantigens. Sci. Immunol. 2021, 6, eabd5515. [Google Scholar] [CrossRef]
- Congdon, K.L.; Gedeon, P.C.; Suryadevara, C.M.; Caruso, H.G.; Cooper, L.J.N.; Heimberger, A.B.; Sampson, J.H. Epidermal Growth Factor Receptor and Variant III Targeted Immunotherapy. Neuro-Oncology 2014, 16 (Suppl. S8), viii20–viii25. [Google Scholar] [CrossRef] [Green Version]
- Heimberger, A.B.; Hlatky, R.; Suki, D.; Yang, D.; Weinberg, J.; Gilbert, M.; Sawaya, R.; Aldape, K. Prognostic Effect of Epidermal Growth Factor Receptor and EGFRvIII in Glioblastoma Multiforme Patients. Clin. Cancer Res. 2005, 11, 1462–1466. [Google Scholar] [CrossRef] [Green Version]
- Eskilsson, E.; Rosland, G.V.; Talasila, K.M.; Knappskog, S.; Keunen, O.; Sottoriva, A.; Foerster, S.; Solecki, G.; Taxt, T.; Jirik, R.; et al. EGFRvIII Mutations can Emerge as Late and Heterogenous Events in Glioblastoma Development and Promote Angiogenesis through Src Activation. Neuro-Oncology 2016, 18, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.; Weiss, W.A. Epidermal Growth Factor Receptor and EGFRvIII in Glioblastoma: Signaling Pathways and Targeted Therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Thorne, A.H.; Zanca, C.; Furnari, F. Epidermal Growth Factor Receptor Targeting and Challenges in Glioblastoma. Neuro-Oncology 2016, 18, 914–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chistiakov, D.A.; Chekhonin, I.V.; Chekhonin, V.P. The EGFR Variant III Mutant as a Target for Immunotherapy of Glioblastoma Multiforme. Eur. J. Pharmacol. 2017, 810, 70–82. [Google Scholar] [CrossRef]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-Transduced T Cells Targeting EGFRvIII in Patients with Glioblastoma. J. Immunother. 2019, 42, 126–135. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, D.M.; Nasrallah, M.P.; Desai, A.; Melenhorst, J.J.; Mansfield, K.; Morrissette, J.J.D.; Martinez-Lage, M.; Brem, S.; Maloney, E.; Shen, A.; et al. A Single Dose of Peripherally Infused EGFRvIII-Directed CAR T Cells Mediates Antigen Loss and Induces Adaptive Resistance in Patients with Recurrent Glioblastoma. Sci. Transl. Med. 2017, 9, eaaa0984. [Google Scholar] [CrossRef] [Green Version]
- Durgin, J.S.; Henderson, F.J.; Nasrallah, M.P.; Mohan, S.; Wang, S.; Lacey, S.F.; Melenhorst, J.J.; Desai, A.S.; Lee, J.Y.K.; Maus, M.V.; et al. Case Report: Prolonged Survival Following EGFRvIII CAR T Cell Treatment for Recurrent Glioblastoma. Front. Oncol. 2021, 11, 669071. [Google Scholar] [CrossRef]
- Thaci, B.; Brown, C.E.; Binello, E.; Werbaneth, K.; Sampath, P.; Sengupta, S. Significance of Interleukin-13 Receptor Alpha 2-Targeted Glioblastoma Therapy. Neuro-Oncology 2014, 16, 1304–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- His, L.C.; Kundu, S.; Palomo, J.; Xu, B.; Ficco, R.; Vogelbaum, M.A.; Cathcart, M.K. Silencing IL-13Rα2 Promotes Glioblastoma Cell Death via Endogenous Signaling. Mol. Cancer Ther. 2011, 10, 1149–1160. [Google Scholar]
- Sattiraju, A.; Solingapuram Sai, K.K.; Xuan, A.; Pandya, D.N.; Almaguel, F.G.; Wadas, T.J.; Herpai, D.M.; Debinski, W.; Mintz, A. IL13RA2 Targeted Alpha Particle Therapy Against Glioblastomas. Oncotarget 2017, 8, 42997–43007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krenciute, G.; Prinzing, B.L.; Yi, Z.; Wu, M.; Liu, H.; Dotti, G.; Balyasnikova, I.V.; Gottschalk, S. Transgenic Expression of IL15 Improves Antiglioma Activity of IL13Rα2-CAR T Cells but Results in Antigen Loss Variants. Cancer Immunol. Res. 2017, 5, 571–581. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.E.; Badie, B.; Barish, M.E.; Weng, L.; Ostberg, J.R.; Chang, W.; Naranjo, A.; Starr, R.; Wagner, J.; Wright, C.; et al. Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2015, 21, 4062–4072. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.E.; Alizadeh, D.; Starr, R.; Weng, L.; Wagner, J.R.; Naranjo, A.; Ostberg, J.R.; Blanchard, M.S.; Kilpatrick, J.; Simpson, J.; et al. Regression of Glioblastoma After Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016, 375, 2561–2569. [Google Scholar] [CrossRef]
- Ahmed, N.; Salsman, V.S.; Kew, Y.; Shaffer, D.; Powell, S.; Zhang, Y.J.; Grossman, R.G.; Heslop, H.E.; Gottschalk, S. HER2-Specific T Cells Target Primary Glioblastoma Stem Cells and Induce Regression of Autologous Experimental Tumors. Clin. Cancer Res. 2010, 16, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced with a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor-Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Teachey, D.T.; Porter, D.L.; Grupp, S.A. CD19-Targeted Chimeric Antigen Receptor T-Cell Therapy for Acute Lymphoblastic Leukemia. Blood 2015, 125, 4017–4023. [Google Scholar] [CrossRef] [Green Version]
- Majzner, R.G.; Mackall, C.L. Tumor Antigen Escape from CAR T-Cell Therapy. Cancer Discov. 2018, 8, 1219–1226. [Google Scholar] [CrossRef] [Green Version]
- Sotillo, E.; Barrett, D.M.; Black, K.L.; Bagashev, A.; Oldridge, D.; Wu, G.; Sussman, R.; Lanauze, C.; Ruella, M.; Gazzara, M.R.; et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015, 5, 1282–1295. [Google Scholar] [CrossRef] [Green Version]
- Sampson, J.H.; Heimberger, A.B.; Archer, G.E.; Aldape, K.D.; Friedman, A.H.; Friedman, H.S.; Gilbert, M.R.; Herndon, J.E., II; McLendon, R.E.; Mitchell, D.A.; et al. Immunologic Escape After Prolonged Progression-Free Survival with Epidermal Growth Factor Receptor Variant III Peptide Vaccination in Patients with Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2010, 28, 4722–4729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-Cell RNA-Seq Highlights Intratumoral Heterogeneity in Primary Glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef] [Green Version]
- Barish, M.E.; Weng, L.; Awabdeh, D.; Zhai, Y.; Starr, R.; D’Apuzzo, M.; Rockne, R.C.; Li, H.; Badie, B.; Forman, S.J.; et al. Spatial Organization of Heterogeneous Immunotherapy Target Antigen Expression in High-Grade Glioma. Neoplasia 2022, 30, 100801. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.A.; Vora, P.; Venugopal, C.; Sidhu, S.S.; Moffat, J.; Swanton, C.; Singh, S.K. Intratumoral Heterogeneity: Pathways to Treatment Resistance and Relapse in Human Glioblastoma. Ann. Oncol. 2017, 28, 1448–1456. [Google Scholar] [CrossRef]
- Bar-Ephraim, Y.E.; Kretzschmar, K.; Clevers, H. Organoids in Immunological Research. Nat. Rev. Immunol. 2020, 20, 279–293. [Google Scholar] [CrossRef]
- Jacob, F.; Ming, G.; Song, H. Generation and Biobanking of Patient-Derived Glioblastoma Organoids and their Application in CAR T Cell Testing. Nat. Protoc. 2020, 15, 4000–4033. [Google Scholar] [CrossRef]
- Wei, W.; Shin, Y.S.; Xue, M.; Matsutani, T.; Masui, K.; Yang, H.; Ikegami, S.; Gu, Y.; Herrmann, K.; Johnson, D.; et al. Single-Cell Phosphoproteomics Resolves Adaptive Signaling Dynamics and Informs Targeted Combination Therapy in Glioblastoma. Cancer Cell 2016, 29, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Zhao, S.; Zhang, Y.; Wang, Y.; Zhang, Z.; Yang, M.; Zhu, Y.; Zhang, G.; Guo, G.; Tong, A.; et al. B7-H3 as a Novel CAR-T Therapeutic Target for Glioblastoma. Mol. Ther. Oncolytics 2019, 14, 279–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Zhang, Z.; Li, F.; Shen, Z.; Qiao, Y.; Li, L.; Liu, S.; Song, M.; Zhao, X.; Ren, F.; et al. Large-Scale Analysis Reveals the Specific Clinical and Immune Features of B7-H3 in Glioma. Oncoimmunology 2018, 7, e1461304. [Google Scholar] [CrossRef] [Green Version]
- Nehama, D.; Di Ianni, N.; Musio, S.; Du, H.; Patané, M.; Pollo, B.; Finocchiaro, G.; Park, J.J.H.; Dunn, D.E.; Edwards, D.S.; et al. B7-H3-Redirected Chimeric Antigen Receptor T Cells Target Glioblastoma and Neurospheres. EBioMedicine 2019, 47, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykosky, J.; Gibo, D.M.; Stanton, C.; Debinski, W. EphA2 as a Novel Molecular Marker and Target in Glioblastoma Multiforme. Mol. Cancer Res. 2005, 3, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Chow, K.K.H.; Naik, S.; Kakarla, S.; Brawley, V.S.; Shaffer, D.R.; Yi, Z.; Rainusso, N.; Wu, M.; Liu, H.; Kew, Y.; et al. T Cells Redirected to EphA2 for the Immunotherapy of Glioblastoma. Mol. Ther. 2013, 21, 629–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Z.; Prinzing, B.L.; Cao, F.; Gottschalk, S.; Krenciute, G. Optimizing EphA2-CAR T Cells for the Adoptive Immunotherapy of Glioma. Mol. Ther. Methods Clin. Dev. 2018, 9, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Ge, H.; Long, Y.; Yang, C.; Chang, Y.E.; Mu, L.; Sayour, E.J.; De Leon, G.; Wang, Q.J.; Yang, J.C.; et al. CD70, a Novel Target of CAR T-Cell Therapy for Gliomas. Neuro-Oncology 2018, 20, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Seyfrid, M.; Maich, W.T.; Shaikh, V.M.; Tatari, N.; Upreti, D.; Piyasena, D.; Subapanditha, M.; Savage, N.; McKenna, D.; Mikolajewicz, N.; et al. CD70 as an Actionable Immunotherapeutic Target in Recurrent Glioblastoma and its Microenvironment. J. ImmunoTher. Cancer 2022, 10, e003289. [Google Scholar] [CrossRef]
- Wang, Q.J.; Yu, Z.; Hanada, K.; Patel, K.; Kleiner, D.; Restifo, N.P.; Yang, J.C. Preclinical Evaluation of Chimeric Antigen Receptors Targeting CD70-Expressing Cancers. Clin. Cancer Res. 2017, 23, 2267–2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, L.; Tao, H.; Karachi, A.; Long, Y.; Hou, A.Y.; Na, M.; Dyson, K.A.; Grippin, A.J.; Deleyrolle, L.P.; Zhang, W.; et al. CXCR1- Or CXCR2-Modified CAR T Cells Co-Opt IL-8 for Maximal Antitumor Efficacy in Solid Tumors. Nat. Commun. 2019, 10, 4016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Lemoi, B.A.; Sentman, C.L. Chimeric NK-Receptor-Bearing T Cells Mediate Antitumor Immunotherapy. Blood 2005, 106, 1544–1551. [Google Scholar] [CrossRef]
- Crane, C.A.; Austgen, K.; Haberthur, K.; Hofmann, C.; Moyes, K.W.; Avanesyan, L.; Fong, L.; Campbell, M.J.; Cooper, S.; Oakes, S.A.; et al. Immune Evasion Mediated by Tumor-Derived Lactate Dehydrogenase Induction of NKG2D Ligands on Myeloid Cells in Glioblastoma Patients. Proc. Natl. Acad. Sci. USA 2014, 111, 12823–12828. [Google Scholar] [CrossRef] [Green Version]
- Weiss, T.; Schneider, H.; Silginer, M.; Steinle, A.; Pruschy, M.; Polić, B.; Weller, M.; Roth, P. NKG2D-Dependent Antitumor Effects of Chemotherapy and Radiotherapy Against Glioblastoma. Clin. Cancer Res. 2018, 24, 882–895. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Sun, B.; Dai, H.; Li, W.; Shi, L.; Zhang, P.; Li, S.; Zhao, X. T Cells Expressing NKG2D Chimeric Antigen Receptors Efficiently Eliminate Glioblastoma and Cancer Stem Cells. J. ImmunoTher. Cancer 2019, 7, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Zhang, H.; Cao, H.; Tang, L.; Mo, H.; Liu, F.; Zhang, L.; Yi, Z.; Long, L.; Yan, L.; et al. Tumor Buster—Where Will the CAR-T Cell Therapy ‘Missile’ Go? Mol. Cancer 2022, 21, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Prapa, M.; Chiavelli, C.; Golinelli, G.; Grisendi, G.; Bestagno, M.; Di Tinco, R.; Dall’Ora, M.; Neri, G.; Candini, O.; Spano, C.; et al. GD2 CAR T Cells Against Human Glioblastoma. NPJ Precis. Oncol. 2021, 5, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gargett, T.; Ebert, L.M.; Truong, N.T.H.; Kollis, P.M.; Sedivakova, K.; Yu, W.; Yeo, E.C.F.; Wittwer, N.L.; Gliddon, B.L.; Tea, M.N.; et al. GD2-Targeting CAR-T Cells Enhanced by Transgenic IL-15 Expression are an Effective and Clinically Feasible Therapy for Glioblastoma. J. ImmunoTher. Cancer 2022, 10, e005187. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Yang, X.; Liu, Y.; Zou, C.; Lv, W.; Chen, C.; Cheng, K.K.; Chen, T.; Chang, L.; et al. Safety and Antitumor Activity of GD2-Specific 4SCAR-T Cells in Patients with Glioblastoma. Mol. Cancer. 2023, 22, 3–9. [Google Scholar] [CrossRef]
- Pellegatta, S.; Savoldo, B.; Di Ianni, N.; Corbetta, C.; Chen, Y.; Patané, M.; Sun, C.; Pollo, B.; Ferrone, S.; DiMeco, F.; et al. Constitutive and TNFα-Inducible Expression of Chondroitin Sulfate Proteoglycan 4 in Glioblastoma and Neurospheres: Implications for CAR-T Cell Therapy. Sci. Transl. Med. 2018, 10, eaao2731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mayhani, M.T.F.; Grenfell, R.; Narita, M.; Piccirillo, S.; Kenney-Herbert, E.; Fawcett, J.W.; Collins, V.P.; Ichimura, K.; Watts, C. NG2 Expression in Glioblastoma Identifies an Actively Proliferating Population with an Aggressive Molecular Signature. Neuro-Oncology 2011, 13, 830–845. [Google Scholar] [CrossRef] [Green Version]
- Chekenya, M.; Krakstad, C.; Svendsen, A.; Netland, I.A.; Staalesen, V.; Tysnes, B.B.; Selheim, F.; Wang, J.; Sakariassen, P.Ø.; Sandal, T.; et al. The Progenitor Cell Marker NG2/MPG Promotes Chemoresistance by Activation of Integrin-Dependent PI3K/Akt Signaling. Oncogene 2008, 27, 5182–5194. [Google Scholar] [CrossRef] [Green Version]
- Lyons, S.A.; O’Neal, J.; Sontheimer, H. Chlorotoxin, a Scorpion-Derived Peptide, Specifically Binds to Gliomas and Tumors of Neuroectodermal Origin. Glia 2002, 39, 162–173. [Google Scholar] [CrossRef]
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults with Newly Diagnosed Or Recurrent Gliomas. Neurosurgery 2019, 85, E641–E649. [Google Scholar] [CrossRef]
- Wang, D.; Starr, R.; Chang, W.; Aguilar, B.; Alizadeh, D.; Wright, S.L.; Yang, X.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; et al. Chlorotoxin-Directed CAR T Cells for Specific and Effective Targeting of Glioblastoma. Sci. Transl. Med. 2020, 12, eaaw2672. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Rossi, J.M.; Chou, J.; Wang, L.; Poddar, S.; Han, G.; Wang, Z.; Kuang, S.; Chu, F.; Davis, R.E.; et al. CD19 Target Evasion as a Mechanism of Relapse in Large B-Cell Lymphoma Treated with Axicabtagene Ciloleucel. Blood 2021, 138, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Corder, A.; Chow, K.K.H.; Mukherjee, M.; Ashoori, A.; Kew, Y.; Zhang, Y.J.; Baskin, D.S.; Merchant, F.A.; Brawley, V.S.; et al. Combinational Targeting Offsets Antigen Escape and Enhances Effector Functions of Adoptively Transferred T Cells in Glioblastoma. Mol. Ther. 2013, 21, 2087–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Tang, X.; Zhang, Z.; Gu, L.; Wei, H.; Zhao, S.; Zhong, K.; Mu, M.; Huang, C.; Jiang, C.; et al. Tandem CAR-T Cells Targeting CD70 and B7-H3 Exhibit Potent Preclinical Activity against Multiple Solid Tumors. Theranostics 2020, 10, 7622–7634. [Google Scholar] [CrossRef] [PubMed]
- Bielamowicz, K.; Fousek, K.; Byrd, T.T.; Samaha, H.; Mukherjee, M.; Aware, N.; Wu, M.; Orange, J.S.; Sumazin, P.; Man, T.; et al. Trivalent CAR T Cells Overcome Interpatient Antigenic Variability in Glioblastoma. Neuro-Oncology 2018, 20, 506–518. [Google Scholar] [CrossRef] [Green Version]
- Morsut, L.; Roybal, K.T.; Xiong, X.; Gordley, R.M.; Coyle, S.M.; Thomson, M.; Lim, W.A. Engineering Customized Cell Sensing and Response Behaviors using Synthetic Notch Receptors. Cell 2016, 164, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Choe, J.H.; Watchmaker, P.B.; Simic, M.S.; Gilbert, R.D.; Li, A.W.; Krasnow, N.A.; Downey, K.M.; Yu, W.; Carrera, D.A.; Celli, A.; et al. SynNotch-CAR T Cells Overcome Challenges of Specificity, Heterogeneity, and Persistence in Treating Glioblastoma. Sci. Transl. Med. 2021, 13, eabe7378. [Google Scholar] [CrossRef]
- Vescovi, A.L.; Galli, R.; Reynolds, B.A. Brain Tumour Stem Cells. Nat. Rev. Cancer 2006, 6, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Esparza, R.; Azad, T.D.; Feroze, A.H.; Mitra, S.S.; Cheshier, S.H. Glioblastoma Stem Cells and Stem Cell-Targeting Immunotherapies. J. Neurooncol. 2015, 123, 449–457. [Google Scholar] [CrossRef]
- Beier, D.; Schulz, J.B.; Beier, C.P. Chemoresistance of Glioblastoma Cancer Stem Cells—Much More Complex than Expected. Mol. Cancer 2011, 10, 128. [Google Scholar]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Starr, R.; Aguilar, B.; Shami, A.F.; Martinez, C.; D’Apuzzo, M.; Barish, M.E.; Forman, S.J.; Jensen, M.C. Stem-Like Tumor-Initiating Cells Isolated from IL13Rα2 Expressing Gliomas are Targeted and Killed by IL13-Zetakine-Redirected T Cells. Clin. Cancer Res. 2012, 18, 2199–2209. [Google Scholar] [CrossRef] [Green Version]
- Emlet, D.R.; Gupta, P.; Holgado-Madruga, M.; Del Vecchio, C.A.; Mitra, S.S.; Han, S.; Li, G.; Jensen, K.C.; Vogel, H.; Xu, L.W.; et al. Targeting a Glioblastoma Cancer Stem-Cell Population Defined by EGF Receptor Variant III. Cancer Res. 2014, 74, 1238–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, T.; Weller, M.; Guckenberger, M.; Sentman, C.L.; Roth, P. NKG2D-Based CAR T Cells and Radiotherapy Exert Synergistic Efficacy in Glioblastoma. Cancer Res. 2018, 78, 1031–1043. [Google Scholar] [CrossRef] [Green Version]
- DeSelm, C.; Palomba, M.L.; Yahalom, J.; Hamieh, M.; Eyquem, J.; Rajasekhar, V.K.; Sadelain, M. Low-Dose Radiation Conditioning Enables CAR T Cells to Mitigate Antigen Escape. Mol. Ther. 2018, 26, 2542–2552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Bowerman, N.A.; Salama, J.K.; Schmidt, H.; Spiotto, M.T.; Schietinger, A.; Yu, P.; Fu, Y.; Weichselbaum, R.R.; Rowley, D.A.; et al. Induced Sensitization of Tumor Stroma Leads to Eradication of Established Cancer by T Cells. J. Exp. Med. 2007, 204, 49–55. [Google Scholar] [CrossRef]
- Guedan, S.; Alemany, R. CAR-T Cells and Oncolytic Viruses: Joining Forces to Overcome the Solid Tumor Challenge. Front. Immunol. 2018, 9, 2460. [Google Scholar] [CrossRef] [Green Version]
- Park, A.K.; Fong, Y.; Kim, S.; Yang, J.; Murad, J.P.; Lu, J.; Jeang, B.; Chang, W.; Chen, N.G.; Thomas, S.H.; et al. Effective Combination Immunotherapy using Oncolytic Viruses to Deliver CAR Targets to Solid Tumors. Sci. Transl. Med. 2020, 12, eaaz1863. [Google Scholar] [CrossRef]
- Cohen-Inbar, O.; Xu, Z.; Sheehan, J.P. Focused Ultrasound-Aided Immunomodulation in Glioblastoma Multiforme: A Therapeutic Concept. J. Ther. Ultrasound 2016, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Mashouf, L.A.; Lim, M. CAR T Cell Therapy in Primary Brain Tumors: Current Investigations and the Future. Front. Immunol. 2022, 13, 817296. [Google Scholar] [CrossRef]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T Cell Exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Gumber, D.; Wang, L.D. Improving CAR-T Immunotherapy: Overcoming the Challenges of T Cell Exhaustion. EBioMedicine 2022, 77, 103941. [Google Scholar] [CrossRef]
- Luksik, A.S.; Maxwell, R.; Garzon-Muvdi, T.; Lim, M. The Role of Immune Checkpoint Inhibition in the Treatment of Brain Tumors. Neurotherapeutics 2017, 14, 1049–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, S.H.; Woroniecka, K.; Barbour, A.B.; Fecci, P.E.; Sanchez-Perez, L.; Sampson, J.H. CAR T Cells and Checkpoint Inhibition for the Treatment of Glioblastoma. Expert Opin. Biol. Ther. 2020, 20, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Boesteanu, A.C.; Binder, Z.A.; Xu, C.; Reid, R.A.; Rodriguez, J.L.; Cook, D.R.; Thokala, R.; Blouch, K.; McGettigan-Croce, B.; et al. Checkpoint Blockade Reverses Anergy in IL-13Rα2 Humanized scFv-Based CAR T Cells to Treat Murine and Canine Gliomas. Mol. Ther. Oncolytics 2018, 11, 20–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, E.W.; Parker, K.R.; Sotillo, E.; Lynn, R.C.; Anbunathan, H.; Lattin, J.; Good, Z.; Belk, J.A.; Daniel, B.; Klysz, D.; et al. Transient Rest Restores Functionality in Exhausted CAR-T Cells through Epigenetic Remodeling. Science 2021, 372, eaba1786. [Google Scholar] [CrossRef]
| Status | Target Antigen | Title | Primary Site | ID |
|---|---|---|---|---|
| Completed | EGFRvIII | CART-EGFRvIII + Pembrolizumab in GBM | Abramson Cancer Center of the University of Pennsylvania, Philadelphia, PA | NCT03726515 |
| Completed | EGFRvIII | CAR T Cell Receptor Immunotherapy Targeting EGFRvIII for Patients with Malignant Gliomas Expressing EGFRvIII | National Institutes of Health Clinical Center, 9000 Rockville Pike, Bethesda, MD | NCT01454596 |
| Completed | IL13Rα2 | Cellular Adoptive Immunotherapy Using Genetically Modified T-Lymphocytes in Treating Patients with Recurrent or Refractory High-Grade Malignant Glioma | City of Hope Medical Center, Duarte, CA | NCT00730613 |
| Completed | HER2 | CMV-specific Cytotoxic T Lymphocytes Expressing CAR Targeting HER2 in Patients With GBM | Houston Methodist Hospital, Houston, TX; Texas Children’s Hospital, Houston, TX | NCT01109095 |
| Active, not recruiting | EGFRvIII | The Efficacy and Safety of Brain-targeting Immune Cells (EGFRvIII-CAR T Cells) in Treating Patients with Leptomeningeal Disease from Glioblastoma. Administering Patients EGFRvIII -CAR T Cells May Help to Recognize and Destroy Brain Tumor Cells in Patients | Jyväskylä Central Hospital, Jyväskylä, Finland; University of Oulu, Oulu, Finland; Apollo Hospital, New Delhi, India | NCT05063682 |
| Active, not recruiting | IL13Rα2 | Genetically Modified T-cells in Treating Patients with Recurrent or Refractory Malignant Glioma | City of Hope Comprehensive Cancer Center, Duarte, CA | NCT02208362 |
| Recruiting | B7-H3 | Safety and Efficacy Study of Anti-B7-H3 CAR-T Cell Therapy for Recurrent Glioblastoma | Beijing Tiantan Hospital, Beijing, China | NCT05241392 |
| Recruiting | B7-H3 | B7-H3 CAR-T for Recurrent or Refractory Glioblastoma | Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang, China; Huzhou Central Hospital, Huzhou, Zhejiang, China; Ningbo Yinzhou People’s Hospital, Ningbo, Zhejiang, China | NCT04077866 |
| Recruiting | B7-H3 | Autologous CAR-T Cells Targeting B7-H3 in Recurrent or Refractory GBM CAR.B7-H3Tc | Lineberger Comprehensive Cancer Center, Chapel Hill, NC | NCT05366179 |
| Recruiting | B7-H3 | B7-H3 Chimeric Antigen Receptor T Cells (B7-H3CART) in Recurrent Glioblastoma Multiforme | Stanford Cancer Institute, Palo Alto, CA | NCT05474378 |
| Recruiting | Chlorotoxin | Chimeric Antigen Receptor (CAR) T Cells with a Chlorotoxin Tumor-Targeting Domain for the Treatment of MMP2+ Recurrent or Progressive Glioblastoma | City of Hope Medical Center, Duarte, CA | NCT04214392 |
| Recruiting | HER2 | Memory-Enriched T Cells in Treating Patients with Recurrent or Refractory Grade III-IV Glioma | City of Hope Medical Center, Duarte, CA | NCT03389230 |
| Recruiting | EGFRvIII | Autologous CAR-T/TCR-T Cell Immunotherapy for Malignancies | The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China | NCT03638206 |
| Recruiting | EGFRvIII | Autologous CAR-T/TCR-T Cell Immunotherapy for Solid Malignancies | Henan Provincial People’s Hospital, Zhengzhou, Henan, China | NCT03941626 |
| Recruiting | EGFRvIII, GD2, IL13Rα2, Her2, EphA2, CD133 | Personalized Chimeric Antigen Receptor T Cell Immunotherapy for Patients with Recurrent Malignant Gliomas | Xuanwu Hospital, Beijing, China | NCT03423992 |
| Recruiting | IL13Rα2 | A Clinical Study of IL13RŒ ± 2 Targeted CAR-T in Patients with Malignant Glioma (MAGIC-I) | National Cancer Center, Korea, Goyang-si, Gyeonggi, Republic of Korea | NCT05540873 |
| Recruiting | IL13Rα2 | IL13Rα2-CAR T Cells with or Without Nivolumab and Ipilimumab in Treating Patients With GBM | City of Hope Medical Center, Duarte, CA | NCT04003649 |
| Recruiting | IL13Rα2 | Brain Tumor-Specific Immune Cells (IL13Ralpha2-CAR T Cells) for the Treatment of Leptomeningeal Glioblastoma, Ependymoma, or Medulloblastoma | City of Hope Medical Center, Duarte, CA | NCT04661384 |
| Recruiting | NKG2D | NKG2D-based CAR T-cells Immunotherapy for Patient With r/r NKG2DL+ Solid Tumors | Xunyang Changchun Shihua Hospital, Jiujiang, Jiangxi, China | NCT05131763 |
| Not yet recruiting | CLTX | A Phase 1 Study to Evaluate CHM-1101 CAR T Cells in Patients with MMP2+ Recurrent or Progressive Glioblastoma | City of Hope Medical Center, Duarte, CA | NCT05627323 |
| Not yet recruiting | Dual CD70, IL8 (CXCR2) | Phase I Study of IL-8 Receptor-modified CD70 CAR T Cell Therapy in CD70+ and MGMT-unmethylated Adult Glioblastoma (IMPACT) | University of Florida Health, Gainesville, FL | NCT05353530 |
| Not yet recruiting | EGFRvIII | Long-term Follow-up of Subjects Treated with CARv3-TEAM-E T Cells | Massachusetts General Hospital, Boston, MA | NCT05024175 |
| Not yet recruiting | IL7R1 (CD127) | Tris-CAR-T Cell Therapy for Recurrent Glioblastoma | Beijing Tiantan Hospital, Beijing, China | NCT05577091 |
| Not yet recruiting | NKG2D | Pilot Study of NKG2D CAR-T in Treating Patients with Recurrent Glioblastoma | Not listed | NCT04717999 |
| Not yet recruiting | NKG2D | NKG2D CAR-T(KD-025) in the Treatment of Relapsed or Refractory NKG2DL+ Tumors | The Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School, Nanjing, Jiangsu, China | NCT04550663 |
| Antigen | Full Name | Description | Clinical Trial Status |
|---|---|---|---|
| EGFRvIII | Epidermal growth factor receptor, variant 3 | Transmembrane receptor tyrosine kinase that is amplified in half of GBM tumors, minimally expressed in CNS | First trials completed, ongoing recruitment in multiple other trials |
| IL13Rα2 | Interleukin-13 receptor α chain variant 2 | Receptor that binds IL-13 to activate downstream JAK-STAT signaling to promote apoptosis; acts as decoy receptor in GBM | First trial completed, ongoing recruitment in multiple other trials |
| HER2 | Human epidermal growth factor receptor 2 | Receptor tyrosine kinase normally expressed in epidermal tissue at low levels, upregulated in 80% of GBM tumors | First trial completed, ongoing recruitment in multiple other trials |
| B7-H3 | B7 homolog 3 protein | Transmembrane immune checkpoint protein with mixed stimulatory/inhibitory properties, expressed on over half of GBM tumors | Active recruitment in multiple trials |
| EphA2 | Erythropoietin-producing hepatocellular carcinoma A2 | Protein involved in oncogenesis, expressed in over 90% of GBM but not healthy CNS tissue | Active recruitment in single trial |
| CD70 | Cluster of differentiation 70 | Transmembrane ligand of CD27, a co-stimulatory immune cell receptor that activates TNF pathway, expressed in mesenchymal GBM cells | Not yet recruiting |
| NKG2D | Natural killer group 2-member D | Binds various ligands expressed during cellular stress, including ligands expressed by GBM tumor cells | Active recruitment in single trial |
| GD2 | Disialoganglioside | Carbohydrate-containing sphingolipid seen involved in tumor development, expressed in GBM | Active recruitment in single trial |
| CSPG4 | Chondroitin sulfate proteoglycan 4 | Protein homogeneously expressed in 67% of GBM, involved in tumor progression and chemotherapy/radiotherapy resistance | No clinical trial submitted to date |
| CLTX | Chlorotoxin | Peptide isolated from death stalker scorpion, selectively binds to GBM without binding to normal brain | Active recruitment in single trial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luksik, A.S.; Yazigi, E.; Shah, P.; Jackson, C.M. CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. Cancers 2023, 15, 1414. https://doi.org/10.3390/cancers15051414
Luksik AS, Yazigi E, Shah P, Jackson CM. CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression. Cancers. 2023; 15(5):1414. https://doi.org/10.3390/cancers15051414
Chicago/Turabian StyleLuksik, Andrew S., Eli Yazigi, Pavan Shah, and Christopher M. Jackson. 2023. "CAR T Cell Therapy in Glioblastoma: Overcoming Challenges Related to Antigen Expression" Cancers 15, no. 5: 1414. https://doi.org/10.3390/cancers15051414




